Abstract
While almost two-thirds of all strokes and one-half of all myocardial infarctions could be prevented if hypertensive individuals had their blood pressures optimally controlled, only a minority of hypertensive individuals (even in publicly funded health care systems with subsidization of medication costs) achieve target blood pressures. Traditional hypertension guidelines have had limited impact on hypertension management and control rates. As a result, the Canadian Hypertension Education Program was developed to address the perceived flaws in the traditional hypertension guideline approach. In the present article, the key features of the Canadian Hypertension Education Program methodology are reviewed, with attention to those factors thought to be critical to the successful translation of recommendations into practice.
Keywords: Evidence-based, Guidelines, Hypertension
Abstract
Presque les deux tiers des accidents vasculaires cérébraux et la moitié des infarctus du myocarde pourraient être évités si seulement la pression artérielle était bien maîtrisée chez les patients hypertendus; à l’inverse, seule une minorité de personnes hypertendues (même dans les systèmes publics de santé dotés d’un régime de médicaments subventionné) réussissent à atteindre les valeurs cibles de la pression artérielle. Force est de reconnaître que les anciennes lignes directrices ont une faible incidence sur la prise en charge de l’hypertension et sur la normalisation des valeurs. Le Programme d’éducation canadien sur l’hypertension a donc été élaboré dans l’optique de combler les lacunes perçues dans l’ancienne approche. Le présent article porte sur les principaux éléments du Programme et, en particulier, sur les facteurs que l’on croit essentiels à l’application concrète des recommandations.
World Hypertension Day is an appropriate time to emphasize some basic facts about hypertension – some well known and some less so. For example, while it is well documented (and often quoted) that over one-fifth of Canadians have hypertension (1), it is less well appreciated that the lifetime risk of developing hypertension for an otherwise healthy, North American adult now exceeds 90% (2). Second, while hypertension is recognized as an important, modifiable risk factor for cardiovascular disease, particularly stroke and myocardial infarction (3,4), some clinicians and many members of the lay press express surprise when they learn that it is the single leading cause of mortality in the world (and trails only malnutrition and unsafe sex as contributors to the global burden of disease) (5). Third, while readers of this journal would not be surprised to hear that more patients visit physicians and receive prescriptions for the treatment of hypertension than any other medical disorder (6), the magnitude is staggering – every month in Canada, over four million prescriptions are written for antihypertensive agents (7).
Despite the wealth of evidence emphasizing the importance of hypertension (both to the individual and the health care system) and the robust evidence base proving that various blood pressure-lowering agents prevent cardiovascular morbidity and mortality, it remains an uncomfortable truth that only a minority of hypertensive individuals (even in publicly funded health care systems with subsidization of medication costs) have their blood pressures treated and controlled to target levels (1,8). Indeed, it has been estimated that, worldwide, approximately two-thirds of strokes and one-half of all ischemic heart disease could be prevented if hypertensive individuals had their blood pressures optimally controlled (9). Moreover, even those hypertensive individuals who have their blood pressures treated and well controlled still exhibit an increased risk of cardiovascular events compared with age- and sex-matched controls due to the under-treatment of their other atherosclerotic risk factors (particularly hyperlipidemia) (10,11).
As a result, policy-makers and health care professional organizations have made extensive efforts to improve hypertension detection and management through the development of ongoing national knowledge translation strategies, of which the Canadian Hypertension Education Program (CHEP) is arguably the most rigorous and successful. While hypertension clinical practice guidelines have been produced and published in Canada (and most other Western nations) for over 20 years, it is important to recognize that the CHEP is not just another hypertension guideline. Indeed, the CHEP process was carefully designed to address six issues that often proved to be critical shortfalls in previous Canadian (and current national or international) hypertension guidelines.
ENDORSEMENT
It is not uncommon for guidelines from different groups to offer the clinician conflicting advice for the diagnosis and treatment of the same condition. The CHEP is guided by a multistakeholder steering committee that includes representatives from the Canadian Hypertension Society, Blood Pressure Canada (formerly the Canadian Coalition for High Blood Pressure Prevention and Control), The College of Family Physicians of Canada, the Canadian Pharmacy Association, the Canadian Council of Cardiovascular Nurses, the Heart & Stroke Foundation of Canada and the Public Health Agency of Canada. The CHEP Steering Committee ensures conformity of hypertension recommendations across member organizations and sets strategic directions for the CHEP, particularly the choice of each year’s ‘key messages’ for marketing to the public and to health care providers.
PERIODICITY
Traditionally, hypertension guidelines from national and international organizations are updated every five to seven years. As a result, there are frequently substantial delays before new evidence is incorporated into guidelines (12). As the number of therapeutic trials increases and the pace of publication accelerates over time (13), this delay is likely to continue to lengthen and to play an increasingly important role in the failure of new evidence to be translated into practice. To address this shortfall, at the heart of the CHEP process is a commitment to annually update the evidence-based recommendations for all aspects of hypertension diagnosis and management, a goal that the CHEP has achieved every year since it was established in 1999.
METHODOLOGY
Clinicians not infrequently express skepticism about the motivation behind (at least some) guideline recommendations (14), and it has been shown that those guideline recommendations that are perceived by clinicians to be most evidence-based are also those most likely to be followed (15). As a result, it seems reasonable to suppose that one of the factors contributing to the poor uptake of hypertension guidelines are clinician recognition of (and reaction to) flaws in their development (16). As such, the CHEP instituted a novel process for creating recommendations that, to my knowledge, has not yet been matched by other national or international hypertension guideline developers.
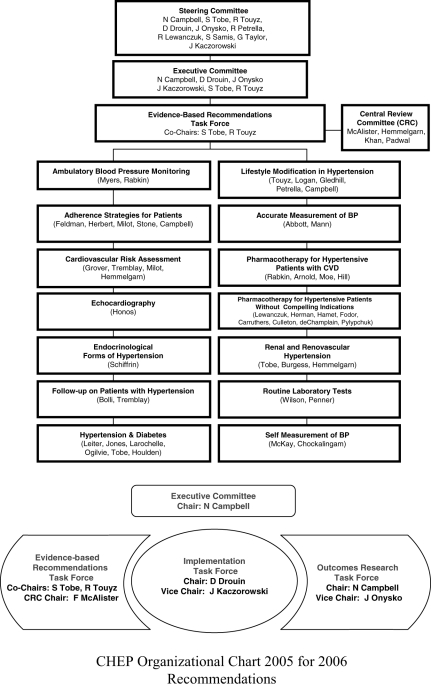
The CHEP Evidence-Based Recommendations Task Force (see Figure 1 for organizational chart) consists of 14 subcommittees with topic-specific content experts (see Appendix for listing of members). Each subcommittee is charged to review literature searches conducted by a Cochrane librarian (using standard systematic review search strategies with search terms suggested by the subcommittee) and interpret the evidence arising from identified studies. The draft conclusions derived by the content expert subcommittees are then independently validated by the Central Review Committee (clinical epidemiologists applying a priori standardized rules of evidence – see Figures 2, 3, 4 and 5 for algorithms used to grade recommendations), and in an iterative process between the Central Review Committee and each subcommittee, final draft recommendations are developed. These draft recommendations are presented and debated at a meeting of all members of the CHEP Evidence-Based Recommendations Task Force held each October at the Canadian Cardiovascular Congress (CCC). Based on discussions at the consensus conference, that year’s recommendations are finalized and presented publicly at the CCC meeting. After these presentations (which permit attendees of the CCC to provide input into the recommendations), all members of the CHEP Task Force vote to accept or reject each recommendation individually. Only those recommendations receiving at least 70% support are included in each year’s finalized recommendations. These recommendations, and the scientific rationale behind each, are published annually in The Canadian Journal of Cardiology and other Canadian health care professional journals, as well as at <www.hypertension.ca>.
Figure 1.
Canadian Hypertension Education Program 2005 organizational chart for the 2006 Recommendations Task Force. BP Blood pressure; CVD Cardiovascular disease
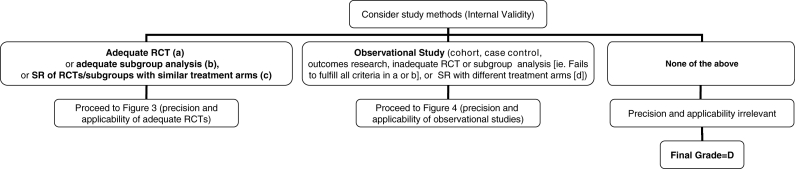
Figure 2.
Algorithm for assigning evidence grades to therapy recommendations (step 1). (a) Randomized, controlled trial (RCT) with blinded assessment of outcomes, intention-to-treat analysis, adequate follow-up (ie, at least 90%, or losses to follow-up are too few to materially affect the results) and sufficient sample size to detect a clinically important difference with power greater than 80%. (b) Subgroup analysis was a priori, done within an adequate RCT, one of only a few tested, and there was sufficient sample size within the examined subgroup to detect a clinically important difference with power greater than 80%. (c) Systematic review (SR, also known as meta-analysis) in which the comparison arms were derived from head-to-head comparisons within the same RCT. (d) SR in which the comparison arms were derived from different placebo-controlled RCTs, then extrapolations were made across RCTs
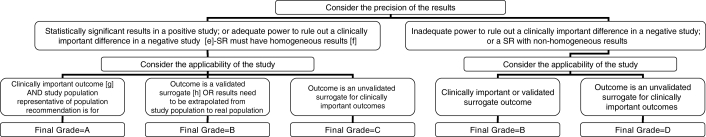
Figure 3.
Algorithm for assigning evidence grades to recommendations (continued from Figure 2 – for adequate randomized controlled trials [RCTs], systematic reviews [SRs] or subgroup analyses). (e) Adequate power in a negative study implies that 95% CIs exclude a clinically important difference. (f) Effect estimates in each study included in the systematic review were qualitatively similar (ie, in the same direction). (g) ‘Hard’ end points such as death, stroke, myocardial infarction and hospitalization. (h) End points that have been consistently shown to be associated with the clinical end point in multiple studies (observational or RCT), and RCTs consistently demonstrated that improvement in the surrogate translated into a consistent and predictable improvement in the clinical end point
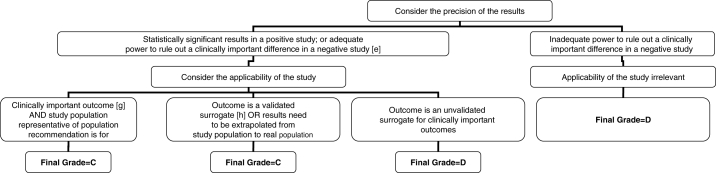
Figure 4.
Algorithm for assigning evidence grades to recommendations (continued from Figure 2 – for observational studies). (e) Adequate power in a negative study implies that 95% CI exclude a clinically important difference. (f) Effect estimates in each study included in the systematic review are qualitatively similar (ie, in the same direction). (g) ‘Hard’ end points such as death, stroke, myocardial infarction and hospitalization. (h) End points that have been consistently shown to be associated with the clinical end point in multiple studies (observational or RCT), and RCTs consistently demonstrated that improvement in the surrogate translated into a consistent and predictable improvement in the clinical end point
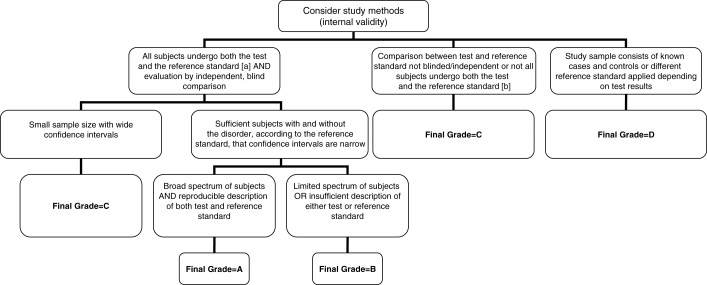
Figure 5.
Algorithm for assigning evidence grades to diagnostic recommendations. (a) The gold standard. This can be either another test which is currently accepted as the gold standard or analysis of a representative cohort of patients who underwent the test of interest and are followed for a sufficient length of time that occurrence of the target outcome is likely if the diagnosis is present (with adjustment for covariates associated with prognosis). (b) Note that if follow-up of a cohort is not sufficiently long or complete enough to rule out diagnostic errors, or if data are not adjusted for covariates, then this category would apply
Because it is a source of some confusion, it deserves emphasis here that the CHEP grading scheme for recommendations is based on the strength of the underlying evidence (derived by assessing the internal validity, precision and applicability of each study supporting a recommendation, using Figures 2, 3 and 4 for therapy recommendations and Figure 5 for diagnosis recommendations) and not the clinical importance of the recommendation. Thus, recommendations that have been assigned a grade D ranking because of lack of evidence (such as the importance of measuring blood pressure) should not be interpreted as being any less clinically important than those assigned a grade A. To avoid confusion when marketing the key messages to patients and health care providers, evidence grades are removed from recommendations at the implementation stage.
CONFLICTS OF INTEREST
Increasingly, attention is being focused on the potential influence of relationships between the pharmaceutical industry and experts who sit on guideline panels (17). The CHEP has been cognizant of the potential for such conflicts of interest since its inception, and operates a transparent system to ensure the integrity of the process and the quality of the recommendations made. Thus, while CHEP volunteers (all scientific members of the CHEP listed in the Appendix are unpaid volunteers) are not barred from accepting honoraria for speaking engagements or from serving as consultants or advisory board members for pharmaceutical companies, all draft recommendations from the expert subcommittees are vetted through the Central Review Committee before presentation at the consensus conference each October. This is an important step, because members of the Central Review Committee deliberately do not serve as consultants or advisory board members for industry, nor do they accept honoraria for industry-initiated activities – the only pharmaceutical industry funding any member of the central review committee has received was for an investigator-initiated trial, which was funded two-thirds by a national peer-review funding organization and one-third by an industrial partner. At the October consensus meeting, the potential conflicts of interest of all members are disclosed in writing and distributed to the group. Members are asked to recuse themselves from voting on any recommendations with which they have potential conflicts (or if others around the table perceive them to have a potential conflict based on their disclosure statements). While a number of pharmaceutical companies do provide financial support to the CHEP to defray the costs of developing the recommendations and carrying out the implementation processes (including a Web site with educational materials for patients and physicians, as well as sponsored continuing medical education events throughout Canada), it is important to clarify that the two largest financial supporters of the CHEP are the Public Health Agency of Canada and the Canadian Hypertension Society. Furthermore, it is also important to stress that CHEP financial sponsors from industry do not have any representation at the consensus conferences and do not have any input into the identification or interpretation of the evidence, the generation and approval of the recommendations or the writing and approval of the manuscripts. Furthermore, none of the sponsors receive copies of the recommendations prior to their public presentation at the CCC every October.
IMPLEMENTATION
It has been well documented that hypertension guidelines have had limited impact on physician practice patterns, even when clinicians are aware of and profess agreement with the recommendations (18–20). As described in detail in a separate article in this series (21), a key aspect of the CHEP is the extensive implementation program that attempts to enhance guideline uptake after the recommendations are produced (and that incorporates local opinion leader-led, small group workshops and individual academic detailing alongside traditional passive dissemination techniques such as journal publications and mailed information packages).
EVALUATION
Finally, as described in detail in a separate article in this series (22), the CHEP is unique in including an evaluative component to monitor for changes in hypertension management and determine whether the implementation processes are working.
SUMMARY
The CHEP is driven by family physicians, specialist physicians, pharmacists, clinical pharmacologists, clinical epidemiologists, nurses, physiatrists, an exercise physiologist, a psychologist and a biostatistician from both university and community settings; these individuals share a common interest in hypertension and cardiovascular disease prevention, and volunteer their time and efforts to the process outlined above. The CHEP was designed to specifically address several shortcomings in traditional hypertension guidelines and, as discussed in a separate article in this series (22), there are encouraging signs that the CHEP is achieving the goal of improving hypertension management in Canada. The future is bright for this unique Canadian initiative.
APPENDIX
MEMBERS OF THE 2005/2006 CANADIAN HYPERTENSION EDUCATION PROGRAM (CHEP) WORKING GROUP
Steering Committee: N Campbell (Chair), S Tobe, R Touyz, D Drouin, J Onysko, R Petrella, R Lewanczuk, S Samis.
Executive Committee: N Campbell (Chair), D Drouin, J Onysko, S Tobe, R Touyz.
Evidence-Based Recommendations Task Force: S Tobe (Co-Chair), R Touyz (Co-Chair).
Central Review Committee: F McAlister (Chair), B Hemmelgarn, N Khan, R Padwal.
Subcommittees
Ambulatory Blood Pressure Monitoring: M Myers (Chair), S Rabkin.
Lifestyle Modification in Hypertension: R Touyz (Chair), A Logan, N Gledhill, R Petrella, N Campbell.
Adherence Strategies for Patients: R Feldman (Chair), T Campbell, C Herbert, A Milot, J Stone.
Accurate Measurement of BP: C Abbott (Chair), K Mann.
Global Cardiovascular Risk Assessment: S Grover (Chair), G Tremblay, A Milot.
Pharmacotherapy for Hypertensive Patients with CVD: S Rabkin (Chair), M Arnold, G Moe, M Hill.
Echocardiography: G Honos (Chair).
Pharmacotherapy for Hypertensive Patients Without Compelling Indications: R Lewanczuk (Chair), R Herman, P Hamet, G Fodor, G Carruthers, B Culleton, J deChamplain, G Pylypchuk.
Endocrinological Forms of Hypertension: E Schiffrin (Chair).
Renal and Renovascular Hypertension: S Tobe (Chair), E Burgess.
Follow-up on Patients with Hypertension: P Bolli (Chair), G Tremblay.
Routine Laboratory Tests: T Wilson (Chair), B Penner.
Hypertension & Diabetes: L Leiter (Chair), C Jones, P Larochelle, R Ogilvie, S Tobe, R Houlden.
Self Measurement of BP: D McKay (Chair), A Chockalingam.
Implementation Task Force: D Drouin (Co-Chair), N Campbell (Co-Chair), J Kaczarowski (Co-Chair), A Milot, C Repchinsky, T Ruddy, G Tremblay, B Semchuk, J Stone, E Wilson, S Chander, R Petrella, R Feldman (Ex officio)
Outcomes Research Task Force: N Campbell (Co-Chair), J Onysko (Co-Chair), E Amankwah, G Bartlett, T Bhattia, R Brant, A Chockalingam (Ex officio), D Drouin (Ex officio), M Eliasziw, W Ghali, R Gao, B Hemmelgarn, M Hill, H Johansen, N Khan, C Maxwell, F McAlister, H Quan, S Phillips, M Smith, L Svenson, G Taylor, K Tu, A Wielgosz.
Industry Sponsorship (in the form of unrestricted educational grants)
Abbott Laboratories Ltd
AstraZeneca Canada Inc
Bayer Inc
Biovail Pharmaceuticals Canada
Boehringer Ingelheim (Canada) Ltd
Bristol-Myers Squibb Canada Inc
Fournier Pharma Inc
Merck Frosst Canada Inc
Novartis Pharmaceuticals Canada Inc
Pfizer Canada Inc
Servier Canada Inc
Solvay Pharma Inc
Footnotes
CONFLICTS OF INTEREST: Dr Finlay A McAlister holds career salary support from the Alberta Heritage Foundation for Medical Research, the Canadian Institutes of Health Research and the Merck Frosst/Aventis Patient Health Management Chair at the University of Alberta. He has received operating grant funding from Pfizer Canada for an investigator-initiated trial co-funded by the Heart and Stroke Foundation of Canada and Pfizer Canada.
NOTES: Dr McAlister works in the Division of General Internal Medicine, University of Alberta, and chaired the Central Review Committee of the Evidence-Based Recommendations Task Force of the CHEP for the 2006 recommendations.
Competing Interests: Signed conflict of interest forms for each participant in the 2005-2006 Canadian Hypertension Guidelines are on file with Dr S Tobe.
REFERENCES
- 1.Joffres MR, Ghadirian P, Fodor JG, Petrasovits A, Chockalingam A, Hamet P. Awareness, treatment, and control of hypertension in Canada. Am J Hypertens. 1997;10:1097–102. doi: 10.1016/s0895-7061(97)00224-0. [DOI] [PubMed] [Google Scholar]
- 2.Vasan R, Beiser A, Seshadri S, et al. Residual lifetime risk for developing hypertension in middle-aged women and men. The Framingham Heart Study. JAMA. 2002;287:1003–10. doi: 10.1001/jama.287.8.1003. [DOI] [PubMed] [Google Scholar]
- 3.Yusuf S, Hawken S, Ounpuu S, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 4.Kannel WB. Blood pressure as a cardiovascular risk factor: Prevention and treatment. JAMA. 1996;275:1571–6. [PubMed] [Google Scholar]
- 5.Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murrary CJ Comparative Risks Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 6.Morgan S. Drug spending in Canada: Recent trends and causes. Med Care. 2004;42:635–42. doi: 10.1097/01.mlr.0000129494.36245.4b. [DOI] [PubMed] [Google Scholar]
- 7.Campbell NR, McAlister FA, Brant R, et al. Temporal trends in antihypertensive drug prescriptions in Canada before and after introduction of the Canadian Hypertension Education Program. J Hypertens. 2003;21:1591–7. doi: 10.1097/00004872-200308000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Wolf-Maier K, Cooper RS, Kramer H, et al. Hypertension treatment and control in five European countries, Canada, and the United States. Hypertension. 2004;43:10–7. doi: 10.1161/01.HYP.0000103630.72812.10. [DOI] [PubMed] [Google Scholar]
- 9.Lawes CM, Vander Hoorn S, Law MR, Elliott P, MacMahon S, Rodgers A. Blood pressure and the global burden of disease 2000. Part II: Estimates of attributable burden. Hypertension. 2006;24:423–30. doi: 10.1097/01.hjh.0000209973.67746.f0. [DOI] [PubMed] [Google Scholar]
- 10.Perreault S, Dorais M, Coupal L, Paradis G, Joffres MR, Grover SA. Impact of treating hyperlipidemia or hypertension to reduce the risk of death from coronary artery disease. CMAJ. 1999;160:1449–55. [PMC free article] [PubMed] [Google Scholar]
- 11.Andersson OK, Almgren T, Persson B, Samuelsson O, Hedner T, Wilhelmsen L. Survival in treated hypertension: Follow up study after two decades. BMJ. 1998;317:167–71. doi: 10.1136/bmj.317.7152.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antman EM, Lau J, Kupelnick B, Mosteller F, Chalmers TC. A comparison of results of meta-analyses of randomized control trials and recommendations of clinical experts. Treatments for myocardial infarction. JAMA. 1992;268:240–8. [PubMed] [Google Scholar]
- 13.Toma M, McAlister FA, Bialy L, Adams D, Vandermeer B, Armstrong PW. Transition from meeting abstract to full-length journal article for randomized controlled trials. JAMA. 2006;295:1281–7. doi: 10.1001/jama.295.11.1281. [DOI] [PubMed] [Google Scholar]
- 14.Farquhar CM, Kofa EW, Slutsky JR. Clinicians’ attitudes to clinical practice guidelines: A systematic review. Med J Aust. 2002;177:502–6. doi: 10.5694/j.1326-5377.2002.tb04920.x. [DOI] [PubMed] [Google Scholar]
- 15.Grol R, Dalhuijsen J, Thomas S, in’t Veld C, Rutten G, Mokkink H. Attributes of clinical guidelines that influence use of guidelines in general practice: Observational study. BMJ. 1998;317:858–61. doi: 10.1136/bmj.317.7162.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAlister FA, Campbell NR, Zarnke K, Levine M, Graham I. The management of hypertension in Canada: A review of current guidelines, their shortcomings, and implications for the future. CMAJ. 2001;164:517–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Clinical practice guidelines and conflict of interest. CMAJ. 2005;173:1297–1299. doi: 10.1503/cmaj.051423. (Erratum in 2006;174:67) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu K, Mamdani MM, Tu JV. Hypertension guidelines in elderly patients: Is anybody listening? Am J Med. 2002;113:52–8. doi: 10.1016/s0002-9343(02)01144-0. [DOI] [PubMed] [Google Scholar]
- 19.Oliveria SA, Lapuerta P, McCarthy BD, L’Italien GJ, Berlowitz DR, Asch SM. Physician-related barriers to the effective management of uncontrolled hypertension. Arch Intern Med. 2002;162:413–20. doi: 10.1001/archinte.162.4.413. [DOI] [PubMed] [Google Scholar]
- 20.Majumdar SR, McAlister FA, Furberg CD. From publication to practice in chronic cardiovascular disease: A long and winding road. J Am Coll Cardiol. 2004;43:1738–42. doi: 10.1016/j.jacc.2003.12.043. [DOI] [PubMed] [Google Scholar]
- 21.Drouin D, Campbell NR, Kaczorowski J, et al. for the Canadian Hypertension Education Program and the Implementation Task Force. Implementation of recommendations on hypertension: The Canadian Hypertension Education Program. Can J Cardiol. 2006;22:595–598. doi: 10.1016/s0828-282x(06)70281-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campbell NR, Onysko J for the Canadian Hypertension Education Program and the Outcomes Research Task Force. The Outcomes Research Task Force and the Canadian Hypertension Education Program. Can J Cardiol. 2006;22:556–8. doi: 10.1016/s0828-282x(06)70276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]