Abstract
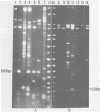
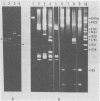
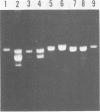
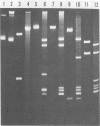
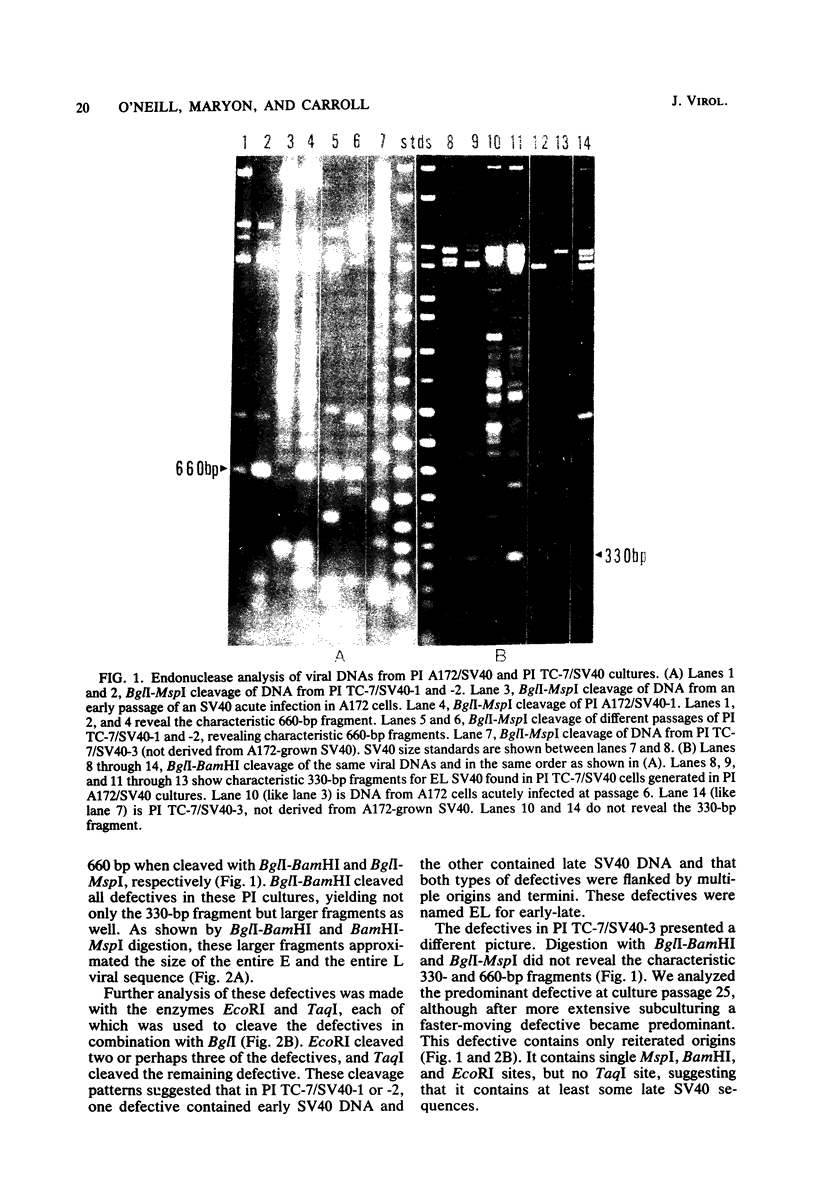
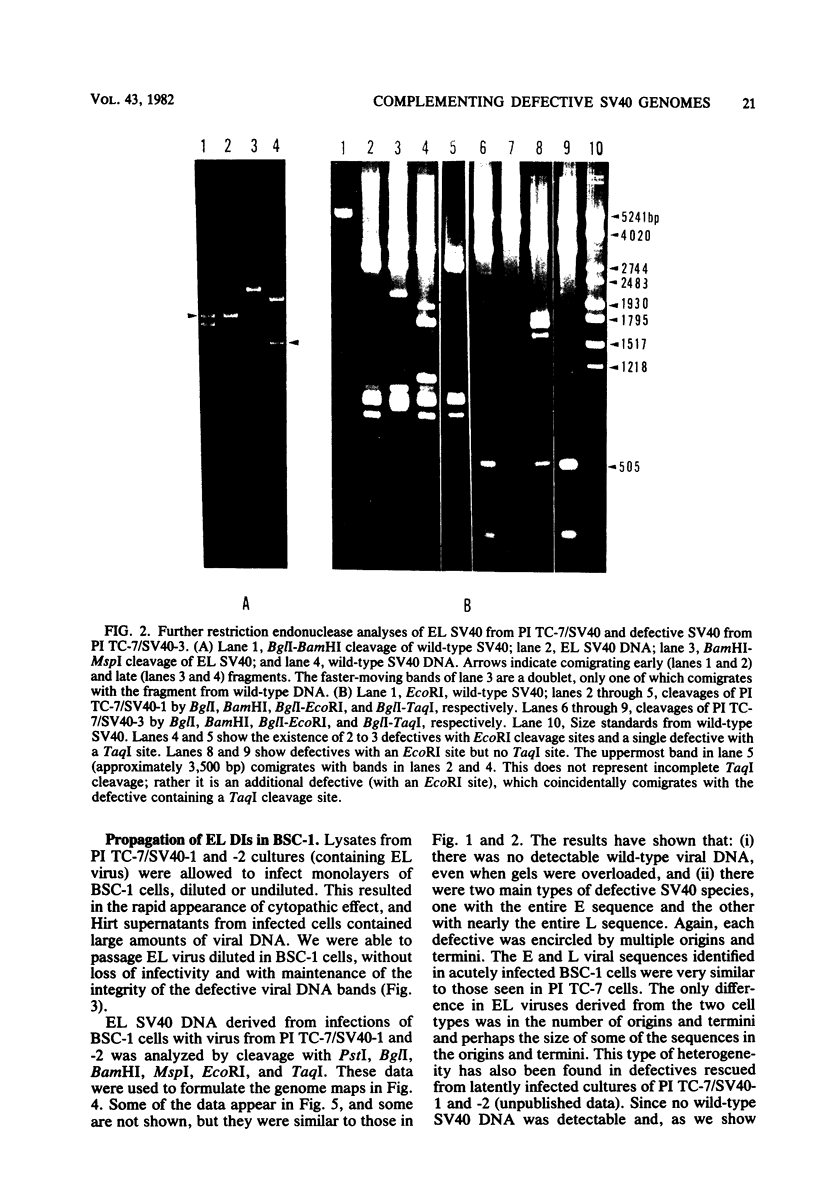
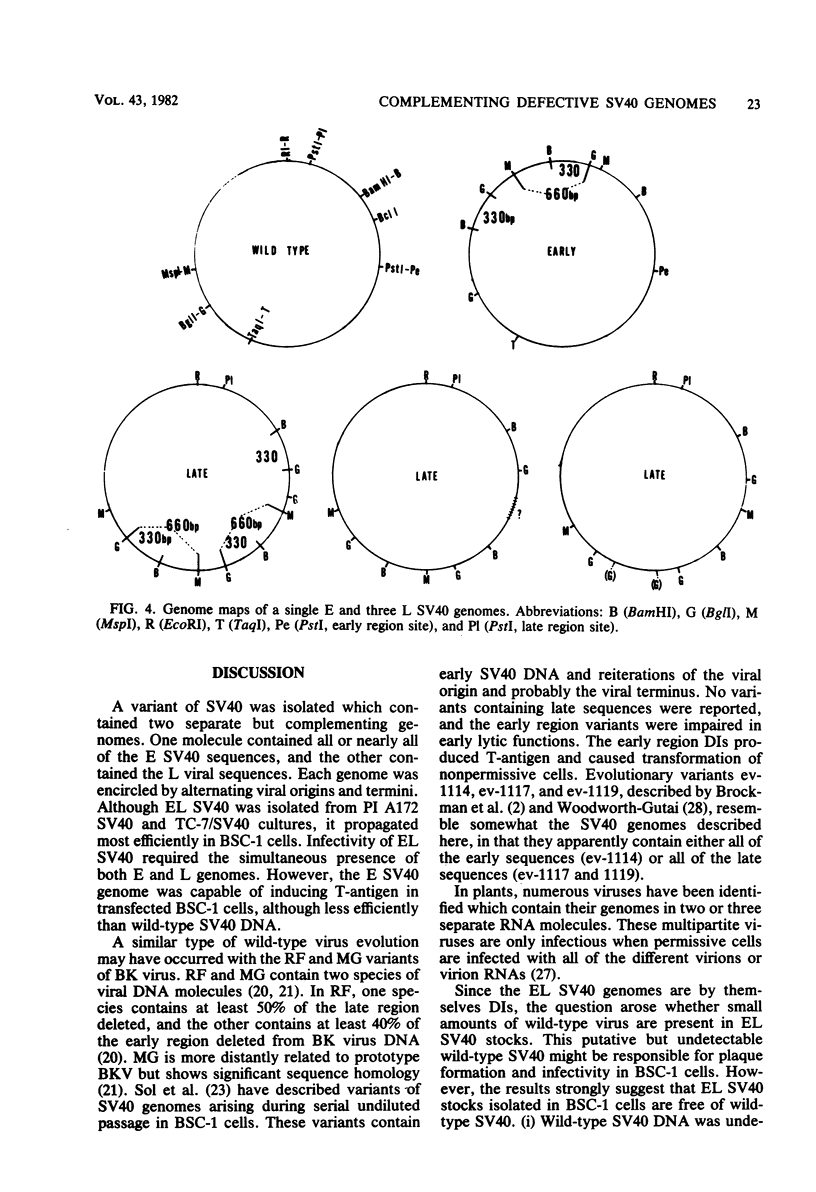
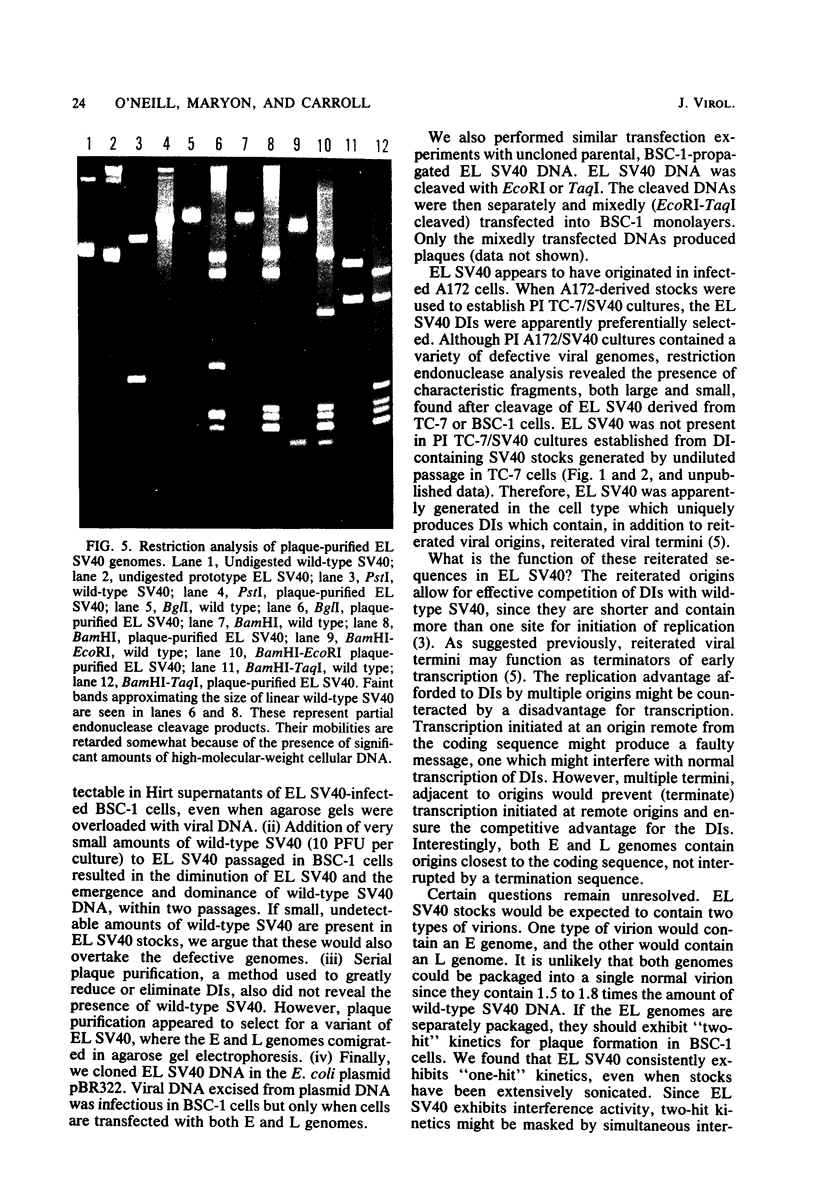
A new variant of simian virus 40 (EL SV40), containing the complete viral DNA separated into two molecules, was isolated. One DNA species contains nearly all of the early (E) SV40 sequences, and the other DNA contains nearly all of the late (L) viral sequences. Each genome was encircled by reiterated viral origins and termini and migrated in agarose gels as covalently closed supercoiled circles. EL SV40 or its progenitor appears to have been generated in human A172 glioblastoma cells, as defective interfering genomes during acute lytic infections, but was selected during the establishment of persistently infected (PI) green monkey cells (TC-7). PI TC-7/SV40 cells contained EL SV40 as the predominant SV40 species. EL SV40 propagated efficiently and rapidly in BSC-1, another line of green monkey cells, where it also formed plaques. EL SV40 stocks generated in BSC-1 cells were shown to be free of wild-type SV40 by a number of criteria. E and L SV40 genomes were also cloned in the bacterial plasmid pBR322. When transfected into BSC-1 cell monolayers, only the combination of E and L genomes produced a lytic infection, followed by the synthesis of EL SV40. However, transfection with E SV40 DNA alone did produce T-antigen, although at reduced frequency.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyd V. A., Butel J. S. Demonstration of infectious deoxyribonucleic acid in transformed cells. I. Recovery of simian virus 40 from yielder and nonyielder transformed cells. J Virol. 1972 Sep;10(3):399–409. doi: 10.1128/jvi.10.3.399-409.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockman W. W., Gutai M. W., Nathans D. Evolutionary variants of simian virus 40: characterization of cloned complementing variants. Virology. 1975 Jul;66(1):36–52. doi: 10.1016/0042-6822(75)90177-4. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Lee T. N., Nathans D. The evolution of new species of viral DNA during serial passage of simian virus 40 at high multiplicity. Virology. 1973 Aug;54(2):384–397. doi: 10.1016/0042-6822(73)90151-7. [DOI] [PubMed] [Google Scholar]
- Carroll D., Hansen J. L., Maryon E. B., O'Neill F. J. SV40 defectives selected during low multiplicity passage on A172 human glioblastoma cells. Virology. 1981 Jul 30;112(2):461–471. doi: 10.1016/0042-6822(81)90293-2. [DOI] [PubMed] [Google Scholar]
- Carroll D., O'Neill F. J. Genome maps of simian virus 40 defectives propagated in human glioblastoma cells. Virology. 1978 Jun 1;87(1):120–129. doi: 10.1016/0042-6822(78)90164-2. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- Hanahan D., Lane D., Lipsich L., Wigler M., Botchan M. Characteristics of an SV40-plasmid recombinant and its movement into and out of the genome of a murine cell. Cell. 1980 Aug;21(1):127–139. doi: 10.1016/0092-8674(80)90120-8. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Huang A. S. Defective interfering viruses. Annu Rev Microbiol. 1973;27:101–117. doi: 10.1146/annurev.mi.27.100173.000533. [DOI] [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Noonan C. A., Brugge J. S., Butel J. S. Characterization of simian cells tranformed by temperature-sensitive mutants of simian virus 40. J Virol. 1976 Jun;18(3):1106–1119. doi: 10.1128/jvi.18.3.1106-1119.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill F. J., Carroll D. Appearance of defective simian virus 40 following infection of cultured human glioblastoma cells. Virology. 1978 Jun 1;87(1):109–119. doi: 10.1016/0042-6822(78)90163-0. [DOI] [PubMed] [Google Scholar]
- O'Neill F. J., Cohen S., Renzetti L. Temperature dependency for maintenance of transformation in mouse cells transformed by simian virus 40 tsA mutants. J Virol. 1980 Jul;35(1):233–245. doi: 10.1128/jvi.35.1.233-245.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater A., Pater M. M., di Mayorca G. Arrangement of the genome of the human papovavirus RF virus. J Virol. 1980 Nov;36(2):480–487. doi: 10.1128/jvi.36.2.480-487.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater M. M., Pater A., di Mayorca G. Genome analysis of MG virus, a human papovavirus. J Virol. 1981 Sep;39(3):968–972. doi: 10.1128/jvi.39.3.968-972.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Sol C. J., Hassing I., Maris W., Walig C., van der Noordaa J. Evolutionary variants of simian virus 40 which are impaired in early lytic functions but transform nonpermissive cells. J Virol. 1981 Jan;37(1):395–410. doi: 10.1128/jvi.37.1.395-410.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Absence of interference during high-multiplicity infection by clonally purified vesicular stomatitis virus. J Virol. 1971 Mar;7(3):409–411. doi: 10.1128/jvi.7.3.409-411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stollar V. Defective interfering particles of togaviruses. Curr Top Microbiol Immunol. 1979;86:35–66. doi: 10.1007/978-3-642-67341-2_2. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Weisblum B. Construction of a colicin E1-R factor composite plasmid in vitro: means for amplification of deoxyribonucleic acid. J Bacteriol. 1975 Jan;121(1):354–362. doi: 10.1128/jb.121.1.354-362.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodworth-Gutai M. Recombination in SV40-infected cells: nucleotide sequences at viral-viral recombinant joints in naturally arising variants. Virology. 1981 Mar;109(2):344–352. doi: 10.1016/0042-6822(81)90505-5. [DOI] [PubMed] [Google Scholar]