Abstract
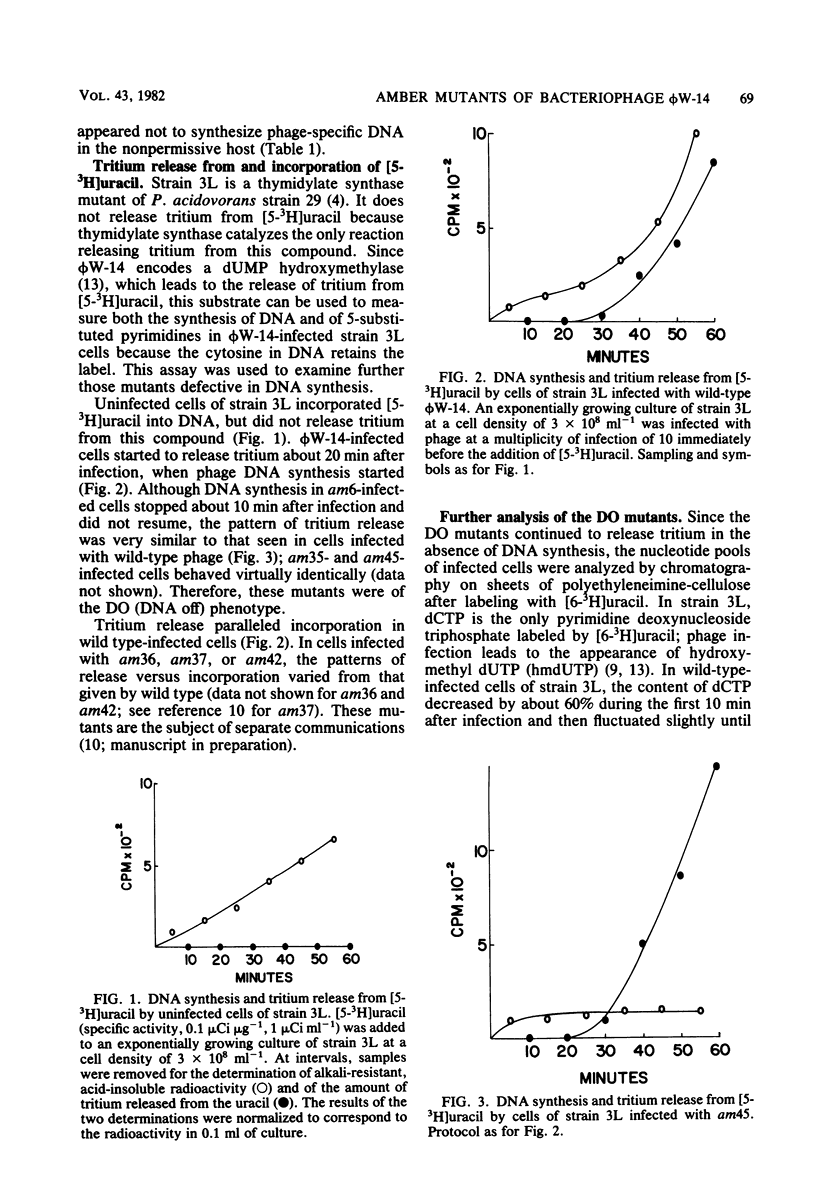
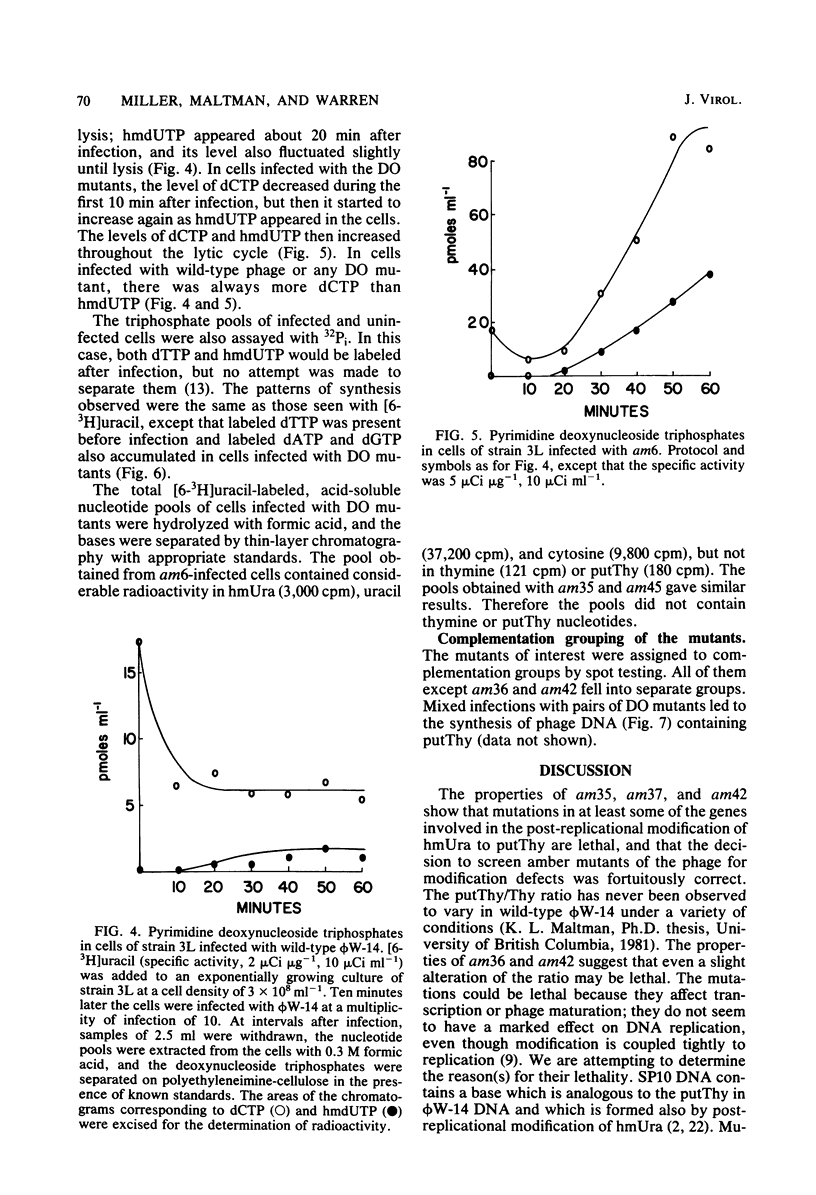
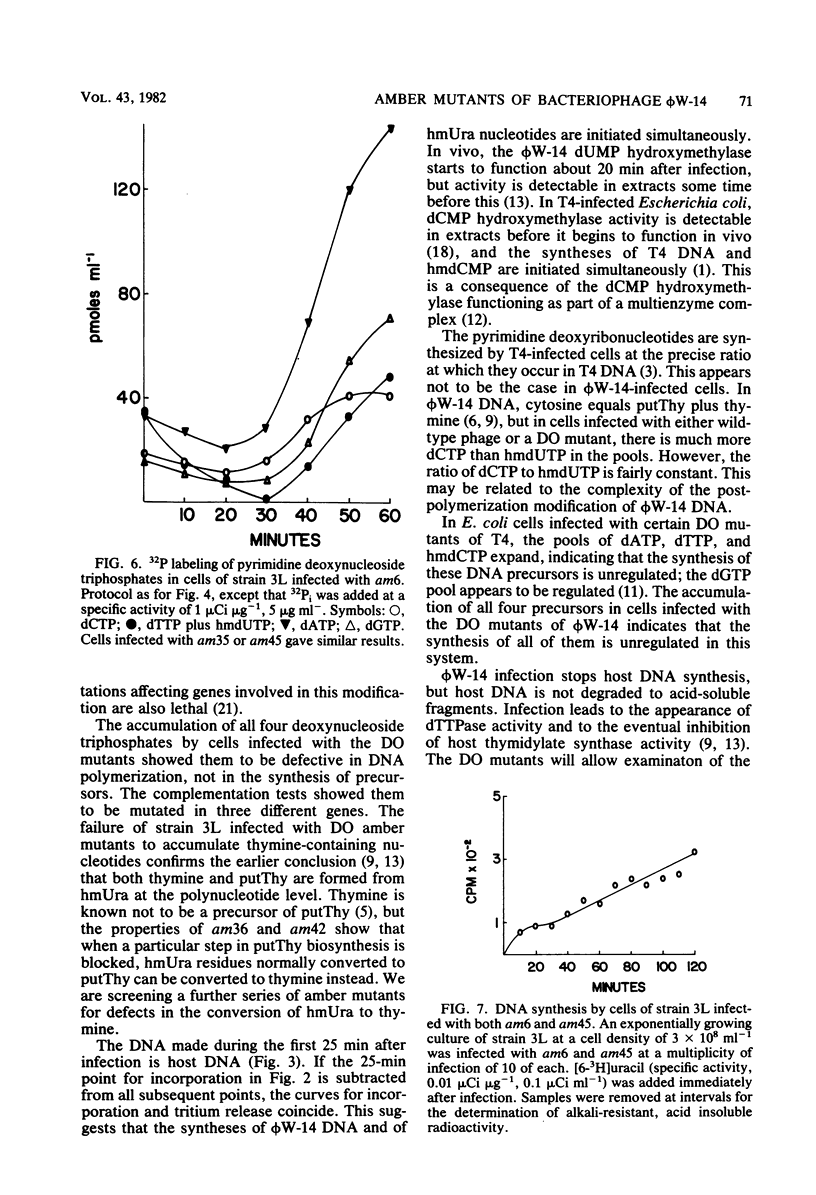
Of 42 amber mutants of bacteriophage phi W-14, 6 were defective in DNA synthesis. Three of the mutants synthesized DNA in the nonpermissive host, but were defective in post-replicational modification of the DNA. The DNA synthesized by two of these mutants, am36 and am42, contained more thymine and less alpha-putrescinylthymine than did wild-type DNA; that synthesized by the third mutant, am37, contained the normal amount of thymine, no alpha-putrescinylthymine, and hydroxymethyluracil. The properties of these mutants suggested that the presence of the normal amount of alpha-putrescinylthymine in phi W-14 DNA was essential for the production of viable progeny. Three of the mutants, am6, am35, and am45, failed to synthesize DNA in the nonpermissive host. These mutants were analogous to the DNA off mutants of T4. Nonpermissive cells infected with DNA off mutants accumulated dATP, dGTP, dCTP, and hydroxymethyl dUTP, but not dTTP or alpha-putrescinyldeoxythymidine triphosphate, confirming that both thymine and alpha-putrescinylthymidine in phi W-14 DNA are formed from hydroxymethyluracil at the polynucleotide level. The synthesis of phi W-14 DNA is unusual because (i) thymine is formed from hydroxymethyluracil at the polynucleotide level, (ii) the hypermodification forming alpha-putrescinylthymine is essential, and (iii) thymine and alpha-putrescinylthymine must be made in the correct proportions. Complementation tests showed that the mutants defined three genes involved in DNA polymerization and two genes involved in post-replicational modification.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chiu C. S., Tomich P. K., Greenberg G. R. Simultaneous initiation of synthesis of bacteriophage T4 DNA and of deoxyribonucleotides. Proc Natl Acad Sci U S A. 1976 Mar;73(3):757–761. doi: 10.1073/pnas.73.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Greenberg G. R. Regulation of deoxyribonucleotide biosynthesis during in vivo bacteriophage T4 DNA replication. Intrinsic control of synthesis of thymine and 5-hydroxymethylcytosine deoxyribonucleotides at precise ratio found in DNA. J Biol Chem. 1977 May 10;252(9):3019–3027. [PubMed] [Google Scholar]
- Kelln R. A., Warren R. A. Obligate thymidine auxotrophs of Pseudomonas acidovorans. J Bacteriol. 1973 Jan;113(1):510–511. doi: 10.1128/jb.113.1.510-511.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelln R. A., Warren R. A. Studies on the biosynthesis of alpha-putrescinylthymine in bacteriophage phi W-14-infected Pseudomonas acidovorans. J Virol. 1973 Dec;12(6):1427–1433. doi: 10.1128/jvi.12.6.1427-1433.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropinski A. M., Bose R. J., Warren R. A. 5-(4-Aminobutylaminomethyl)uracil, an unusual pyrimidine from the deoxyribonucleic acid of bacteriophage phiW-14. Biochemistry. 1973 Jan 2;12(1):151–157. doi: 10.1021/bi00725a025. [DOI] [PubMed] [Google Scholar]
- Kropinski A. M., Warren R. A. Isolation and properties of a Pseudomonas acidovorans bacteriophage. J Gen Virol. 1970 Jan;6(1):85–93. doi: 10.1099/0022-1317-6-1-85. [DOI] [PubMed] [Google Scholar]
- Lewis H. A., Miller R. C., Jr, Stone J. C., Warren R. A. Alkali lability of bacteriophage phi W-14 DNA. J Virol. 1975 Dec;16(6):1375–1379. doi: 10.1128/jvi.16.6.1375-1379.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Lewis H. A., Warren R. A. Synthesis of thymine and alpha-putrescinylthymine in bacteriophage phi W-14-infected Pseudomonas acidovorans. J Virol. 1980 May;34(2):354–359. doi: 10.1128/jvi.34.2.354-359.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltman K. L., Neuhard J., Warren R. A. 5-[(Hydroxymethyl)-O-pyrophosphoryl]uracil, an intermediate in the biosynthesis of alpha-putrescinylthymine in deoxyribonucleic acid of bacteriophage phi W-14. Biochemistry. 1981 Jun 9;20(12):3586–3591. doi: 10.1021/bi00515a043. [DOI] [PubMed] [Google Scholar]
- Mathews C. K. Biochemistry of deoxyribonucleic acid-defective amber mutants of bacteriophage T4. 3. Nucleotide pools. J Biol Chem. 1972 Nov 25;247(22):7430–7438. [PubMed] [Google Scholar]
- Mathews C. K., North T. W., Prem veer Reddy G. Multienzyme complexes in DNA precursor biosynthesis. Adv Enzyme Regul. 1978;17:133–156. doi: 10.1016/0065-2571(79)90011-6. [DOI] [PubMed] [Google Scholar]
- Neuhard J., Maltman K. L., Warren R. A. Bacteriophage phi W-14-infected Pseudomonas acidovorans synthesizes hydroxymethyldeoxyuridine triphosphate. J Virol. 1980 May;34(2):347–353. doi: 10.1128/jvi.34.2.347-353.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R., Luria S. E. DNA-glucosylation in T-even phage: genetic determination and role in phagehost interaction. Annu Rev Genet. 1970;4(0):177–192. doi: 10.1146/annurev.ge.04.120170.001141. [DOI] [PubMed] [Google Scholar]
- Russell R. L. Comparative genetics of the T-even bacteriophages. Genetics. 1974 Dec;78(4):967–988. doi: 10.1093/genetics/78.4.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Tomich P. K., Chiu C. S., Wovcha M. G., Greenberg G. R. Evidence for a complex regulating the in vivo activities of early enzymes induced by bacteriophage T4. J Biol Chem. 1974 Dec 10;249(23):7613–7622. [PubMed] [Google Scholar]
- Warren R. A. Amber suppressor mutations in Pseudomonas acidovorans. J Bacteriol. 1979 Feb;137(2):1053–1055. doi: 10.1128/jb.137.2.1053-1055.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R. A. Modified bases in bacteriophage DNAs. Annu Rev Microbiol. 1980;34:137–158. doi: 10.1146/annurev.mi.34.100180.001033. [DOI] [PubMed] [Google Scholar]
- Witmer H. Synthesis of deoxythymidylate and the unusual deoxynucleotide in mature DNA of Bacillus subtilis bacteriophage SP10 occurs by postreplicational modification of 5-hydroxymethyldeoxyuridylate. J Virol. 1981 Aug;39(2):536–547. doi: 10.1128/jvi.39.2.536-547.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]



