Abstract
Nitric oxide-donating nonsteroidal anti-inflammatory drugs (NO-NSAIDs) consist of a conventional NSAID to which an NO-releasing moiety is attached covalently, often via a spacer molecule. NO-NSAIDs represent an emerging class of compounds with chemopreventive properties against a variety of cancers, demonstrated in preclinical models including cell culture systems and animal tumor models; their potential efficacy in humans has not been assessed. Their mechanism of action appears complex and involves the generation of reactive oxygen species, suppression of microsatellite instability in mismatch repair-deficient cells, and modulation of several signaling cascades that culminate in inhibited cell renewal and enhanced apoptosis. NO, long appreciated to be able to protect from and also promote cancer, is released form NO-NSAIDs and constitutes their defining property. Existing data are consistent with the notion that NO may mediate their anticancer effect. In addition there is evidence that long term administration of NO-donating compounds is not associated with increased incidence of colon cancer. Whether NO release is required for the anticancer effect of NO-NSAIDs has being questioned by recent data indicating that, at least in the case of NO-aspirin, the NO-releasing moiety may serve as a leaving group while the spacer actually being the moiety responsible for its pharmacological action. Regardless of mechanistic issues, these compounds promise to contribute to the control of cancer.
Keywords: nitric oxide, cancer, NO-NSAIDs, NO-aspirin, chemoprevention
Introduction
Cancer represents perhaps the defining medical challenge of our times. The search for pharmacological agents that can control cancer, either as chemotherapeutic or as chemopreventive agents is intense and to date has yielded significant results. Nevertheless, as the continuing morbidity and mortality form cancer indicate, there is a pressing need to identify new agents. Rational design of pharmacological agents includes, among others, modification of known agents in order to optimize their pharmacological properties, mainly their safety and efficacy. Nitric oxide-donating nonsteroidal anti-inflammatory drugs (NO-NSAIDs) represent a case in point (reviewed in [1]). This emerging class of compounds was designed based on the known properties of NSAIDs and those of the then-newly discovered NO, a molecule that plays an important role not only in the cardiovascular system but also in much of human physiology. The expectation was that these molecules would harness the properties of both achieving an enhanced, if not synergistic, effect.
The concept of modifying known pharmacological compounds to make them capable of releasing NO is broader than NSAIDs and encompasses many others, such as steroids, statins, prostaglandin F2α analogs, and antihypertensive agents. Besides cancer, NO-donating compounds hold promise for the control of other diseases, including cardiovascular diseases [2], asthma [3], hypoxic–ischemic brain injury [4], glaucoma [5], and Alzheimer's disease [6]. To date, an extensive body of work including studies in preclinical models and relevant clinical trials underscores their therapeutic potential.
The ability of NO-NSAIDs to release NO is their defining (and name-giving) property. Here, we summarize the current status of NO-NSAIDs as anticancer agents and examine the intriguing question of whether NO is indeed required for their action in cancer.
NO-NSAIDs: Rationale and Structure
The rationale for the initial synthesis of NO-NSAIDs was simple, brilliant and probably inaccurate. NSAIDs damage the gastroduodenal mucosa by inhibiting the synthesis of cytoprotective prostaglandins [7]. Since the action of NO on this mucosa is similar to that of prostaglandins, it was reasoned that if an NSAID could provide locally NO, its mucosal damaging effect would be averted. Numerous animal and some human studies of NO-NSAIDs have clearly demonstrated that NO-NSAIDs indeed protected the gastric mucosa from the damage that the parent NSAIDs were expected to inflict [8,9]. As discussed later, however, there is serious doubt as to whether this notion is correct.
NO-NSAIDs consist of a conventional NSAID to which an NO-releasing moiety is attached covalently, often via a spacer molecule. The first NO-NSAIDs were synthesized by del Soldato and his colleagues (reviewed in [7]); NO-ASA and NO-indomethacin are depicted in Fig. 1. Various molecules, both aliphatic and aromatic, have been used as spacer molecules in NO-NSAIDs
Fig.1. The structures of NO-ASA and NO-indomethacin.
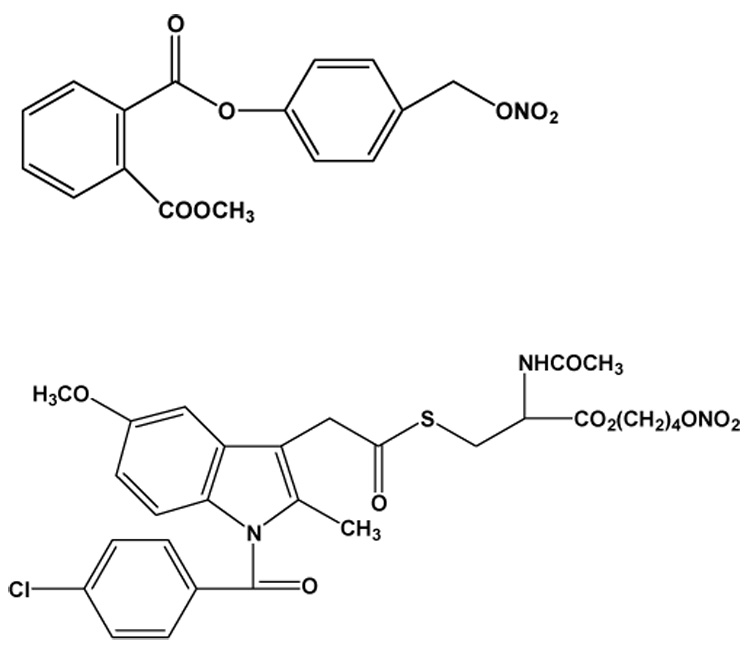
The spacer molecules differ between NO-ASA (top) and NO-indomethacin (below) but the NO-releasing moiety is the same (−ONO2).
Additional versions of NO-donating molecules, including NO-NSAIDs have been synthesized by various teams attempting to capitalize either on the pharmacological properties of NO per se or on the enhanced efficacy of NO-releasing compounds.
Thatcher’s team has also synthesized chimeras able to release NO [10–12]. GT 1061, an NO mimetic compound that contains an ancillary, synergistic pharmacophore, is being investigated as neuroprotective in patients with Alzheimer's disease [6]. In addition, this group has synthesized the NO chimera GT-094. This compound, a novel nitrate containing an NSAID and disulfide pharmacophores, exhibits antiproliferative activity and exerts a G2/M cell cycle block in cultured colon cancer cells [13]. Finally, the so-called NONO-NSAIDs have been synthesized and studied for their anti-inflammatory and anticancer properties [14–16]. It should not be forgotten that the organic nitrates are also NO-donating compounds. Nitroglycerin, the prototypical pharmacological agent of this category, has been used successfully for cardiac indications for over a century, long before its mechanism of action was unraveled following the discovery of NO as a vasodilating agent [12].
The straightforward mechanism outlined above that involves the release from NO-NSAIDs of cytoprotective NO within the stomach may not be accurate. The strongest evidence against this mechanism comes form the pharmacokinetic studies of NO-aspirin (NO-ASA). Carini et al have shown that NO-ASA traverses the stomach intact, thus ruling out this mechanism, at least in the case of NO-ASA [17,18]. The alternative explanation that NO, released beyond the upper GI tract, can reach the gastric mucosa via the circulation remains unproven; elevated NO levels in the circulation following the administration of NO-NSAIDs have been repeatedly demonstrated (e.g., [18]).
Evidence for the Anticancer Effect of NO-NSAIDs
During the last several years we and other groups have studied NO-NSAIDs as potential agents for the control of cancer. There is ample evidence of their efficacy in preclinical models of cancer. Williams et al provided the first evidence that NO-NSAIDs possess chemopreventive properties by showing that NO-ASA, NO-sulindac, and NO-ibuprofen reduce the growth of cultured HT-29 human colon adenocarcinoma cells much more potently than their corresponding NSAIDs [19]. This observation was later extended to additional NO-NSAIDs [20] and additional cell lines [21–23].
Several animal studies, all congruent, have generated results consistent with the conclusions derived from the cell culture studies. For colon cancer, three animal tumor models documented the chemopreventive effect of NO-NSAIDs. Tumor incidence and multiplicity were reduced in Min mice and in an azoxymethane model of colon cancer, whereas the size of tumor xenografts was significantly reduced in response to NO-ASA [24–27]. In pancreatic cancer meta NO-ASA displayed an astonishing effect, reducing the incidence of pancreatic cancer by 89.5%, while conventional ASA, studied in parallel, was totally ineffective [28].
The mechanism of action of NO-NSAIDs against cancer (mainly of the colon and pancreas) has been pursued from several angles: their cell kinetic effect, effects on cell signaling pathways, and effects on detoxifying enzymes. To a first approximation, NO-NSAIDs have a strong cell growth inhibitory effect, which is the end result of three individual effects: inhibition of cell proliferation, induction of apoptosis and deceleration of cell cycle phase transitions [19,29–31]. The most detailed study on the induction of apoptosis was conducted by Gao et al [32], whose work on NO-ASA highlighted three important aspects of this effect: a) the first recognizable effect was the induction of oxidative stress; b) this was followed by activation of the intrinsic apoptosis pathway; and c) inhibition of Wnt signaling was a major component of the proapoptotic effect of NO-ASA. Animal studies confirmed the importance of the induction of apoptosis for the chemopreventive effect of NO-ASA [24,28].
NO-NSAIDs, in particular NO-ASA, the compound that has attracted the greatest number of mechanistic studies, modulate a large array of molecular targets. Their action includes effects on mitogen-activated protein kinase (MAPK) signaling [33]; inhibition of inducible nitric oxide synthase (NOS2) [34]; and inhibition of NF-κB activation [35]. The effect of NO-NSAIDs on COX-2 is still unclear, since some in vitro studies indicate its induction by NO-NSAIDs and some suggest that the opposite effect is likely [25,36]. Finally, NO-ASA modulates drug metabolizing enzymes, including induction of the activity and expression of NAD(P)H:quinone oxireductase (NQO) and glutathione S-transferase (GST) and translocation of Nrf2 into the nucleus, likely by binding to Keap1, the protein that anchors Nrf2 in the cytoplasm [37]. Finally, it was recently shown that NO-ASA isomers were more effective at suppressing microsatellite instability in mismatch repair-deficient cell lines than conventional ASA, raising the possibility that NO-ASA could be an effective chemopreventive agent for hereditary nonpolyposis colorectal cancer (HNPCC) carriers [38].
What is both remarkable and perplexing is that in theory each of these effects could prevent cancer. It remains, however, unclear whether only one, more than one or all of these effects are required for the drug’s overall pharmacological effect. Resolving this dilemma, formulated as mechanistic dominance (one effect) versus redundancy (multiple effects) [39], will require further work.
Regardless of preclinical effects and mechanistic studies, only clinical trials will definitively assess the role of NO-NSAIDs in cancer control. Unfortunately, a clinical trial of NO-ASA for the prevention of colon cancer was recently terminated prematurely due to concerns about its potential genotoxicity [40]. The expectation is that resumption of this trial should follow the successful resolution of this issue.
NO Release from NO-NSAIDs and their Anticancer Effect
NO is long appreciated to have a dichotomous effect on cancer, being able to protect from and also promote cancer [41]. The outcome depends on a host of factors, including mainly the concentration of NO and the location where it is released. Consequently, the presence of the NO-releasing moiety on the various permutations of NO-donating compounds intended to be used as anticancer agents engenders three questions:
Does the NO that NO-NSAIDs ultimately release have an anticancer effect or simply a cytoprotective effect in the stomach as initially envisioned?
Could the NO derived form NO-NSAIDs instead of protecting form cancer promote carcinogenesis, especially after their long-term administration? And, finally,
Is the NO actually required for the anticancer effect of NO-NSAIDs?
NO as the mediator of the action of NO-NSAIDs
There is clear evidence that NO-NSAIDs do release their NO. First, NO levels (measured as NOx) are increased in the circulation following NO-NSAID administration [17,42]. Second, no intact NO-NSAID has been identified in the circulation or target tissues [17,18] and our own unpublished data], suggesting that the breakdown of the NO-NSAID molecule occurs either prior to or rapidly after it reaches the circulation. Third, S-nitrosylation, an unambiguous marker of NO action, following exposure to NO-NSAIDs has been repeatedly identified [43]. Finally, Govoni et al using electron paramagnetic resonance demonstrated that NO-flurbiprofen generated NO in erythrocytes, with hemoglobin mediating this biotransformation [44]. Their work and that of others suggests that the NO-releasing moiety (−ONO2) undergoes 1 e− reduction to NO2−, which is then either converted to NO or oxidized to NO3−. The issue then appears to be whether the NO released from the NO-NSAIDs is the moiety that brings about their cytoprotective effect and/or their anticancer effect.
It is fair to assume that those who conceived the idea of NO-NSAIDs expected them to release NO in the upper GI tract, protecting it from the mucosal damage brought about by conventional NSAIDs [45][46]. As mentioned earlier, NO-ASA traverses the stomach intact [17,18], thus ruling out such a mechanism. Therefore, the only remaining possibility, that could link gastric cytoprotection to the NO that is released from NO-NSAIDs, is that NO reaches the mucosa via the circulation. This is entirely possible as elevated circulating NO levels have been documented following NO-NSAID administration [18,42]. Whether, however, such a mechanism is operative alone or as part of a complex series of events remains uncertain; NO is indeed an important mediator of mucosal defense in the stomach [47].
The issue whether NO actually mediates the anticancer effect of NO-NSAIDs is still unresolved. When it was shown that NO-NSAIDs were more potent than their parent NSAIDs, the intuitive interpretation of the data was that NO release accounted for this effect. Similar to gastroprotection, such an effect would also require, to a first approximation, one of two general mechanisms. The first mechanism requires that the NO-NSAIDs have somehow a tropism to the target tissue where they travel intact and deliver their NO in situ, perhaps on account of a property of the neoplastic tissue. The second mechanism will require that NO released from NO-NSAIDs reaches the neoplastic cells and kills them. There is very little support for the first mechanism, as already discussed above. In fact, the most important obstacle in the field of NO donor drugs is represented by the difficulty in targeting NO release, and thereby its effects, to a particular tissue [48].
The second mechanism appears, however, plausible. Various NO donor molecules that are structurally dissimilar to NO-NSAIDs have anticancer properties. For example, S-nitrosoglutathione inhibits the growth of various cancer cells [49] [50]. In addition, organic nitrates [51] diazeniumdiolates [52], syndominides [53], S-nitrosothiols [54] and even metal-NO complexes [55], all display anticancer properties in preclinical models of cancer, mostly cell lines. Since they differ structurally so broadly and their “common denominator” is that they are NO donors, it is fair to conclude that their anticancer properties are due or related to the NO that they release. One can then extrapolate this conclusion to NO-NSAIDs and, in the context of the mechanistic assumptions mentioned above, attribute at least part of their effect to NO. It should be emphasized that stating that NO contributes to the anticancer effect of NO-NSAIDs is not equivalent to stating that the release of NO is required for this effect. Since doubts about such a requirement have been voiced in recent literature, we will examine this question in a later section of this review.
NO-NSAIDs as potential promoters of carcinogenesis
Carcinogenesis is in general a lengthy process, in some cases estimated to require several years until a full blown cancer develops. If the NO released form NO-NSAIDs is to behave as a carcinogen or promoter of carcinogenesis, this would require either a prolonged exposure to NO or some crucial effect on genes whose mutation may be contribute to carcinogenesis. A rather compelling case has been described by Ambs et al [56] who studied the mutations of p53 in the vicinity of NOS and demonstrated that the NO produced through the catalytic activity of this enzyme was responsible for critical mutagenesis.
It was in the face of such considerations that we were prompted to examine the possibility that the NO released by NO-NSAIDs may promote carcinogenesis, even in a manner akin to second tumors developing following anticancer treatment with some chemotherapeutic agents. The immediate problem was the absence of human trials with NO-NSAIDs that had a sufficient lag time between treatment and follow up for cancer development. Our approach was to examine cohorts of patients treated with nitrovasodilators and for whom there were long-term follow up observations. Therefore, we evaluated data from the Framingham study, an on-going population-based cohort study initiated in 1948 which now includes the original participants, and a second cohort (“The Framingham Offspring Study”) of the children of the original participants and their spouses. We analyzed the latter cohort. This database included exam information that included questions on medicinal nitrate use, which started in 1983/1984 through November 1999. There were 195 newly diagnosed incident cases of colon cancer during this period. Three controls were selected for each case, matched by study cohort, age (within five years) and sex. Analysis of the effects of nitrovasodilators on the risk of colorectal cancer showed that the odds ratio for colorectal cancer associated with nitrovasodilator use was 1.2 (95% confidence interval 0.6, 2.2), indicating that NO does not change the risk of colorectal cancer. Aspirin use, used as an internal control, had the expected preventive effect. Perhaps remarkably, nitrovasodilators showed no protection from cancer. These results offer a level of comfort, even as indirect as it is, that medicinal NO may not be carcinogenic in the colon.
The NO requirement for the anticancer effect of NO-NSAIDs
Recently, our group [57]and Hulsman et al [58] raised the possibility that, at least in the case of NO-ASA, the NO releasing moiety is not required for its anti-cancer effect. Both sets of data demonstrate that carboxylic ester hydrolysis of NO-ASA leads to the formation of quinone methide which reacts at least with cellular GSH. It is assumed that the formation of quinone methide is the key biological event in this mechanism and that the −ONO2 moiety serves simply as a good leaving group, simply facilitating the release of quinone methide (Fig. 2)
Fig. 2. Postulated mechanism of action of NO-ASA and the structure of non-NO releasing analogues.
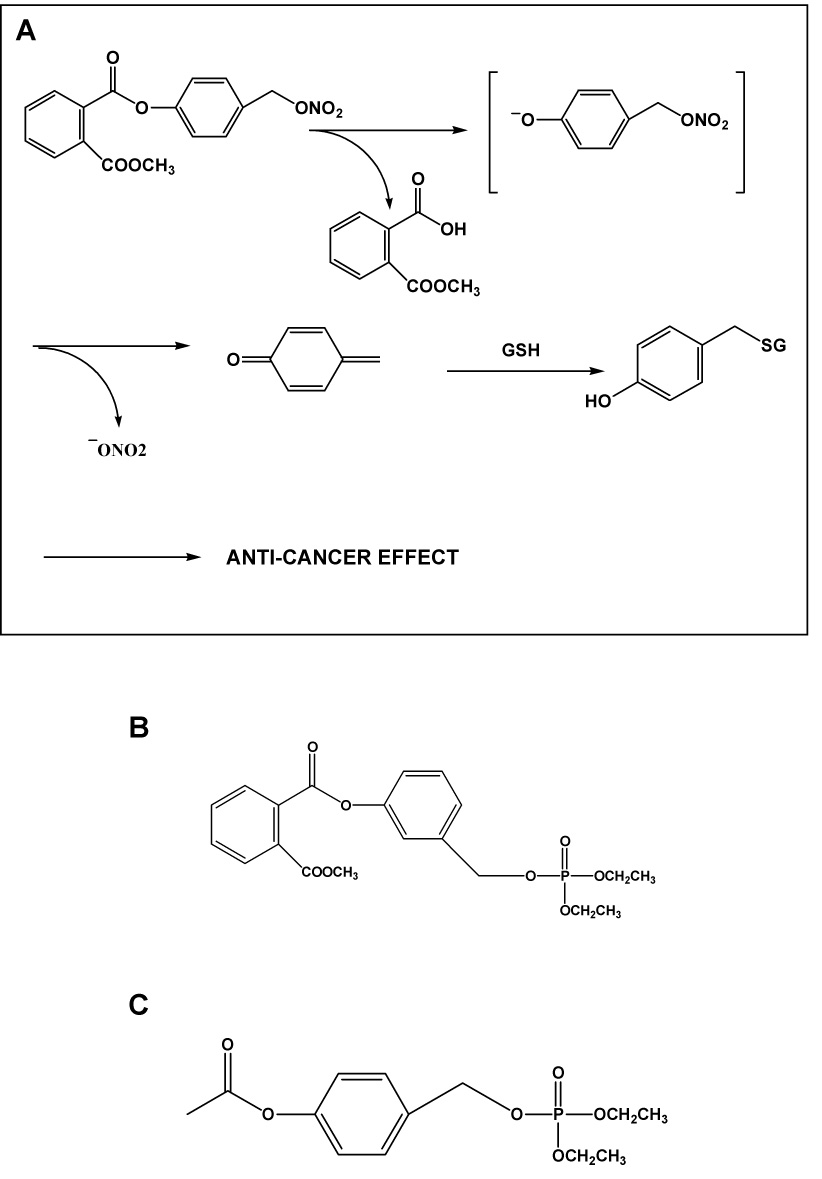
A: NO-ASA is hydrolyzed to release quinone methide which can react with glutathione, ultimately leading to cell death. A carbocation-based mechanism has been proposed for its meta positional isomer [57]. B: This compound has neither aspirin nor the NO-releasing moiety and, similar to C (a derivative of aspirin, but without the NO-releasing moiety), it is effective against colon cancer in preclinical models.
The strongest support for such a mechanism comes from the demonstration that analogues of NO-ASA deprived of the aspirin or the −ONO2 moiety are as potent as the classic NO-ASA against the growth of cancer cells. Very recently, we actually showed that “phosphoaspirin” (a meta isomer of NO-ASA in which −ONO2 was replaced by diethylphosphate) was very effective in inhibiting the growth of colon cancer xenografts in nude mice [59]. Thus, at least in the case of NO-ASA, NO is not required for its anticancer effect.
Concluding Remarks
NO-NSAIDs represent a highly promising development in the area of cancer prevention and treatment. These compounds are part of a larger family of agents sharing their ability to release NO, a molecule which appears to exert a very important, although not always predictable (or even desirable) effect on cancer. The key questions concerning the pharmacology of NO-NSAIDs are three:
Are these compounds effective against cancer in humans?
What is the mechanism underlying their higher potency and limited gastrointestinal toxicity, two practically important pharmacological properties? And
Is the NO-releasing moiety required for their anticancer effect?
All evidence to date, essentially all of it preclinical, suggests that they could prove effective against colon and a host of other cancers. There has been enough background preclinical work to justify evaluating them in suitable groups of patients and it is only through such trials that the eagerly awaited answers will be obtained.
NO-NSAIDs appear safer than their parent compounds, especially with respect to the all-important gastrointestinal toxicity. The question of the potential genotoxicity of NO-ASA is at present just that, a question that needs to be addressed. Regardless of the outcome of such re-evaluation, it should be noted that such genotoxicity may be of concern, if at all, only for chemoprevention applications and not for chemotherapy applications. In the latter case, concerns about genotoxicity are viewed differently because of the underlying disease (full-blown cancer), the duration of treatment (very brief compared to chemoprevention) and their reference point (many currently used chemotherapeutic agents are genotoxic).
Although their precise mechanism of action may not be resolved in sufficient detail for quite some time, it is clear that the ability of NO-NSAIDs to generate ROS and to suppress microsatellite instability may be key proximal events. Delineating the mechanism of action of NO-NSAIDs may guide efforts to develop effective drug combinations or guidelines for chemoprevention.
The most recent development concerns the requirement of NO-NSAIDs to have the NO-releasing moiety. Although at first glance this question is totally iconoclastic, it needs not be perceived as such. The evidence is strong that NO-NSAIDs do release NO and that NO does have an effect against cancer. On the other hand, at least in the case of NO-ASA and perhaps of other such derivatives, the NO-releasing moiety can be replaced by moieties that do not release NO without any drastic change in anti-cancer efficacy in preclinical models. At the very least, this development appears to lead to new classes of compounds and their synthesis may be a useful outcome of the mechanistic studies.
In conclusion, NO-NSAIDs are poised to become a new class of anti-cancer agents and to generate perhaps equally promising and certainly equally puzzling “offspring”, the “non-NO NO-NSAIDs.” Their future seems full of promise, excitement – and more work!
Acknowledgments
Grant support: NIH 2R01 CA92423
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rigas B. The use of nitric oxide-donating nonsteroidal anti-inflammatory drugs in the chemoprevention of colorectal neoplasia. Curr Opin Gastroenterol. 2007;23:55–59. doi: 10.1097/MOG.0b013e32801145b0. [DOI] [PubMed] [Google Scholar]
- 2.Gresele P, Momi S. Pharmacologic profile and therapeutic potential of NCX 4016, a nitric oxide-releasing aspirin, for cardiovascular disorders. Cardiovasc Drug Rev. 2006;24:148–168. doi: 10.1111/j.1527-3466.2006.00148.x. [DOI] [PubMed] [Google Scholar]
- 3.Zhang ZG, Zhang YH, Ji H, Qiu SG, Feng XC. [Design, synthesis and antiasthmatic activities of NO-donating seratrodast derivatives] Yao Xue Xue Bao. 2004;39:705–710. [PubMed] [Google Scholar]
- 4.Kakizawa H, Matsui F, Tokita Y, Hirano K, Ida M, Nakanishi K, Watanabe M, Sato Y, Okumura A, Kojima S, Oohira A. Neuroprotective effect of nipradilol, an NO donor, on hypoxic-ischemic brain injury of neonatal rats. Early Hum Dev. 2007;83:535–540. doi: 10.1016/j.earlhumdev.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Kimura I. Medical benefits of using natural compounds and their derivatives having multiple pharmacological actions. Yakugaku Zasshi. 2006;126:133–143. doi: 10.1248/yakushi.126.133. [DOI] [PubMed] [Google Scholar]
- 6.Thatcher GR, Bennett BM, Reynolds JN. NO chimeras as therapeutic agents in Alzheimer's disease. Curr Alzheimer Res. 2006;3:237–245. doi: 10.2174/156720506777632925. [DOI] [PubMed] [Google Scholar]
- 7.del Soldato P, Sorrentino R, Pinto A. NO-aspirins: a class of new anti-inflammatory and antithrombotic agents. Trends Pharmacol Sci. 1999;20:319–323. doi: 10.1016/s0165-6147(99)01353-x. [DOI] [PubMed] [Google Scholar]
- 8.Ellis JL, Augustyniak ME, Cochran ED, Earl RA, Garvey DS, Gordon LJ, Janero DR, Khanapure SP, Letts LG, Melim TL, Murty MG, Schwalb DJ, Shumway MJ, Selig WM, Trocha AM, Young DV, Zemtseva IS. NMI-1182, a gastro-protective cyclo-oxygenase-inhibiting nitric oxide donor. Inflammopharmacology. 2005;12:521–534. doi: 10.1163/156856005774382661. [DOI] [PubMed] [Google Scholar]
- 9.Fiorucci S, Santucci L, Gresele P, Faccino RM, Del Soldato P, Morelli A. Gastrointestinal safety of NO-aspirin (NCX-4016) in healthy human volunteers: a proof of concept endoscopic study. Gastroenterology. 2003;124:600–607. doi: 10.1053/gast.2003.50096. [DOI] [PubMed] [Google Scholar]
- 10.Thatcher GR. An introduction to NO-related therapeutic agents. Curr Top Med Chem. 2005;5:597–601. doi: 10.2174/1568026054679281. [DOI] [PubMed] [Google Scholar]
- 11.Thatcher GR, Bennett BM, Reynolds JN. Nitric oxide mimetic molecules as therapeutic agents in Alzheimer's disease. Curr Alzheimer Res. 2005;2:171–182. doi: 10.2174/1567205053585945. [DOI] [PubMed] [Google Scholar]
- 12.Thatcher GR, Nicolescu AC, Bennett BM, Toader V. Nitrates and NO release: contemporary aspects in biological and medicinal chemistry. Free Radic Biol Med. 2004;37:1122–1143. doi: 10.1016/j.freeradbiomed.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Hagos GK, Carroll RE, Kouznetsova T, Li Q, Toader V, Fernandez PA, Swanson SM, Thatcher GR. Colon cancer chemoprevention by a novel NO chimera that shows anti-inflammatory and antiproliferative activity in vitro and in vivo. Mol Cancer Ther. 2007;6:2230–2239. doi: 10.1158/1535-7163.MCT-07-0069. [DOI] [PubMed] [Google Scholar]
- 14.Velazquez CA, Praveen Rao PN, Citro ML, Keefer LK, Knaus EE. O2-acetoxymethyl-protected diazeniumdiolate-based NSAIDs (NONO-NSAIDs): synthesis, nitric oxide release, and biological evaluation studies. Bioorg Med Chem. 2007;15:4767–4774. doi: 10.1016/j.bmc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiziltepe T, Hideshima T, Ishitsuka K, Ocio EM, Raje N, Catley L, Li CQ, Trudel LJ, Yasui H, Vallet S, Kutok JL, Chauhan D, Mitsiades CS, Saavedra JE, Wogan GN, Keefer LK, Shami PJ, Anderson KC. JS-K, a GST-activated nitric oxide generator, induces DNA double-strand breaks, activates DNA damage response pathways, and induces apoptosis in vitro and in vivo in human multiple myeloma cells. Blood. 2007;110:709–718. doi: 10.1182/blood-2006-10-052845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chakrapani H, Wilde TC, Citro ML, Goodblatt MM, Keefer LK, Saavedra JE. Synthesis, nitric oxide release, and anti-leukemic activity of glutathione-activated nitric oxide prodrugs: Structural analogues of PABA/NO, an anti-cancer lead compound. Bioorg Med Chem. 2007 doi: 10.1016/j.bmc.2007.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carini M, Aldini G, Orioli M, Piccoli A, Rossoni G, Maffei Facino R. Nitric oxide release and distribution following oral and intraperitoneal administration of nitroaspirin (NCX 4016) in the rat. Life Sci. 2004;74:3291–3305. doi: 10.1016/j.lfs.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Carini M, Aldini G, Orioli M, Piccoli A, Tocchetti P, Facino RM. Chemiluminescence and LC-MS/MS analyses for the study of nitric oxide release and distribution following oral administration of nitroaspirin (NCX 4016) in healthy volunteers. J Pharm Biomed Anal. 2004;35:277–287. doi: 10.1016/S0731-7085(03)00531-4. [DOI] [PubMed] [Google Scholar]
- 19.Williams JL, Borgo S, Hasan I, Castillo E, Traganos F, Rigas B. Nitric oxide-releasing nonsteroidal anti-inflammatory drugs (NSAIDs) alter the kinetics of human colon cancer cell lines more effectively than traditional NSAIDs: implications for colon cancer chemoprevention. Cancer Res. 2001;61:3285–3289. [PubMed] [Google Scholar]
- 20.Yeh RK, Chen J, Williams JL, Baluch M, Hundley TR, Rosenbaum RE, Kalala S, Traganos F, Benardini F, del Soldato P, Kashfi K, Rigas B. NO-donating nonsteroidal antiinflammatory drugs (NSAIDs) inhibit colon cancer cell growth more potently than traditional NSAIDs: a general pharmacological property? Biochem Pharmacol. 2004;67:2197–2205. doi: 10.1016/j.bcp.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 21.Huguenin S, Vacherot F, Fleury-Feith J, Riffaud JP, Chopin DK, Bolla M, Jaurand MC. Evaluation of the antitumoral potential of different nitric oxide-donating non-steroidal anti-inflammatory drugs (NO-NSAIDs) on human urological tumor cell lines. Cancer Lett. 2005;218:163–170. doi: 10.1016/j.canlet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Huguenin S, Vacherot F, Kheuang L, Fleury-Feith J, Jaurand MC, Bolla M, Riffaud JP, Chopin DK. Antiproliferative effect of nitrosulindac (NCX 1102), a new nitric oxide-donating non-steroidal anti-inflammatory drug, on human bladder carcinoma cell lines. Mol Cancer Ther. 2004;3:291–298. [PubMed] [Google Scholar]
- 23.Kashfi K, Ryann Y, Qiao LL, Williams JL, Chen J, Del Soldato P, Traganos F, Rigas B. Nitric oxide-donating nonsteroidal anti-inflammatory drugs inhibit the growth of various cultured human cancer cells: evidence of a tissue type-independent effect. J Pharmacol Exp Ther. 2002;303:1273–1282. doi: 10.1124/jpet.102.042754. [DOI] [PubMed] [Google Scholar]
- 24.Williams JL, Kashfi K, Ouyang N, del Soldato P, Kopelovich L, Rigas B. NO-donating aspirin inhibits intestinal carcinogenesis in Min (APC(Min/+)) mice. Biochem Biophys Res Commun. 2004;313:784–788. doi: 10.1016/j.bbrc.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Rao CV, Reddy BS, Steele VE, Wang CX, Liu X, Ouyang N, Patlolla JM, Simi B, Kopelovich L, Rigas B. Nitric oxide-releasing aspirin and indomethacin are potent inhibitors against colon cancer in azoxymethane-treated rats: effects on molecular targets. Mol Cancer Ther. 2006;5:1530–1538. doi: 10.1158/1535-7163.MCT-06-0061. [DOI] [PubMed] [Google Scholar]
- 26.Tesei A, Ulivi P, Fabbri F, Rosetti M, Leonetti C, Scarsella M, Zupi G, Amadori D, Bolla M, Zoli W. In vitro and in vivo evaluation of NCX 4040 cytotoxic activity in human colon cancer cell lines. J Transl Med. 2005;3:7. doi: 10.1186/1479-5876-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonetti C, Scarsella M, Zupi G, Zoli W, Amadori D, Medri L, Fabbri F, Rosetti M, Ulivi P, Cecconetto L, Bolla M, Tesei A. Efficacy of a nitric oxide-releasing nonsteroidal anti-inflammatory drug and cytotoxic drugs in human colon cancer cell lines in vitro and xenografts. Mol Cancer Ther. 2006;5:919–926. doi: 10.1158/1535-7163.MCT-05-0536. [DOI] [PubMed] [Google Scholar]
- 28.Ouyang N, Williams JL, Rigas B. NO-donating aspirin isomers downregulate peroxisome proliferator-activated receptor (PPAR){delta} expression in APCmin/+ mice proportionally to their tumor inhibitory effect: Implications for the role of PPAR{delta} in carcinogenesis. Carcinogenesis. 2006;27:232–239. doi: 10.1093/carcin/bgi221. [DOI] [PubMed] [Google Scholar]
- 29.Kashfi K, Borgo S, Williams JL, Chen J, Gao J, Glekas A, Benedini F, Del Soldato P, Rigas B. Positional isomerism markedly affects the growth inhibition of colon cancer cells by nitric oxide-donating aspirin in vitro and in vivo. J Pharmacol Exp Ther. 2005;312:978–988. doi: 10.1124/jpet.104.075994. [DOI] [PubMed] [Google Scholar]
- 30.Tesei A, Rosetti M, Ulivi P, Fabbri F, Medri L, Vannini I, Bolla M, Amadori D, Zoli W. Study of molecular mechanisms of pro-apoptotic activity of NCX 4040, a novel nitric oxide-releasing aspirin, in colon cancer cell lines. J Transl Med. 2007;5:52. doi: 10.1186/1479-5876-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosetti M, Tesei A, Ulivi P, Fabbri F, Vannini I, Brigliadori G, Amadori D, Bolla M, Zoli W. Molecular characterization of cytotoxic and resistance mechanisms induced by NCX 4040, a novel NO-NSAID, in pancreatic cancer cell lines. Apoptosis. 2006;11:1321–1330. doi: 10.1007/s10495-006-6986-x. [DOI] [PubMed] [Google Scholar]
- 32.Gao J, Liu X, Rigas B. Nitric oxide-donating aspirin induces apoptosis in human colon cancer cells through induction of oxidative stress. Proc Natl Acad Sci U S A. 2005;102:17207–17212. doi: 10.1073/pnas.0506893102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hundley TR, Rigas B. Nitric oxide-donating aspirin inhibits colon cancer cell growth via mitogen-activated protein kinase activation. J Pharmacol Exp Ther. 2006;316:25–34. doi: 10.1124/jpet.105.091363. [DOI] [PubMed] [Google Scholar]
- 34.Spiegel A, Hundley TR, Chen J, Gao J, Ouyang N, Liu X, Go MF, Tsioulias GJ, Kashfi K, Rigas B. NO-donating aspirin inhibits both the expression and catalytic activity of inducible nitric oxide synthase in HT-29 human colon cancer cells. Biochem Pharmacol. 2005;70:993–1000. doi: 10.1016/j.bcp.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 35.Williams JL, Ji P, Ouyang N, Liu X, Rigas B. NO-donating aspirin inhibits the activation of NF-{kappa}B in human cancer cell lines and Min mice. Carcinogenesis. 2008 doi: 10.1093/carcin/bgm275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Williams JL, Nath N, Chen J, Hundley TR, Gao J, Kopelovich L, Kashfi K, Rigas B. Growth inhibition of human colon cancer cells by nitric oxide (NO)-donating aspirin is associated with cyclooxygenase-2 induction and beta-catenin/T-cell factor signaling, nuclear factor-kappaB, and NO synthase 2 inhibition: implications for chemoprevention. Cancer Res. 2003;63:7613–7618. [PubMed] [Google Scholar]
- 37.Gao J, Kashfi K, Liu X, Rigas B. NO-donating aspirin induces phase II enzymes in vitro and in vivo. Carcinogenesis. 2006;27:803–810. doi: 10.1093/carcin/bgi262. [DOI] [PubMed] [Google Scholar]
- 38.McIlhatton MA, Tyler J, Burkholder S, Ruschoff J, Rigas B, Kopelovich L, Fishel R. Nitric oxide-donating aspirin derivatives suppress microsatellite instability in mismatch repair-deficient and hereditary nonpolyposis colorectal cancer cells. Cancer Res. 2007;67:10966–10975. doi: 10.1158/0008-5472.CAN-07-2562. [DOI] [PubMed] [Google Scholar]
- 39.Rigas B, Kashfi K. Nitric-oxide-donating NSAIDs as agents for cancer prevention. Trends Mol Med. 2004;10:324–330. doi: 10.1016/j.molmed.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 40.NicOx S. [Accessed November 23, 2007];NicOx provides an update on NCX 4016 (Press release) 2007 http://www.nicox.com/upload/NCX4016-EF-180607.pdf;
- 41.Wink DA, Mitchell JB. Nitric oxide and cancer: an introduction. Free Radic Biol Med. 2003;34:951–954. doi: 10.1016/s0891-5849(02)01362-x. [DOI] [PubMed] [Google Scholar]
- 42.Anuar F, Whiteman M, Bhatia M, Moore PK. Flurbiprofen and its nitric oxide-releasing derivative protect against septic shock in rats. Inflamm Res. 2006;55:498–503. doi: 10.1007/s00011-006-5150-y. [DOI] [PubMed] [Google Scholar]
- 43.Carini M, Aldini G, Stefani R, Orioli M, Facino RM. Nitrosylhemoglobin, an unequivocal index of nitric oxide release from nitroaspirin: in vitro and in vivo studies in the rat by ESR spectroscopy. J Pharm Biomed Anal. 2001;26:509–518. doi: 10.1016/s0731-7085(01)00478-2. [DOI] [PubMed] [Google Scholar]
- 44.Govoni M, Casagrande S, Maucci R, Chiroli V, Tocchetti P. In vitro metabolism of (nitrooxy)butyl ester nitric oxide-releasing compounds: comparison with glyceryl trinitrate. J Pharmacol Exp Ther. 2006;317:752–761. doi: 10.1124/jpet.105.097469. [DOI] [PubMed] [Google Scholar]
- 45.Perini R, Fiorucci S, Wallace JL. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastrointestinal injury and repair: a window of opportunity for cyclooxygenase-inhibiting nitric oxide donors. Can J Gastroenterol. 2004;18:229–236. doi: 10.1155/2004/890585. [DOI] [PubMed] [Google Scholar]
- 46.Brzozowski T, Konturek PC, Konturek SJ, Sliwowski Z, Drozdowicz D, Kwiecien S, Pajdo R, Ptak A, Pawlik M, Hahn E. Gastroprotective and ulcer healing effects of nitric oxide-releasing non-steroidal anti-inflammatory drugs. Dig Liver Dis. 2000;32:583–594. doi: 10.1016/s1590-8658(00)80840-3. [DOI] [PubMed] [Google Scholar]
- 47.Wallace JL. Nitric oxide, aspirin-triggered lipoxins and NO-aspirin in gastric protection. Inflamm Allergy Drug Targets. 2006;5:133–137. doi: 10.2174/187152806776383116. [DOI] [PubMed] [Google Scholar]
- 48.Scatena R, Bottoni P, Martorana GE, Giardina B. Nitric oxide donor drugs: an update on pathophysiology and therapeutic potential. Expert Opin Investig Drugs. 2005;14:835–846. doi: 10.1517/13543784.14.7.835. [DOI] [PubMed] [Google Scholar]
- 49.Liu Q, Chan ST, Mahendran R. Nitric oxide induces cyclooxygenase expression and inhibits cell growth in colon cancer cell lines. Carcinogenesis. 2003;24:637–642. doi: 10.1093/carcin/bgg014. [DOI] [PubMed] [Google Scholar]
- 50.Hamilton D, Batist G. Glutathione analogues in cancer treatment. Curr Oncol Rep. 2004;6:116–122. doi: 10.1007/s11912-004-0023-4. [DOI] [PubMed] [Google Scholar]
- 51.Frederiksen LJ, Siemens DR, Heaton JP, Maxwell LR, Adams MA, Graham CH. Hypoxia induced resistance to doxorubicin in prostate cancer cells is inhibited by low concentrations of glyceryl trinitrate. J Urol. 2003;170:1003–1007. doi: 10.1097/01.ju.0000081126.71235.e0. [DOI] [PubMed] [Google Scholar]
- 52.Huerta-Yepez S, Vega M, Jazirehi A, Garban H, Hongo F, Cheng G, Bonavida B. Nitric oxide sensitizes prostate carcinoma cell lines to TRAIL-mediated apoptosis via inactivation of NF-kappa B and inhibition of Bcl-xl expression. Oncogene. 2004;23:4993–5003. doi: 10.1038/sj.onc.1207655. [DOI] [PubMed] [Google Scholar]
- 53.Dairou J, Atmane N, Rodrigues-Lima F, Dupret JM. Peroxynitrite irreversibly inactivates the human xenobiotic-metabolizing enzyme arylamine N-acetyltransferase 1 (NAT1) in human breast cancer cells: a cellular and mechanistic study. J Biol Chem. 2004;279:7708–7714. doi: 10.1074/jbc.M311469200. [DOI] [PubMed] [Google Scholar]
- 54.Park IC, Woo SH, Park MJ, Lee HC, Lee SJ, Hong YJ, Lee SH, Hong SI, Rhee CH. Ionizing radiation and nitric oxide donor sensitize Fas-induced apoptosis via up-regulation of Fas in human cervical cancer cells. Oncol Rep. 2003;10:629–633. [PubMed] [Google Scholar]
- 55.Wang D, Lu S, Dong Z. Regulation of TGF-beta1 gene transcription in human prostate cancer cells by nitric oxide. Prostate. 2007;67:1825–1833. doi: 10.1002/pros.20669. [DOI] [PubMed] [Google Scholar]
- 56.Ambs S, Bennett WP, Merriam WG, Ogunfusika MO, Oser SM, Harrington AM, Shields PG, Felley-Bosco E, Hussain SP, Harris CC. Relationship between p53 mutations and inducible nitric oxide synthase expression in human colorectal cancer. J Natl Cancer Inst. 1999;91:86–88. doi: 10.1093/jnci/91.1.86. [DOI] [PubMed] [Google Scholar]
- 57.Kashfi K, Rigas B. The mechanism of action of nitric oxide-donating aspirin. Biochem Biophys Res Commun. 2007;358:1096–1101. doi: 10.1016/j.bbrc.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 58.Hulsman N, Medema JP, Bos C, Jongejan A, Leurs R, Smit MJ, de Esch IJ, Richel D, Wijtmans M. Chemical insights in the concept of hybrid drugs: the antitumor effect of nitric oxide-donating aspirin involves a quinone methide but not nitric oxide nor aspirin. J Med Chem. 2007;50:2424–2431. doi: 10.1021/jm061371e. [DOI] [PubMed] [Google Scholar]
- 59.Rigas B, Kozoni V. The novel phenylester anticancer compounds: Study of a derivative of aspirin (phoshoaspirin) Int J Oncol. 2008;32:97–100. [PubMed] [Google Scholar]


