Abstract
A fundamental mechanism by which cells can give rise to daughters with different fates is via asymmetric division. During asymmetric division, a mother cell generates daughter cells that go on to adopt different fates due to differential segregation of cell fate determinants. Although originally characterized in invertebrates, asymmetric division has recently been shown to regulate cell fate decisions in the mammalian hematopoietic system, playing critical roles in stem cell renewal, lymphocyte activation and leukemogenesis. These discoveries have opened new doors to understanding how regulation of division pattern contributes to the normal development and function of the immune system as well as how its dysregulation can lead to cancer.
Introduction
The creation of a multicellular organism requires the single celled zygote to undergo a controlled series of proliferation steps coordinated with perfectly timed cell fate decisions. This pattern is often recapitulated in homeostatic tissue growth and maintenance as individual cells must perpetually renew as well as generate a spectrum of differentiated progeny. One important mechanism by which cells can give rise to daughters with different fates, is via asymmetric division [1,2]. During asymmetric division, a dividing cell polarizes intracellular fate determinants so that the daughters inherit different amounts, thus generating daughters that go on to adopt different fates. In contrast, a symmetric division yields identical progeny. While elegantly simple in theory, in practice this type of division depends on a complex mechanism by which the cell is able to segregate the appropriate components, as well as divide upon the established axis of polarity. Numerous examples of symmetric and asymmetric division have been identified in invertebrates; however, whether and the extent to which asymmetric division occurs in mammals is only beginning to be revealed. In this review we discuss the new advances in the immune system which lend important insight into mammalian asymmetry and the consequences of its aberrant regulation.
Asymmetric Division in Invertebrates
A classic example of invertebrate asymmetric division occurs Jim_Cornelius@bd.com during drosophila neural development. Drosophila neuronal progenitor cells, neuroblasts, divide asymmetrically to form one new neuroblast and one ganglion mother cell (GMC), which subsequently generates mature neurons and glia[3]. This process is coordinated in part by an evolutionarily conserved protein-complex, the Par complex, which is responsible for establishing and maintaining neuroblast apical-basal polarity. In drosophila, key Par members consist of Bazooka/Par3, Par-6 and atypical protein kinase C (aPKC). Apical orientation of the Par complex occurs during polarization of the neuroectodermal epithelium; neuroblasts appear to inherit this apical localization during their specification and delamination from the epithelium[1]. This orientation is necessary for both the ensuing basal localization of cell fate determinants and proper orientation of the mitotic spindle. Basal localization is driven in part by phosphorylation of lethal (2) giant larvae (Lgl) by aPKC, leading to its apical inactivity. Along with Scribble (Scrib) and Discs large (Dlg), Lgl helps recruit the adaptor proteins Miranda (Mir) and Partner of numb (Pon) and their binding partners the cell fate determinants Prospero (Pros), Brain Tumor (Brat) and numb which are the critical factors that are responsible for altering the transcriptional and translational activity of the GMC to confer cell identity[3,4]. Proper orientation of the mitotic spindle along this apical to basal axis is initiated in part through the adaptor protein insceutable (Insc) binding to both the Par complex and partner of insceutable (Pins) during neuroblast delamination[1,5]. Apical Pins interacts with a microtubule-binding protein called mushroom body defective (Mud), which along with other factors orients the mitotic spindle along the apical to basal axis. The emerging apical daughter retains neuroblast identity while the basal daughter, containing numb and other commitment determinants, becomes a differentiated GMC. Thus, asymmetric division depends upon a cells ability to initiate and preserve asymmetry, segregate fate determinants along an axis of polarity, and orient the mitotic spindle along this axis (Figure 1). Many of the proteins controlling asymmetric division in the drosophila neuroblast also control asymmetric division in C. elegans; additionally, mammalian homologues have been shown to be involved in asymmetric division during vertebrate development[2]. Because asymmetric division can play a defining role in whether a cell goes on to generate a differentiated daughter or not, it may be a fundamental shared mechanism used in the generation of daughters with alternate fates at different times in the immune and other mammalian systems.
Figure 1. Coordination of Asymmetric Division.
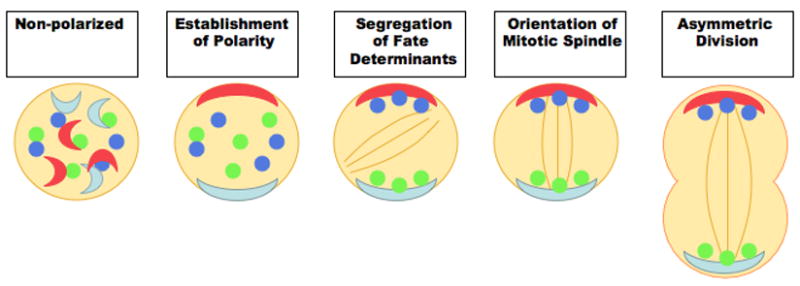
Schematic diagram depicting the requisite steps regulating asymmetric division. For asymmetric division to occur, a cell must initially establish an intrinsic polarity via extrinsic and/or intrinsic cues. Fate determinants responsible for establishing cellular identity must be asymmetrically localized according to the axis of polarity; additionally, orientation of the mitotic spindle must be aligned along the axis of asymmetry to successfully mediate formation of daughter cells with intrinsically different cell fates.
Asymmetric Division during Hematopoietic Stem Cell Development
The generation of the immune system begins with the hematopoietic stem cell (HSC). These well-characterized cells are responsible for the daily production of hundreds of millions of cells of distinct lineages that include the cells of the T, B and myeloid lineages [6,7]. This ability requires the stem cell not only to self-renew to preserve itself but to balance self-renewal with differentiation so that committed daughters are generated. Asymmetric division provides a conceptually attractive mechanism for how self-renewal in HSCs may be balanced with differentiation. However as in most mammalian systems, evidence that this actually occurs in the hematopoietic system has been difficult to obtain. Early work on asymmetric division in the hematopoietic system examined the fates of paired daughter cells separated from a single hematopoietic stem cell containing populations [8–11]. This strategy (termed clone splitting) revealed that paired daughters from individual HSCs were not functionally equivalent and that they could give rise to progeny with different cell-cycle kinetics or multilineage capacity [12,13]. Although this raised the possibility that daughters of distinct fates could arise from one hematopoietic progenitor cell, it was unclear whether the fates could have changed following an equivalent symmetric division, potentially via a differential extrinsic encounter not specifically linked to a mitotic mechanism. Since the work did not reveal whether cellular determinants within progenitors or stem cells were differentially segregated to emerging daughters during mitosis it left the question of whether asymmetric division can occur unresolved [14].
This question has been difficult to resolve because the committed and uncommitted daughters in the immune system following asymmetric division are not morphologically distinct and thus not amenable to direct imaging or fate tracing. The recent description of the Transgenic Notch Reporter (TNR) mice in which Notch signaling induces the expression of GFP, serendipitously turned out to be a system where GFP expression acts as a surrogate marker for HSC identity* [15]. While HSCs are preferentially GFP+, committed cells are GFP−, allowing live GFP+ HSCs to be traced through time-lapse microscopy and the fate of the daughters analyzed [16]. This approach revealed that all three possible modes of division, i.e. asymmetric divisions (one GFP+ and one GFP−daughter), symmetric renewal (two GFP+ progeny) or symmetric commitment (two GFP−daughters) occurs in HSCs. These studies also showed that the cell fate determinant numb was asymmetrically segregated into the committed daughter suggesting that the asymmetry observed in HSCs can be established intrinsically, and was not necessarily a consequence of a symmetric division followed by asymmetric encounter with different microenvironmental cues. It is highly likely that other proteins are also involved is establishing this asymmetry; in fact recent work on human hematopoietic progenitors identified four proteins that segregated asymmetrically in 20% of mitotic human hematopoietic precursors* [17]. Whether and how these and other as yet unidentified proteins interact with numb will be an interesting area of further study.
The real time imaging approach also allowed testing whether the division pattern in HSCs is held constant or can be influenced by the microenvironment. This was carried out by co-culturing cells plated on pro-differentiation or pro-renewal stroma. Interestingly, the cells cultured on pro-differentiation stroma primarily underwent asymmetric divisions, whereas those on pro-renewal stroma primarily divided by symmetric renewal. This data indicates that control of divisional symmetry may be a key mechanism that can be altered to regulate the ultimate outcome of stem cell renewal and commitment. Additionally, this demonstrates that control of asymmetric and symmetric division is responsive to extrinsic signals, corroborating the data from certain invertebrate models such as C. elegans [2,18,19] and paired daughter cell studies [9,12,13].
Dysregulation of Asymmetric Division in Hematopoietic Transformation
During oncogenesis cellular properties such as growth and death are often targets of dysregulation. The finding discussed above that alterations in the balance of asymmetric and symmetric division can result in increased or decreased renewal, suggested the possibility that mammalian oncogenes may also act to cause cancer growth by changing the balance between asymmetric and symmetric division. The effects of two oncogenes were tested in this context: BCR-ABL a translocation product predominantly associated with a slow growing chronic myelogenous leukemia, and Nup98-Hoxa9 a translocation associated primarily with the more aggressive blast crisis phase of CML or de novo acute myeloid leukemia. The introduction of BCR-ABL increased growth and survival consistent with the literature [20] [21] but did not alter division pattern; however, Nup98-HoxA9 did not affect cell cycle kinetics but significantly increased the frequency of symmetric renewal [16]. This work showed that certain oncogenes can in fact subvert the balance between symmetric and asymmetric division, but it also suggested that not all oncogenes functioned similarly in this context. Since BCR-ABL driven leukemias retain the differentiation pattern of the tissue, while Nup98-HoxA9 promotes more immature leukemias, an exciting implication of the work described above is that the ability of Nup98-HoxA9 to shift the balance from asymmetric division to symmetric renewal may underlie its ability to block differentiation In contrast the fact that BCR-ABL cannot readily shift the balance between asymmetric and symmetric division may underlie its ability to maintain a normal rate of differentiation. Interestingly, in drosophila neuroblasts, many proteins involved in specifying asymmetric division function as tumor suppressors. Loss of intrinsic commitment determinants like Numb and Miranda, or loss of spindle alignment via MUD deficiency, leads to tumor-like neuroblast overgrowth[5]. In addition many genes that function in invertebrate asymmetric division have been shown to be dysregulated in human malignancies. For example, atypical PKCι is overexpressed in non-small cell lung cancer[22], atypical PKCζ and human Lgl (Hugl-1) lose their apical and basal localization in cancers of the ovarian epithelia[23], and both human Scrib and Dlg show initial mislocalization with a final loss of expression during progressive dysplasia of colon cancer [24]. Although the precise functional consequence of these changes remains to be elucidated, in context of the finding that altering asymmetric division can lead to transformed growth in drosophila and that certain mammalian oncogenes have the ability to alter asymmetric division as a means to transformation, they strengthen the idea that asymmetric division may indeed be an important target of oncogenic transformation.
Asymmetric Division during activation of the immune system
While the idea of generation of differentially fated daughters is a common paradigm during development, in fact such binary choices can occur at later times following formation of a tissue as well. The activated immune system is a prime example of such a need to make continued fate decisions when T and B cells respond to antigenic stimuli. Specifically, T and B cells of the adaptive immune system must produce effector and memory daughters in response to antigen stimulation. In context of T lymphocytes, the cells are activated during an immune response through contact with the antigen presenting cell (APC) via the immunological synapse[25]. Formation of the immunological synapse causes recruitment of cell surface receptors as well as cytoskeletal polarization of actin and microtubules. But how this could lead to the generation of two distinct cell types that are generated during an immune response was unknown. A recent study tested whether the orientation of the mitotic spindle perpendicular to synapse formation could initiate an asymmetric division generating two lymphocytes with different functional capacities* [26]. Specifically, naive T cells were transplanted into antigen challenged recipients and activated donor T cells that had not yet divided were sorted out. In mitotic cells, proteins known to be part of the immunological synapse still demonstrated an asymmetric distribution that colocalized with one of the microtubule organizing centers (MTOCs). This indicated that: (1) polarity from synapse formation had been retained, similar to drosophila neuroblasts, where apical basal polarity is maintained after delamination from the epithelia [3,27]and (2) the mitotic spindle may be oriented perpendicular to the synapse. Investigation of atypical PKCζ in T cells arrested in cytokinesis revealed that atypical PKCζ was distributed to one daughter cell while the other retained the synapse-derived proteins. Intriguingly, Scrib1 (which has been demonstrated to be vital to T cell polarization during migration and a member of the immunological synapse[28]) as well as Numb, were asymmetrically colocalized to the daughter cell without atypical PKCζ. The functional significance of the segregation of the fate determinant was demonstrated by showing that the synapse-associated daughter inherited more factors found in effector T cells, and the distal daughter inherited more factors of the memory lineage, and that mice transplanted with distal daughter cells did indeed show a significant reduction in bacterial burden after latent challenge in comparison to recipients transplanted with synapse-associated effector daughters. These data demonstrate that asymmetric division of activated T cells was responsible for the production of daughter lymphocytes with functionally different capacities, and additionally suggest that mammalian asymmetric division is linked to a mitotic mechanism seemingly conserved throughout evolution (Figure 2).
Figure 2. Evolutionary Conservation of Molecular Mechanisms Regulating Asymmetric Division.
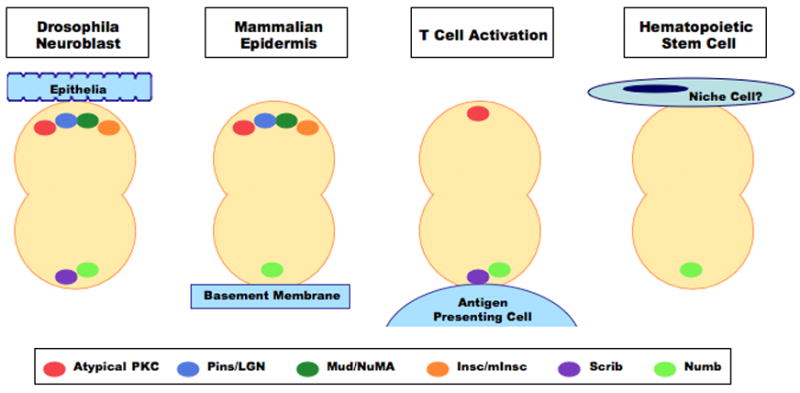
Factors regulating the establishment of polarity, the endowment of cellular identity, and the alignment of the mitotic spindle within invertebrate asymmetric divisions may also be responsible for the coordination of asymmetric divisions in mammalian systems. In particular, oppositional segregation of Par complex components, such as atypical PKC, versus cell fate determinants, such as Numb, appears to be maintained in the asymmetric divisions of: drosophila neuroblasts, mammalian stratified epithelium, and T cell activation. As the asymmetric segregation of Numb may play a role in coordinating the division pattern of HSCs, it will be important to determine what other proteins involved in the establishing polarity are present in the hematopoietic system and how they interact with hematopoietic specific factors.
Perspectives
The hematopoietic system is a complex organ in which correct fate decisions at various stages are critical for normal function. Recent work has shown that asymmetric division underlies these decisions in at least three contexts: during development and differentiation of HSCs, during T cell activation and during leukemogenesis.
HSCs have the ability to generate both committed and uncommitted daughters but how this decision is mediated remains largely unknown. The demonstration that asymmetric division may underlie this decision in HSCs raises many new questions. Is the machinery that controls asymmetric division in invertebrates conserved in HSCs? Are other cell fate determinants besides Numb involved in fate specification in the hematopoietic system? How do these molecules interact with other known signals that regulate self-renewal such as Bmi or elements of the Notch pathway? Are there specific niche-driven signals that initiate polarity and alter the balance between asymmetric and symmetric division, how are these connected to the intrinsic machinery?
In the mature hematopoietic system, the mechanism by which activated lymphocytes generate effector cells and memory cells has also been a mystery. Recent work has demonstrated that the immunological synapse may set up the initial asymmetry that defines different fates for the two daughter cells. An exciting aspect of this work is the finding that Numb is segregated into the daughter that goes on to adopt the fate of the effector cell. This suggests that the memory cell, much like the HSC, may keep the Notch pathway on and thereby preserve the properties of the parent cell. Whether binary fate decisions in other mature cells of the immune system (such as T helper cell subsets or B cells as they differentiate into plasma and memory cells) are also driven by asymmetric division remains to be determined. Differentiation of activated B cells into memory or antibody secreting cell subsets has been proposed to occur at the centrocyte stage where germinal center T cells stimulate the transition from centroblasts to centrocytes [29]. This interaction could additionally initiate a fundamental polarization establishing the memory and effector lineages via asymmetric division, mimicking the role of the immunological synapse between T cells and APCs. Examination of conserved members of the asymmetric division machinery during this cellular interaction may yield important clues as to whether or not binary cell fate decisions also regulate the differentiation of mature B cells.
That asymmetry is fundamentally important to binary fate decisions both during development and during the normal function of the immune response raises the possibility that aberrant asymmetric division could lead to dysfunction of the immune system. In support of this, the exciting finding that asymmetry can be subverted by mammalian oncogenes suggests that asymmetric division can in fact be a critical driving force in leukemogenesis and perhaps other cancers. Interestingly the work to date also suggests that the ability to alter the normal balance of asymmetric and symmetric division may be more a characteristic property of oncogenes that drive immature and aggressive cancers which are typically associated with inhibition of differentiation. Whether such aberrant regulation of the balance between asymmetric and symmetric division may occur within the cancer stem cell fraction of slow growing chronic leukemias will also be an interesting area of investigation. In addition, elucidation of how an oncogene links to the cell polarity machinery to alter its normal function and allowing both daughter cells to adopt similar immature fates will be critical in identifying ways to target this disruption for therapy. The work highlighted here likely represents just the beginning of what will undoubtedly be a new wave of exciting discoveries into the fundamental ways in which cell division can direct and shape the fate of the hematopoietic system.
Footnotes
Annotations
Beckmann Paper: Special Interest
This work identifies four proteins asymmetrically localized in a percentage of mitotic human hematopoietic progenitors, demonstrating that hematopoietic cells may possess the ability to polarize and divide asymmetrically.
Wu Paper: Outstanding interest
Utilizing Notch signaling as a sensor for HSC identity, real-time imaging of hematopoietic precursors demonstrates that they undergo both symmetric and asymmetric divisions and that divisional symmetry is responsive to both extrinsic cues and intrinsic subversion through the expression of oncogenes.
Chang Paper: Outstanding interest
This work demonstrates that formation of the immunological synapse during T cell activation establishes a retained polarization resulting in the differential segregation of cell fate determinants and the asymmetric division of activated T cells, producing individual daughter cells of both the memory and effector lineages.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Betschinger J, Knoblich JA. Dare to be different: asymmetric cell division in Drosophila, C. elegans and vertebrates. Curr Biol. 2004;14:R674–685. doi: 10.1016/j.cub.2004.08.017. [DOI] [PubMed] [Google Scholar]
- 2.Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- 3.Yu F, Kuo CT, Jan YN. Drosophila neuroblast asymmetric cell division: recent advances and implications for stem cell biology. Neuron. 2006;51:13–20. doi: 10.1016/j.neuron.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Chia W, Somers WG, Wang H. Drosophila neuroblast asymmetric divisions: cell cycle regulators, asymmetric protein localization, and tumorigenesis. J Cell Biol. 2008;180:267–272. doi: 10.1083/jcb.200708159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez C. Spindle orientation, asymmetric division and tumour suppression in Drosophila stem cells. Nat Rev Genet. 2007;8:462–472. doi: 10.1038/nrg2103. [DOI] [PubMed] [Google Scholar]
- 6.Spangrude GJ, Heimfeld S, Weissman IL. Purification and characterization of mouse hematopoietic stem cells. Science. 1988;241:58–62. doi: 10.1126/science.2898810. [DOI] [PubMed] [Google Scholar]
- 7.Uchida N, Weissman IL. Searching for hematopoietic stem cells: evidence that Thy-1.1lo Lin-Sca-1+ cells are the only stem cells in C57BL/Ka-Thy-1.1 bone marrow. Journal of Experimental Medicine. 1992;175:175–184. doi: 10.1084/jem.175.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho AD. Kinetics and symmetry of divisions of hematopoietic stem cells. Exp Hematol. 2005;33:1–8. doi: 10.1016/j.exphem.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Takano H, Ema H, Sudo K, Nakauchi H. Asymmetric division and lineage commitment at the level of hematopoietic stem cells: inference from differentiation in daughter cell and granddaughter cell pairs. J Exp Med. 2004;199:295–302. doi: 10.1084/jem.20030929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Punzel M, Zhang T, Liu D, Eckstein V, Ho AD. Functional analysis of initial cell divisions defines the subsequent fate of individual human CD34(+)CD38(−) cells. Exp Hematol. 2002;30:464–472. doi: 10.1016/s0301-472x(02)00781-6. [DOI] [PubMed] [Google Scholar]
- 11.Giebel B, Zhang T, Beckmann J, Spanholtz J, Wernet P, Ho AD, Punzel M. Primitive human hematopoietic cells give rise to differentially specified daughter cells upon their initial cell division. Blood. 2006;107:2146–2152. doi: 10.1182/blood-2005-08-3139. [DOI] [PubMed] [Google Scholar]
- 12.Punzel M, Liu D, Zhang T, Eckstein V, Miesala K, Ho AD. The symmetry of initial divisions of human hematopoietic progenitors is altered only by the cellular microenvironment. Exp Hematol. 2003;31:339–347. doi: 10.1016/s0301-472x(03)00024-9. [DOI] [PubMed] [Google Scholar]
- 13.Ema H, Takano H, Sudo K, Nakauchi H. In vitro self-renewal division of hematopoietic stem cells. J Exp Med. 2000;192:1281–1288. doi: 10.1084/jem.192.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schroeder T. Asymmetric Cell Division in Normal and Malignant Hematopoietic Precursor Cells. Cell Stem Cell. 2007;1:479–481. doi: 10.1016/j.stem.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Duncan AW, Rattis FM, DiMascio LN, Congdon KL, Pazianos G, Zhao C, Yoon K, Cook JM, Willert K, Gaiano N, et al. Integration of Notch and Wnt signaling in hematopoietic stem cell maintenance. Nat Immunol. 2005;6:314–322. doi: 10.1038/ni1164. [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Kwon H, Rattis F, Blum JM, Zhao C, Ashkenazi R, Jackson TL, Gaiano N, Oliver T, Reya T. Imaging Hematopoietic Precursor Division in Real Time. Cell Stem Cell. 2007;1:541–554. doi: 10.1016/j.stem.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beckmann J, Scheitza S, Wernet P, Fischer JC, Giebel B. Asymmetric cell division within the human hematopoietic stem and progenitor cell compartment: identification of asymmetrically segregating proteins. Blood. 2007;109:5494–5501. doi: 10.1182/blood-2006-11-055921. [DOI] [PubMed] [Google Scholar]
- 18.Kai T, Spradling A. Differentiating germ cells can revert into functional stem cells in Drosophila melanogaster ovaries. Nature. 2004;428:564–569. doi: 10.1038/nature02436. [DOI] [PubMed] [Google Scholar]
- 19.Brawley C, Matunis E. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science. 2004;304:1331–1334. doi: 10.1126/science.1097676. [DOI] [PubMed] [Google Scholar]
- 20.Amarante-Mendes GP, McGahon AJ, Nishioka WK, Afar DE, Witte ON, Green DR. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene. 1998;16:1383–1390. doi: 10.1038/sj.onc.1201664. [DOI] [PubMed] [Google Scholar]
- 21.Daley GQ, Van Etten RA, Baltimore D. Induction of chronic myelogenous leukemia in mice by the P210bcr/abl gene of the Philadelphia chromosome. Science. 1990;247:824–830. doi: 10.1126/science.2406902. [DOI] [PubMed] [Google Scholar]
- 22.Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM, Fields AP. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res. 2005;65:8905–8911. doi: 10.1158/0008-5472.CAN-05-2372. [DOI] [PubMed] [Google Scholar]
- 23.Grifoni D, Garoia F, Bellosta P, Parisi F, De Biase D, Collina G, Strand D, Cavicchi S, Pession A. aPKCzeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila and human epithelia. Oncogene. 2007;26:5960–5965. doi: 10.1038/sj.onc.1210389. [DOI] [PubMed] [Google Scholar]
- 24.Gardiol D, Zacchi A, Petrera F, Stanta G, Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer. 2006;119:1285–1290. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- 25.Saito T, Yokosuka T. Immunological synapse and microclusters: the site for recognition and activation of T cells. Curr Opin Immunol. 2006;18:305–313. doi: 10.1016/j.coi.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 27.Siegrist SE, Doe CQ. Extrinsic cues orient the cell division axis in Drosophila embryonic neuroblasts. Development. 2006;133:529–536. doi: 10.1242/dev.02211. [DOI] [PubMed] [Google Scholar]
- 28.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, Pasam A, Iazzolino R, Dow LE, Waterhouse NJ, et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 29.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]


