Abstract
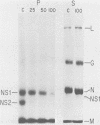
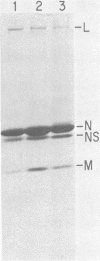
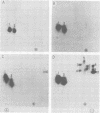
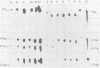
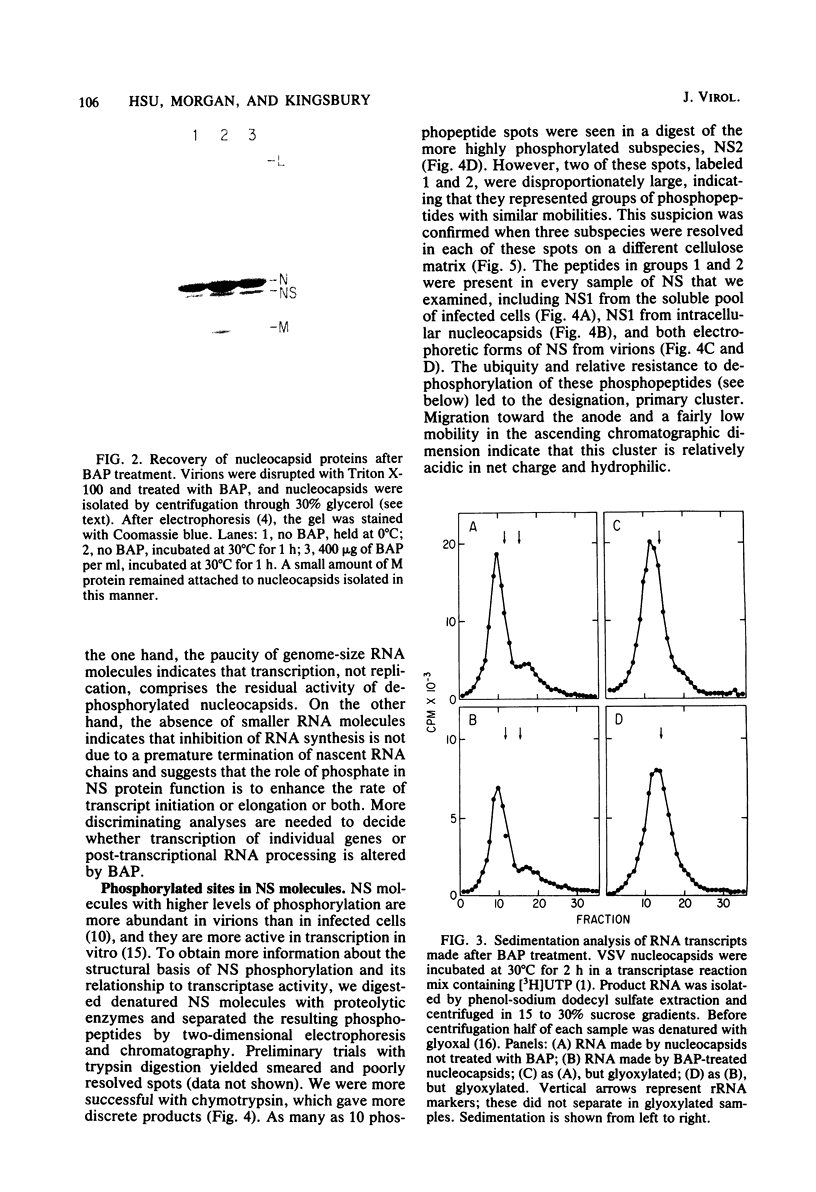
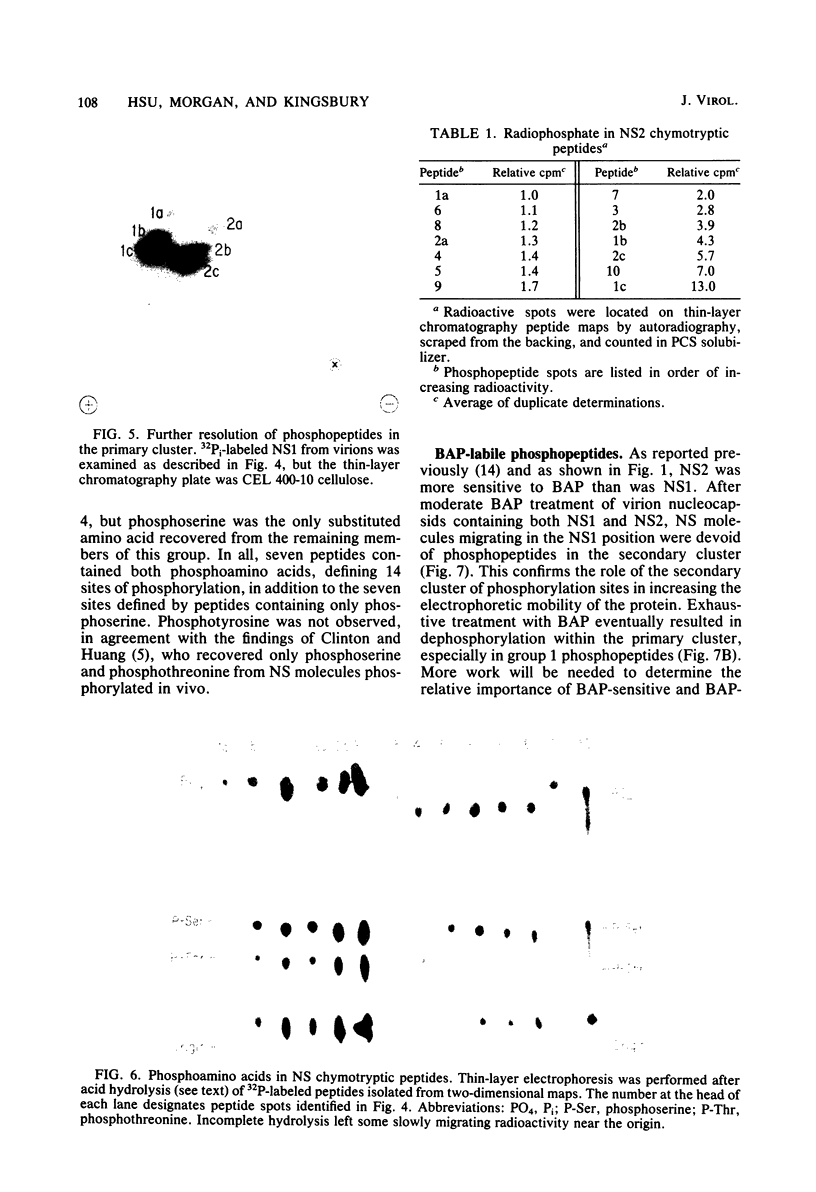
In vitro transcription by vesicular stomatitis virus nucleocapsids is inhibited by enzymatic dephosphorylation of the NS protein. We provide evidence that specific, partial dephosphorylation of NS molecules is the only detectable change in nucleocapsids treated with bacterial alkaline phosphatase under conditions that prevent the action of adventitious protease. Dephosphorylation appeared to affect only the rate of transcription; there were no changes in sedimentation rates of transcripts. To identify the sites of phosphorylation required for NS activity in transcription, we examined phosphopeptides produced by chymotrypsin digestion of the two electrophoretic classes of NS molecules found in virions and infected cells. The electrophoretically slower class, NS1, abundant in the intracellular soluble pool, has a lower activity in transcription; it contained six chymotryptic phosphopeptides. Five of these peptides contained both phosphoserine and phosphothreonine, indicating that this peptide cluster represents at least 11 separate sites of phosphorylation. In the electrophoretically faster nucleocapsid-associated NS2 class of molecules, which support a higher rate of transcription, another group of eight phosphopeptides was superimposed on this pattern. Two of these peptides contained both phosphoserine and phosphothreonine, so this cluster of peptides represents at least 10 additional phosphorylation sites. These sites were especially sensitive to dephosphorylation by bacterial alkaline phosphatase. One or more of them appears to be responsible for the higher transcription rates medicated by NS2 molecules.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K., Rhodes D. P. In vitro synthesis of RNA that contains polyadenylate by virion-associated RNA polymerase of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3566–3570. doi: 10.1073/pnas.70.12.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
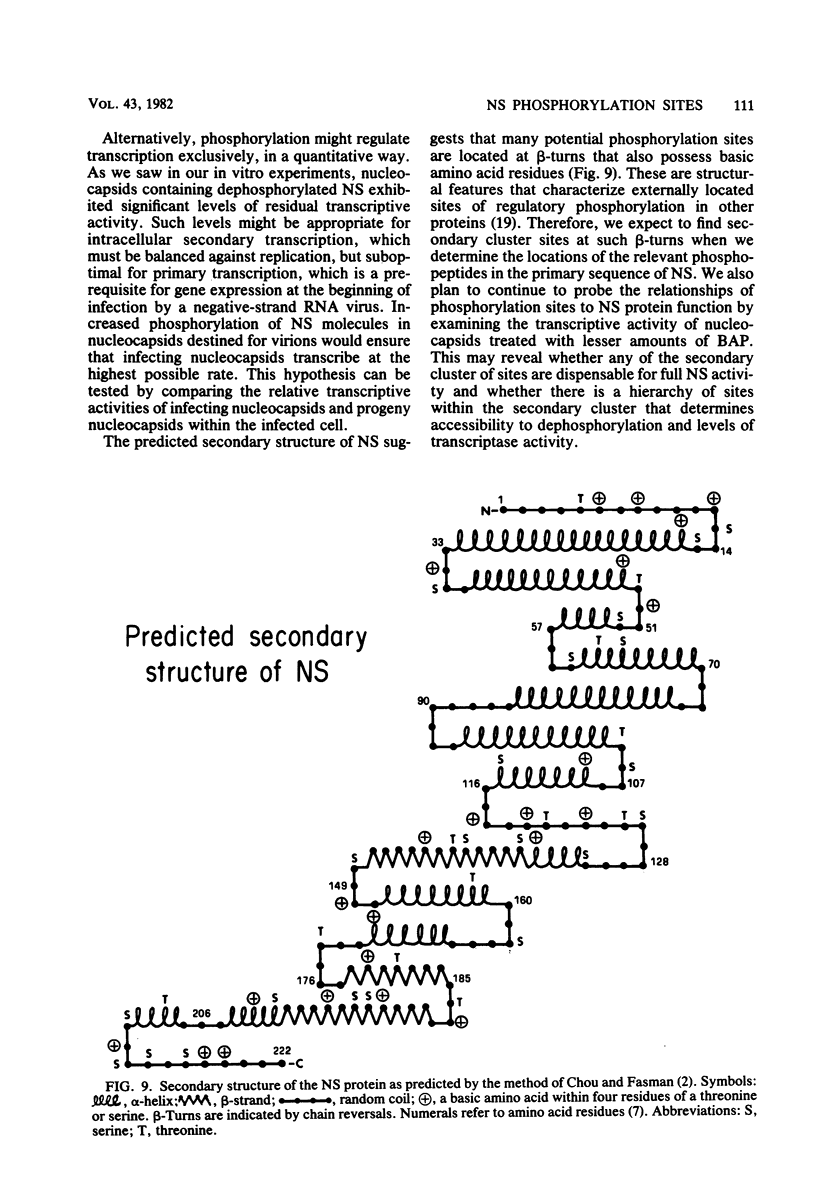
- Chou P. Y., Fasman G. D. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol Relat Areas Mol Biol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Phosphoproteins of vesicular stomatitis virus: identity and interconversion of phosphorylated forms. Virology. 1979 Nov;99(1):84–94. doi: 10.1016/0042-6822(79)90039-4. [DOI] [PubMed] [Google Scholar]
- Clinton G. M., Huang A. S. Distribution of phosphoserine, phosphothreonine and phosphotyrosine in proteins of vesicular stomatitis virus. Virology. 1981 Jan 30;108(2):510–514. doi: 10.1016/0042-6822(81)90459-1. [DOI] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracy R. W. Two-dimensional thin-layer methods. Methods Enzymol. 1977;47:195–204. doi: 10.1016/0076-6879(77)47024-1. [DOI] [PubMed] [Google Scholar]
- Hsu C. H., Kingsbury D. W. NS phosphoprotein of vesicular stomatitis virus: subspecies separated by electrophoresis and isoelectric focusing. J Virol. 1982 Apr;42(1):342–345. doi: 10.1128/jvi.42.1.342-345.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imblum R. L., Wagner R. R. Protein kinase and phosphoproteins of vesicular stomatitis virus. J Virol. 1974 Jan;13(1):113–124. doi: 10.1128/jvi.13.1.113-124.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Asso J., Baltimore D. Further evidence on the formation of poliovirus proteins. J Mol Biol. 1970 May 14;49(3):657–669. doi: 10.1016/0022-2836(70)90289-5. [DOI] [PubMed] [Google Scholar]
- Kingsford L., Emerson S. U. Transcriptional activities of different phosphorylated species of NS protein purified from vesicular stomatitis virions and cytoplasm of infected cells. J Virol. 1980 Mar;33(3):1097–1105. doi: 10.1128/jvi.33.3.1097-1105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer S. A., Summers D. F. Phosphorylation of vesicular stomatitis virus in vivo and in vitro. J Virol. 1974 Feb;13(2):455–465. doi: 10.1128/jvi.13.2.455-465.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. A., Pease L. G. Reverse turns in peptides and proteins. CRC Crit Rev Biochem. 1980;8(4):315–399. doi: 10.3109/10409238009105470. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. In vitro synthesis of the full-length complement of the negative-strand genome RNA of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1980 Jan;77(1):294–298. doi: 10.1073/pnas.77.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]