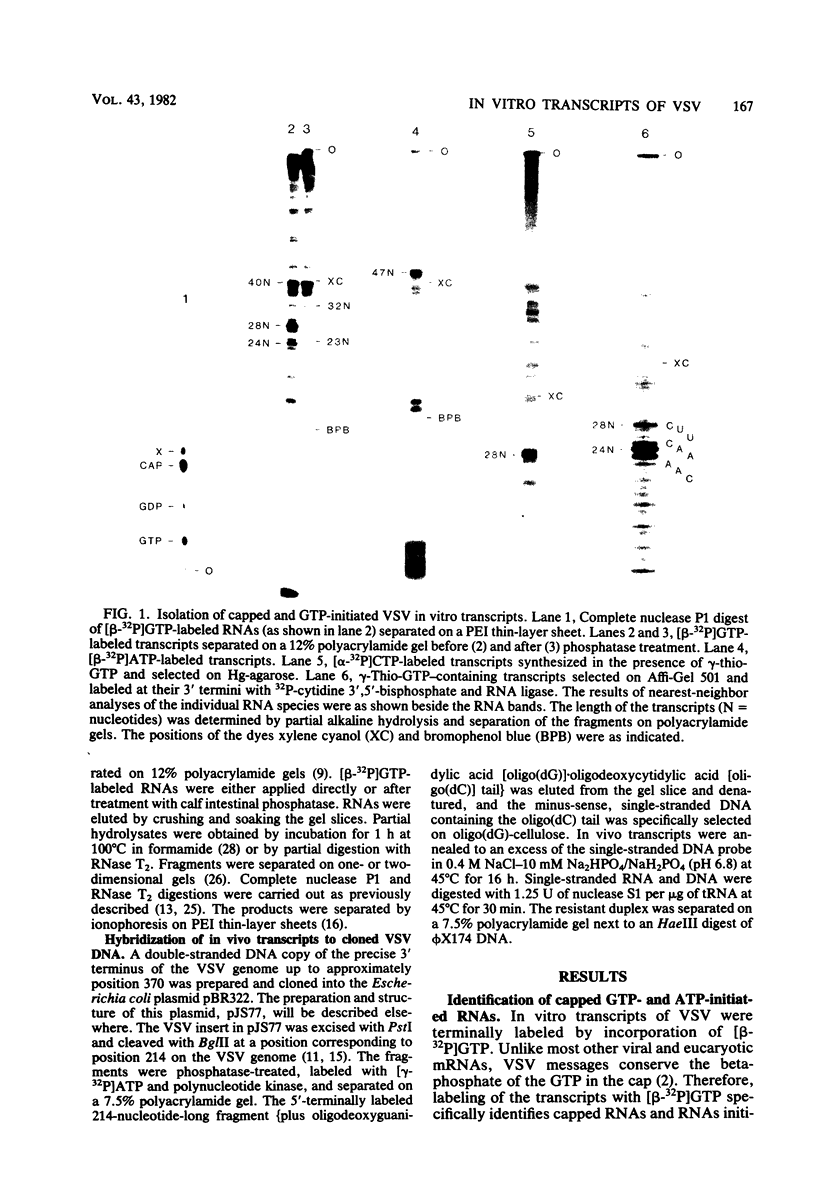
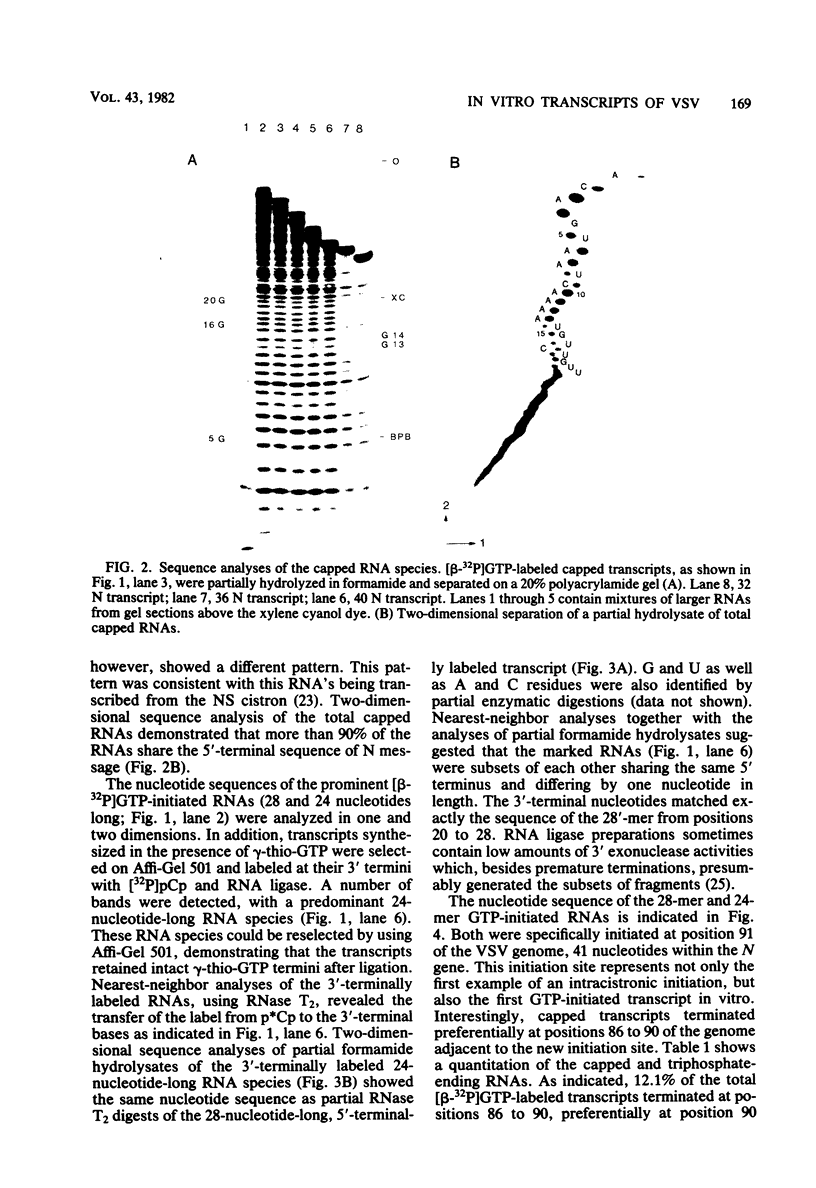
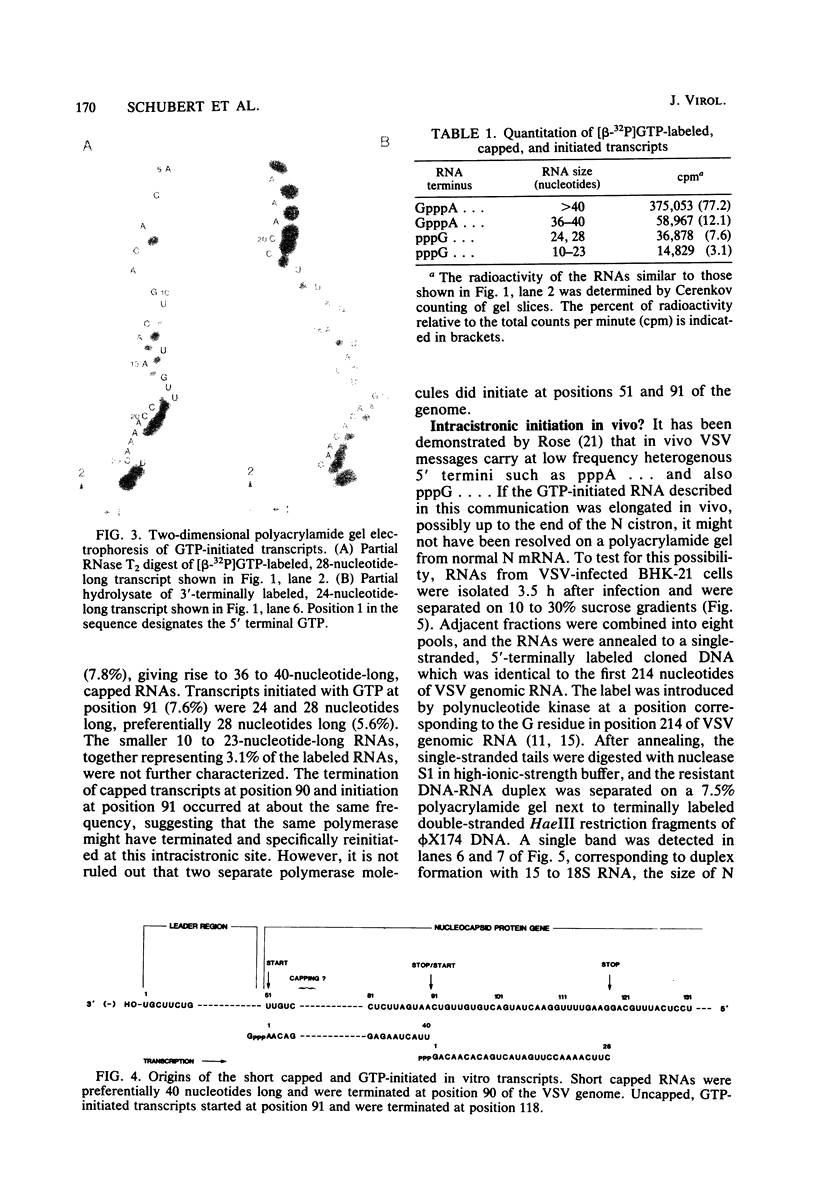
Abstract
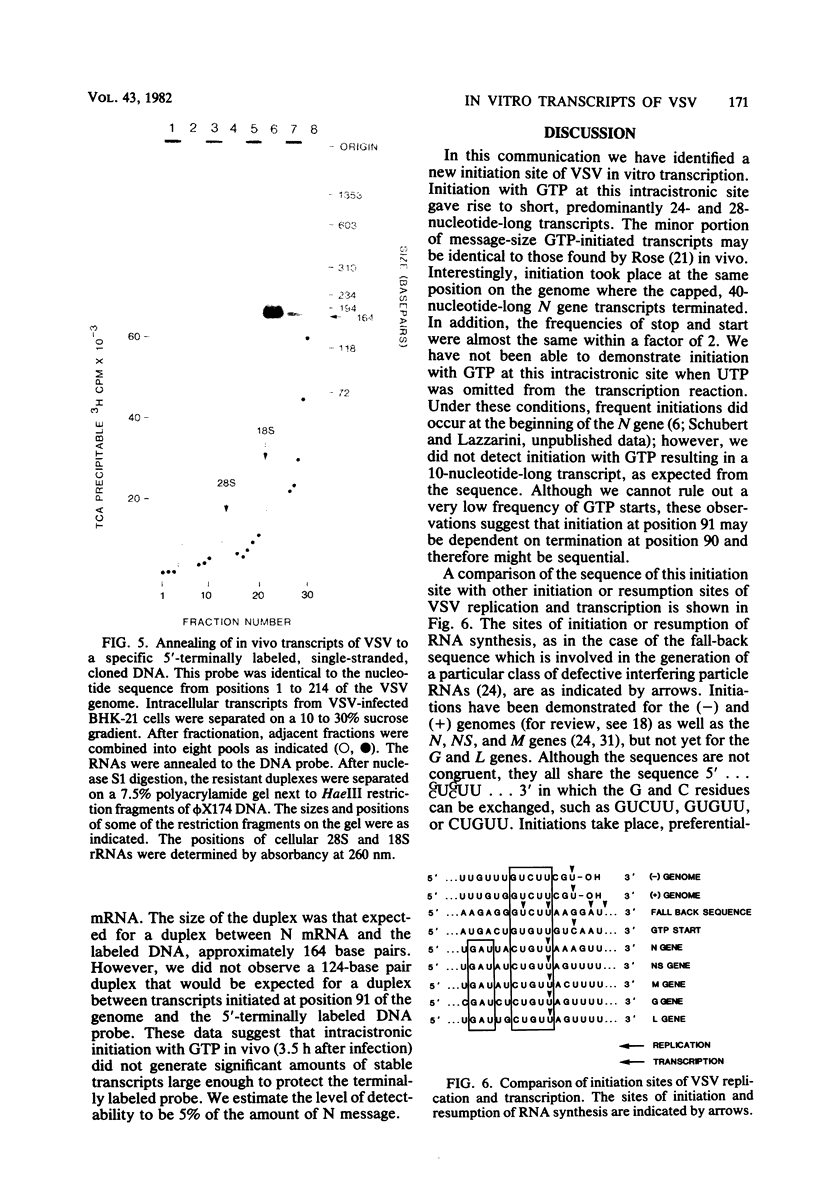
In vitro transcripts of vesicular stomatitis virus (VSV) were either 5′-terminally labeled by incorporation of [β-32P]GTP or were selected on Hg-agarose after incorporation of γ-thio-GTP. Capped RNAs ranged in size from 23 nucleotides, the shortest capped RNA detected, to full-length message size. The 5′-terminal sequences corresponded to those of N message and to a small amount of NS message. Approximately 14% of the capped N gene transcripts were terminated at positions 86 to 90 of the VSV genome, giving rise to specific, 36 to 40-nucleotide-long, capped RNA species. The GTP-initiated RNAs were short with a predominant 28-nucleotide-long RNA species. A minor portion was as large as mRNAs. Nucleotide sequence analyses of the short RNA revealed that it was specifically initiated at positon 91 of the VSV genome, 41 nucleotides within the N cistron. This corresponds exactly to the site where transcription of the 40-nucleotide-long, capped RNA terminated. Initiation with GTP at position 91 occurred at approximately the same frequency as termination of the capped RNA at position 90, suggesting that intracistronic initiation at position 91 may depend upon termination of transcription of the 5′-proximal region and therefore may be sequential. This unique RNA represents the first transcript of VSV which was initiated at an intracistronic site with GTP, and may also represent the first example of a transcript derived from a stop/start mechanism of VSV transcription in vitro. Although initiation occurred frequently at the beginning of the N cistron yielding 11 to 14-nucleotide-long, [β-32P]ATP-labeled transcripts (D. F. Pinney and S. U. Emerson, J. Virol. 42:889-896, 1982), capping of these short RNAs was not detected. This suggests that transcripts may have to be 15 to 23 nucleotides long to be accepted as substrates by the guanyltransferase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G., Banerjee A. K. Sequential transcription of the genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 May;73(5):1504–1508. doi: 10.1073/pnas.73.5.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham G., Rhodes D. P., Banerjee A. K. The 5' terminal structure of the methylated mRNA synthesized in vitro by vesicular stomatitis virus. Cell. 1975 May;5(1):51–58. doi: 10.1016/0092-8674(75)90091-4. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A. D., Abraham G., Colonno R. J. Vesicular stomatitis virus: mode of transcription. J Gen Virol. 1977 Jan;34(1):1–8. doi: 10.1099/0022-1317-34-1-1. [DOI] [PubMed] [Google Scholar]
- Carroll A. R., Wagner R. R. Adenosine-5'-O-(3-thiotriphosphate) as an affinity probe for studying leader RNA's transcribed by vesicular stomatitis virus. J Biol Chem. 1979 Oct 10;254(19):9339–9341. [PubMed] [Google Scholar]
- Chanda P. K., Banerjee A. K. Identification of promoter-proximal oligonucleotides and a unique dinucleotide, pppGpC, from in vitro transcription products of vesicular stomatitis virus. J Virol. 1981 Jul;39(1):93–103. doi: 10.1128/jvi.39.1.93-103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978 Sep;15(1):93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Gumport R. I., Uhlenbeck O. C. Dinucleoside pyrophosphate are substrates for T4-induced RNA ligase. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4839–4842. doi: 10.1073/pnas.74.11.4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Greene J. R., Iverson L. E., Rose J. K. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus N and NS proteins. J Virol. 1981 Aug;39(2):529–535. doi: 10.1128/jvi.39.2.529-535.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefti E., Bishop D. H. The 5' sequences of VSV in vitro transcription product RNA (+/-SAM). Biochem Biophys Res Commun. 1976 Jan 26;68(2):393–400. doi: 10.1016/0006-291x(76)91158-x. [DOI] [PubMed] [Google Scholar]
- Johnson L. D., Lazzarini R. A. The 5' terminal nucleotide of RNA from vesicular stomatitis virus defective interfering particles. Virology. 1977 Apr;77(2):863–866. doi: 10.1016/0042-6822(77)90508-6. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Choder M., Groner Y. Synthesis of carrier-free beta-32P-nucleosides-triphosphate in almost quantitative yields. Anal Biochem. 1980 Nov 15;109(1):198–202. doi: 10.1016/0003-2697(80)90029-9. [DOI] [PubMed] [Google Scholar]
- Keene J. D., Schubert M., Lazzarini R. A. Intervening sequence between the leader region and the nucleopcapsid gene of vesicular stomatitis virus RNA. J Virol. 1980 Feb;33(2):789–794. doi: 10.1128/jvi.33.2.789-794.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W., Crouch R. Degradation of DNA RNA hybrids by ribonuclease H and DNA polymerases of cellular and viral origin. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3360–3364. doi: 10.1073/pnas.69.11.3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini R. A., Chien I., Yang F., Keene J. D. The metabolic fate of independently initiated VSV mRNA transcripts. J Gen Virol. 1982 Feb;58(Pt 2):429–441. doi: 10.1099/0022-1317-58-2-429. [DOI] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. Identification and characterization of a group of discrete initiated oligonucleotides transcribed in vitro from the 3' terminus of the N-gene of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):889–896. doi: 10.1128/jvi.42.3.889-896.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinney D. F., Emerson S. U. In vitro synthesis of triphosphate-initiated N-gene mRNA oligonucleotides is regulated by the matrix protein of vesicular stomatitis virus. J Virol. 1982 Jun;42(3):897–904. doi: 10.1128/jvi.42.3.897-904.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes D. P., Banerjee A. K. 5'-terminal sequence of vesicular stomatitis virus mRNA's synthesized in vitro. J Virol. 1975 Jan;17(1):33–42. doi: 10.1128/jvi.17.1.33-42.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K. Complete intergenic and flanking gene sequences from the genome of vesicular stomatitis virus. Cell. 1980 Feb;19(2):415–421. doi: 10.1016/0092-8674(80)90515-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Complete sequences of the ribosome recognition sites in vesicular stomatitis virus mRNAs: recognition by the 40S and 80S complexes. Cell. 1978 Jun;14(2):345–353. doi: 10.1016/0092-8674(78)90120-4. [DOI] [PubMed] [Google Scholar]
- Rose J. K. Heterogneeous 5'-terminal structures occur on vesicular stomatitis virus mRNAs. J Biol Chem. 1975 Oct 25;250(20):8098–8104. [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A. A specific internal RNA polymerase recognition site of VSV RNA is involved in the generation of DI particles. Cell. 1979 Nov;18(3):749–757. doi: 10.1016/0092-8674(79)90128-4. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J. D., Lazzarini R. A., Emerson S. U. The complete sequence of a unique RNA species synthesized by a DI particle of VSV. Cell. 1978 Sep;15(1):103–112. doi: 10.1016/0092-8674(78)90086-7. [DOI] [PubMed] [Google Scholar]
- Schubert M., Lazzarini R. A. In vivo transcription of the 5'-terminal extracistronic region of vesicular stomatitis virus RNA. J Virol. 1981 Apr;38(1):256–262. doi: 10.1128/jvi.38.1.256-262.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert M., Lazzarini R. A. Structure and origin of a snapback defective interfering particle RNA of vesicular stomatitis virus. J Virol. 1981 Feb;37(2):661–672. doi: 10.1128/jvi.37.2.661-672.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Smith M. M., Reeve A. E., Huang R. C. Analysis of RNA initiated in isolated mouse myeloma nuclei using purine nucleoside 5'[gamma-S]triphosphates as affinity probes. Cell. 1978 Oct;15(2):615–626. doi: 10.1016/0092-8674(78)90030-2. [DOI] [PubMed] [Google Scholar]
- Testa D., Chanda P. K., Banerjee A. K. Unique mode of transcription in vitro by Vesicular stomatitis virus. Cell. 1980 Aug;21(1):267–275. doi: 10.1016/0092-8674(80)90134-8. [DOI] [PubMed] [Google Scholar]