Abstract
Objectives
To measure the efficacy of a program combining mental and physical practice with the efficacy of a program composed of only constraint-induced movement therapy (CIMT) or only mental practice on stroke patients’ levels of upper-extremity impairment and upper-extremity functional outcomes and to establish the relationship between changes in blood-oxygen–level dependent (BOLD) functional magnetic resonance imaging response during a specific motor or imagery task and improvement in motor function between intervention groups.
Design
Case series.
Setting
Licensed, 56-bed, freestanding, university-affiliated rehabilitation hospital.
Participants
Three men and 1 woman with moderate upper-limb hemiparesis after stroke were randomized.
Interventions
Two patients received mental practice and CIMT, 1 patient received only mental practice, and 1 received only CIMT.
Main Outcome Measures
Wolf Motor Function Test (WMFT), Motor Activity Log (MAL), Sirigu break test, Movement Imagery Questionnaire–Revised, and Vividness of Movement Imagery Questionnaire.
Results
The mental practice intervention alone led to slight improvement in certain functional and mental imagery measures (Sirigu, MAL, WMFT) but did not result in a clinically meaningful improvement with notable right cerebellar hemisphere activation that was not present before intervention. After CIMT, only the single patient showed clinically meaningful improvement of his affected hand as exhibited by decreased times on the MAL and WMFT. The patient showed increased bilateral cortical activation in both the motor and premotor areas during execution of a finger flexion and extension task. In contrast, during a second task, which was an imagined flexion and extension task, motor, occipital, and inferior parietal activation mainly in the contralateral hemisphere were observed. After 2 weeks of CIMT plus mental practice a patient with a lesion restricted to the parietal cortex showed little improvement in upper-extremity function and mental imagery in comparison with the patient with damage to nonparietal areas, who showed clinically meaningful improvement. The pattern of activation after 2 weeks of CIMT plus mental practice in the patient with nonparietal damage led to more focal contralateral activation in primary motor cortex when executing a voluntary flexion and extension task.
Conclusions
The case series indicates that for these patients with chronic, moderate upper-extremity impairment after stroke, a 2-week regimen of CIMT or CIMT plus mental practice only (in 1 case) resulted in modest changes occurring as a decrease in impairment, with functional improvement. Mental practice alone did not result in a clinically meaningful improvement in upper-limb impairment. We describe how these interventions may elicit “plastic” changes in the brain. Further investigations to determine the appropriate delivery and dosing of both physical and mental practice, as well as to determine whether mental practice–induced changes positively correlate with distinct patterns of cortical activation, should be undertaken before the efficacy of their use can be ascertained among patients with limitations comparable with these participants.
Keywords: Brain, Magnetic resonance imaging, Rehabilitation, Stroke
Stroke can cause substantial motor dysfunction that compromises ability to perform valued activities. This diminished ability is a primary reason why stroke is the leading cause of chronic disability in the United States. Presently, little evidence determines which patients with stroke should receive motor therapy, what elements those therapies should contain, or when they should be implemented. Moreover, despite a projected increase in stroke incidence, there continue to be few scientifically validated therapeutic techniques with evidence for improvement in motor function after stroke.1 Thus, identifying evidence-based interventions that can improve motor function remains a priority.
In recent years, mental practice with motor imagery, the cognitive rehearsal of physical movements, has emerged as a promising technique to improve motor skill performance (for reviews, see Braun2 and Sharma3 and colleagues). Beginning with studies performed on healthy volunteers, participants who trained mentally on a specific task have consistently displayed increased motor performance and motor skill acquisition compared with a no-practice condition.4 Recently, attempts to apply mental practice in a rehabilitation context have been made. For example, a 2001 study5 in subacute stroke patients (>3mo and <1y poststroke) compared the feasibility and efficacy of a program that combined mental practice and physical therapy (PT) with a program composed only of PT, showing that combining the 2 therapies was a clinically feasible, cost-effective complement to therapy and may improve functional outcomes more than participation in PT only. Other studies have similarly suggested that mental practice led to an increase in more affected arm use and function and thus, offers a potential means to promote motor recovery, even years after central nervous system damage.
PT and mental practice may be promising when combined, yet the neural changes that may accompany these programs remain unclear. A few studies using positron emission tomography (PET)6 and functional magnetic resonance imaging (fMRI)7–9 have shown cerebral blood flow changes during motor task performance during repetitive task practice regimens. However, the mechanisms underlying recovery of motor function after mental practice in patients with hemiparesis after stroke remain unknown. If these mechanisms were better understood, more rational decisions could be made regarding appropriate selection of specific treatment strategies, either in combination with mental practice or in isolation.
The primary aim of this study is to discuss the theoretical bases for mental practice in poststroke rehabilitation, that is, the similarities between executed and imagined movements. This article will also describe how mental practice may induce “plastic” changes in the brain and discuss techniques to measure this change in patients recovering from stroke. We will also introduce outcome measures that can be used to estimate clinical effectiveness of physical practice when combined with mental practice in patients with stroke.
Although no voluntary movements occur during mental practice, decades of evidence suggest that activations occur on several levels when mental practice is performed. For example, in 1931, Jacobsen10 showed that activations occur in the biceps brachii when volunteers were asked to visualize bending their right arms. More recent studies by Hale,11 Bakker et al,12 and Livesay and Samaras13 have replicated the finding using electromyography. They observed minute activations in the targeted musculature as if the activity were being physically performed. Moreover, not only are the appropriate muscles fired during mental practice, but electromyographic amplitude during mental practice is proportional to that observed during actual task performance.12 Recent study results show that vegetative responses (eg, heart rate, oxygen consumption) during mental practice covary with the degree of imagined effort on a particular task. In other words, during mental practice, not only are the appropriate muscle patterns and neural events exhibited as if the activity was actually being performed, but these activations are also exhibited in the appropriate proportions (ie, a more strenuous imagined task elicits greater physiologic reactions than a less strenuous task). Chronometry studies have also been performed in healthy subjects, in which the time taken to physically perform a given task is compared with the time taken to mentally execute the same task.12,14 These studies consistently show that the time taken to physically perform a given task is nearly identical to the time taken to mentally rehearse the same task.
Because imagined actions elicit the same vegetative responses as physical actions, and the time needed to mentally execute actions is nearly identical to the time needed to physically perform them, it has been suggested that the same neural substrates are activated during mental practice as during physical practice of the same task. In the next section, we review data supporting this hypothesis.
Neural Substrates of Mental Practice
There is strong evidence that mental practice can modify motor performance,15 and many studies have sought to delineate the underlying mechanisms and cortical correlates of mental practice. The possibility that the neuronal network involved in movement execution may also be active during mental practice raises a number of issues addressing the origin of this activation. Many cognitive neuroscientists believe that the central nervous system may form a template of movements without actually activating the appropriate motor plan, sharing partly overlapping networks for motor preparation and execution.16,17 Alternatively, mental rehearsal of a particular motor skill may partially activate the descending corticospinal pathway, the spinal machinery, and effector muscles.18,19 In line with this hypothesis are the observations that spinal circuits are activated by transcranial magnetic stimulation in a similar manner during motor imagery and motor execution.20–27 This finding, however, is challenged by other studies, showing modulation of the motor cortical excitability without evoking descending volleys to the spinal cord.28–31 Others believe that cortical activation observed during mental practice may be caused by plastic changes in cortical excitability induced by the absence of somatosensory input, particularly kinesthetic feedback in covert movements. Indeed, recent results32 have revealed an increase of motor cortical excitability after experimental deafferentation, thus confirming earlier findings.33 Last, the primary motor cortex activation observed during mental practice as compared with motor execution may be explained by the cortico-cortical inhibition required to prevent activation of the peripheral motor apparatus during mental practice.34 It appears clear that mental practice does have a neural correlate that controls at least some aspects of movement execution. Later, we discuss the neural responses that we have observed during mental practice in patients with stroke.
Although task-specific, repetitive regimens of PT and mental practice may be promising, we do not clearly comprehend the changes that may occur in the brains of patients who have sustained stroke after any physical therapeutic program. A few studies using imaging methods, such as PET and fMRI, have shown cerebral blood flow changes during motor task performance after constraint-induced movement therapy (CIMT),6–9 as well as other task-specific training protocols.35,36 Several lines of evidence developed in animal models suggest that experience influences change in the motor cortex after stroke and that anatomic enlargement of cortical motor maps may be correlated with improved behavioral or functional recovery.37,38 If the mechanisms underlying recovery of motor function after stroke were better understood, more rational decisions could be made regarding appropriate selection of specific treatment strategies.
Much of the recent knowledge about the plasticity of cortical neural networks stems from the work of Merzenich et al39 in the early and mid-1980s. Using microelectrodes to record neuronal activity in primate somatosensory cortex, sensory representational maps of the palm and fingers were shown to undergo substantial reorganization after peripheral nerve injury40,41 and after repetitive differential sensory stimulation of restricted skin surfaces.40,41 Recent work by Nudo et al38,42 using microelectrodes in monkeys have shown changes in motor maps of the upper extremity after focal cortical lesions. Of potential clinical importance, motor maps in the vicinity of the lesion shrink after inactivity but often expand after physical activity of the limb affected by the lesion. After such reorganization, electric stimulation of cortex adjacent to the lesion results in the motor activity previously associated with stimulation of the damaged area.43 The use of fMRI in the future studies will provide access to the elements contributing to massed practice (either mental or physical) cortical reorganization.
Brain Activations After Mental Practice in Stroke
It is generally held that mental practice with motor imagery is the internal simulation of movements involving one’s own body in the absence of overt execution. Consistent with this hypothesis, results from numerous functional imaging studies indicate that mental practice activates a large variety of motor-related brain regions in both the upper and lower limbs.44–47 There is evidence from fMRI studies that mental practice involves virtually all stages of motor control and that there are no significant differences between the 2 hands with either execution or imagination of executed movements.48,49 In healthy people activation is consistently seen in supplementary motor area (SMA), the premotor cortex, and the primary motor cortex (M1) during both executed and imagined movement.50 Recent study has shown that hand preparation for covert movement simulation activates a large network of motor-related areas located primarily within the left cerebral and right cerebellar hemispheres. By contrast, imagined grip selection activates a distinct parietofrontal circuit that includes the bilateral dorsal premotor cortex, contralateral intraparietal sulcus, and right superior parietal lobule. Because these areas are highly consistent with the frontoparietal reach circuit identified in monkeys, it has been suggested that motor imagery involves action-specific motor representations computed in parietofrontal circuits.48
As the paretic hand regains function in patients with post-stroke hemiparesis, a higher ratio of contralateral to ipsilateral (stroke-affected hemisphere to unaffected hemisphere) activity is seen in the M1 during a movement task. fMRI51–57 and PET58–60 studies in patients who have sustained stroke have generally shown a profound ipsilateral activation of prefrontal cortex, SMA, and cingulate cortex. Few functional imaging studies, however, have investigated the effects of physical rehabilitation interventions,61 specifically mental practice of the upper extremity, on cortical reorganization after stroke.
It has also been shown that mental practice with motor imagery of a novel foot-sequence task can improve through training, thereby improving the efficiency with which the known sequence of movements is associated with a specific and repeated pattern of motor responses.45 These data revealed significant activations in the right medial orbitofrontal cortex in both sequence execution and sequence imagination conditions suggesting that learning had taken place in the frontal pole during the sequence imagination and the random imagination conditions.
The cortical changes observed after undergoing task-specific, repetitive physical practice coupled with mental practice with motor imagery could be similar or even greater to those seen following other protocols, in turn leading to greater amount of cortical reorganization and improved functional outcome. Data being gathered from the authors’ ongoing investigations will permit us to affirm the notion that fMRI can be used to elucidate mechanisms of movement recovery while using these interventions through defining cortical substrates that contribute to recapturing movement control. If investigators see consistency in cortical structures that become engaged with mental practice when used as a therapeutic approach, future investigators will be positioned to develop specific hypotheses about how these therapies work in the context of a clinical trial.
Several studies have shown that a regimen combining mental practice with physical practice of the same skills produce improved function in the more affected arm.
In a recent pilot study,62 8 chronic stroke patients with right-arm hemiparesis were enrolled in a 4-week program combining mental practice and occupational therapy (OT) (group 1), while 8 other patients received only exposure to stroke information and OT (group 2). At the pretesting period, mean scores of group 1 and group 2 on the upper-extremity section of the Fugl-Meyer Assessment (FMA)63 were nearly identical (22.13 and 22.23, respectively). However, after treatment, scores indicated that patients in group 1 exhibited significantly greater reductions in upper-extremity impairment than patients in group 2 (F1,14=14.71, P<.05).
In a follow-up, randomized controlled trial (S.J. Page et al., unpublished data, 2006), the same trend was shown with a larger sample of chronic stroke patients (N=40). Using a multiple baseline design, all subjects were administered the FMA and the Action Research Arm Test (ARAT), a stroke-specific test of fine motor function, on 2 occasions. Patients were then randomly assigned to a group combining mental practice with a traditional arm therapy regimen (n=20) or a regimen of traditional arm therapy only (n=20). Whereas all patients showed stable hemiparesis, and both groups showed equal baseline FMA and ARAT scores, the group receiving mental practice showed significant increases on the FMA and ARAT after intervention. Functionally patients receiving mental practice were able to perform activities of daily living (ADLs) that they had not performed in years, such as writing, using a computer keyboard, and feeding themselves with the more affected hand. Kinematics on randomly selected patients performing functional reaching tasks showed significant increases in linear hand velocity and ability to reach up and reach out64 with their more affected hand.
Page et al65 also compared the arm impairment level and functional outcomes of a patient with a subacute stroke who participated in a regimen of mental practice combined with motor therapy versus those of a patient receiving only arm therapy. Patient A received motor therapy for the affected side 3 times a week for 6 weeks. In addition, 2 times a week, the patient listened to a 10-minute audiotape containing stroke information. Patient B was an age-matched man who received identical amounts and types of therapy for the affected side from the same therapist as patient A. However, 2 times a week, patient B listened to an audiotape during therapy and at home in which he imagined himself using the affected limb. Pre- and postintervention measures used to assess clinical function were the upper-extremity scale of the FMA and ARAT. After intervention, patient B, who received mental practice, exhibited a higher score on the FMA and considerable improvements in fine motor skill, as measured by the ARAT, whereas patient A’s scores remained unchanged.
Expanding the work with patients in the subacute phase after stroke, Page et al5 conducted a randomized pilot study examining mental practice efficacy in reducing impairment and improving functional outcomes. Upper-extremity impairment, as measured by the upper-extremity component of the FMA, remained unchanged for all subjects during pretesting, suggesting a stable motor deficit. Specifically, patients randomized to the control group averaged 35.00 on the FMA after the first pretesting session (pre-1) and 37.00 on the FMA during the second pretesting session (pre-2). Patients randomized to the experimental group scored averages of 19.4 and 19.75 during pre-1 and pre-2, respectively, which were conducted 1 week apart. Postintervention average scores in the control group remained stable at 37.00. However, experimental group subjects exhibited considerable improvement, scoring a mean of 35.5. Functionally, patients receiving mental practice were able to perform ADLs that they had not performed since before their strokes.
Together, the above data suggest that mental practice reduces impairment and increases kinematics, use, and function in the more affected limb. However, although physical practice combined with mental training may be a promising treatment, we do not clearly comprehend the changes that may occur in the brains of patients who have sustained stroke after any physical therapeutic program. As mentioned earlier, a few studies using PET and fMRI have shown cerebral blood flow changes during motor task performance after task-specific, repetitive task practice. If the mechanisms underlying recovery of motor function after stroke were better understood, more rational decisions could be made regarding appropriate selection of specific treatment strategies.
Combining Mental Practice With CIMT
Our lab recently conducted a case series in which 4 patients were subjected to 3 therapy protocols. We compared the efficacy of a program combining mental practice and PT (CIMT) with the efficacy of a program composed solely of CIMT, and a program composed solely of mental practice, on patients’ levels of upper-extremity impairment and upper-extremity functional outcomes. This case series also evaluated the effects of these different training paradigms on cortical reorganization after stroke using fMRI.
METHODS
We randomly assigned 4 stroke patients into groups: 2 patients received mental practice and PT (mental practice + CIMT), 1 patient received only mental practice and 1 received only PT (CIMT). The main outcome measures were the Wolf Motor Function Test (WMFT), Motor Activity Log (MAL), Sirigu break test, Movement Imagery Questionnaire–Revised (MIQ-R), and Vividness of Movement Imagery Questionnaire (VMIQ). The WMFT is a validated test that measures time (15 tasks) or strength (2 tasks) in completing upper-extremity joint specific or multiple joint movements or functions.66 The MAL is an upper-extremity disability measure (self-report interview). The MAL67,68 is a semi-structured interview during which participants are asked to rate how much and how well (6-point scale; range, 0 [worst] to 5 [best]) they use their more affected arm for 30 ADL items in the home environment over a specified period. Mental imagery was assessed using 3 measures. The primary mental imagery outcome measures was the revised MIQ-R69 and VMIQ.70,71 The secondary outcome measure of mental imagery was the Sirigu break test.72,73 Only pre- and immediate post-intervention measures were recorded, with no long-term follow-up.
RESULTS
Motor Recovery After CIMT Only
A 73-year-old, right-handed man, who suffered a stroke in the left basal ganglia and internal capsule 4 months previously, was randomized to the CIMT-only treatment intervention (CIMT progressing to 3h/d). He had received conventional treatment at an acute rehabilitation unit and was discharged to his home. His mean pre- and post-treatment WMFT score did not change for the less impaired arm. For the more affected arm the WMFT scores were 12.4 seconds for mean pretreatment and 6.7 seconds for mean posttreatment (where lower numbers indicate less time to complete tasks). All 17 items in the WMFT, including key turning and maximum grip strength, showed marked improvement. MAL scores also increased from 0.6 and 1.1 to 3.1 and 3.0 on the amount of use and how well scores, respectively (where higher numbers indicate more and better use of the affected arm). Imagery outcome measures were not recorded for this person. The preliminary clinical data suggest that the CIMT intervention favorably impacted upper-extremity functional behavior. However, these changes may be amplified further by combining CIMT with mental training.
Cortical Change Associated With CIMT Only
After 2 weeks of CIMT only, our patient showed increased bilateral cortical activation in both the motor and premotor areas during execution of a finger flexion and extension task paced at 1Hz (as monitored by a fiberoptic position sensor). Activation of these areas are similar to those found in previous studies44,47 and may be due to the recruitment of the healthy (left) hemisphere to complete the task57 (fig 1B). (Note, we observed no mirror movement of the right hand during these tasks.) Interestingly, in this patient who received CIMT alone, we observed motor, occipital, and inferior parietal activation mainly in the contralateral hemisphere during a second task, which was an imagined flexion and extension task paced at 1Hz (fig 1D). This contralateral activation may be due to the lack of mental imagery training and the use of prestroke motor pathways.
Fig 1.
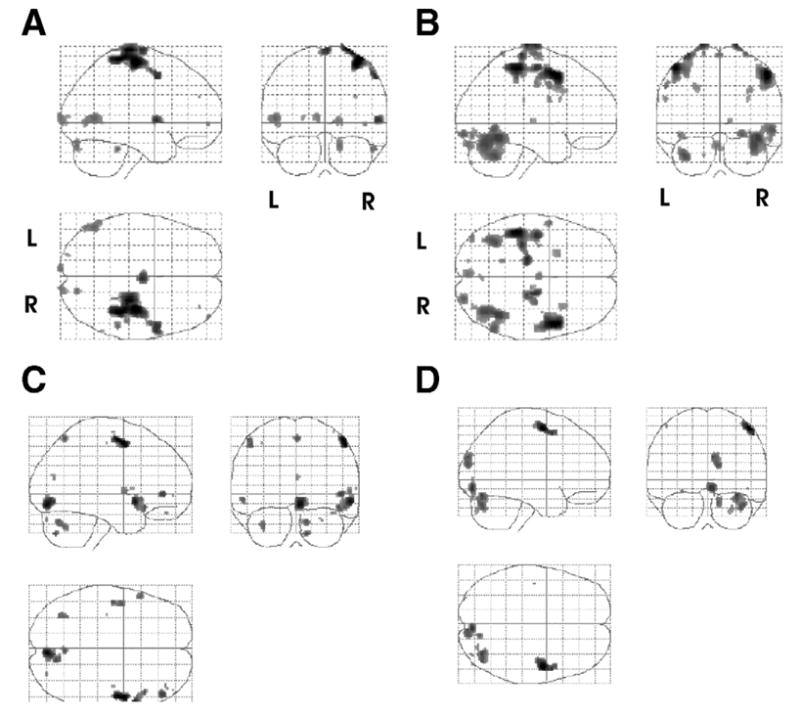
Brain activity associated with CIMT only. Activation maps are shown as a through-projection onto a lateral, sagittal, and horizontal representation of standard stereotactic space. Images reflecting the activations in 4 subtractions. The top row of images depicts the sites of activation by subtracting the rest condition from the actual movement of the left hand condition (A) pretreatment (move affected > rest) and (B) posttreatment (move affected > rest). Note (B) increased bilateral cortical activation following treatment. The second row depicts the sites from the subtraction of the rest from imagine moving the left hand condition both (C) pretreatment (imagine move affected > rest) and (D) posttreatment (imagine move affected > rest). Shown are all activations that passed a criterion of P <.05 corrected for multiple comparisons with an extent threshold of 0. Abbreviations: L, left; R, right.
Motor Recovery After Mental Practice Only
This 60-year-old, right-handed man, who suffered bilateral subcortical lacunar infarcts 3 months previously, was randomized to the mental practice– only intervention, which was of the same duration as CIMT intervention described above (ie, 3h/d for 2 consecutive weeks). He presented with right upper-extremity weakness. After a 3-week intervention of mental practice only, this patient did not show improvements in the affected hand, as expressed by decreased FMA, but showed slight improvements on the MAL and WMFT (table 1). He showed no improvement in ability to mentally image as exhibited by a decreased self-perception of imaging abilities on the VMIQ and MIQ-R. He did not show improvement in the ability to imagine hand movement (decreased Sirigu times) but did execute movements with the fingers of his affected hand faster after 2 weeks of mental practice (see table 1). The mental practice intervention alone did lead to slight improvement in certain functional and mental imagery measures (Sirigu, MAL, WMFT) but did not result in a clinically meaningful improvement.
Table 1.
Pre- and Immediately Postintervention Clinical and Imagery Scores
| Mental Practice Plus CIMT
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Mental Practice
|
CIMT
|
Patient 1
|
Patient 2
|
|||||
| Groups | Pre | Post | Pre | Post | Pre | Post | Pre | Post |
| VMIQ (avg) | 1.00 | 1.88 | * | * | 3.10 | 1.90 | 2.20 | 2.50 |
| MIQ-R (avg) | 7.00 | 4.88 | * | * | 4.80 | 5.80 | 6.00 | 5.10 |
| Sirigu time of affected hand | ||||||||
| Imagery | 7.10 | 9.97 | * | * | 26.30 | 9.90 | 4.52 | 4.04 |
| Movement (s) | 8.18 | 7.44 | * | * | 5.00 | 4.30 | 4.99 | 4.34 |
| MAL | ||||||||
| Amount | 1.27 | 1.95 | 0.60 | 3.10 | 2.50 | 3.50 | 1.20 | 3.50 |
| How well | 1.02 | 2.77 | 1.10 | 3.00 | 3.00 | 2.50 | 1.97 | 3.05 |
| WMFT of affected (s) | 2.13 | 2.03 | 12.40 | 6.70 | 4.70 | 2.90 | 37.40 | 43.60 |
| FMA | ||||||||
| ROM | 17 | 12 | 20 | 23 | 20 | 18 | 21 | 23 |
| MF | 45 | 37 | 40 | 45 | 21 | 37 | 43 | 44 |
Abbreviations: MF, motor function; ROM, range of motion.
Time points at which no data were collected.
Brain Change Associated With Mental Practice Only
After 2 weeks of mental practice only, the patient showed increased contralateral cortical activation in motor areas during execution of the flexion and extension task (see fig 2B). This activation may be due to the patient’s lack of physical intervention, causing the brain to recruit the same motor pathways used before the stroke. However, bilateral cortical activation of inferior and middle temporal gyrus was observed when the patient was asked to imagine the same flexion and extension task (compare figs 2C, 2D). The most notable activation after mental practice was in the right cerebellar hemisphere that was not activated in the first scan during the imagery task. Ipsilateral activation in the anterior lobe of the cerebellum during an executed task in able-bodied participants reflects the organization of the spinocerebellar pathways.47 Furthermore, evidence to support our data comes from a study in which hemiparetic patients with good recovery showed changes in the activation of the cerebellar hemisphere opposite the injured corticospinal tract, whereas patients with poor recovery did not show such changes in cerebellar activation.74 The appearance of cerebellar activation after mental practice may reflect unmasking of pre-existing connections75 or mechanisms of long-term potentiation, because certain forms of learning lead to an enhancement of synaptic responses in a variety of brain structures.76–78 Recently, long-term potentiation has been shown to be involved in learning new motor skills79 and provides compelling evidence for long-term potentiation to be a mechanism involved in natural learning.
Fig 2.

Brain activity associated with mental practice only. Images reflecting the activations in 4 subtractions. The top row of images depicts the sites of activation by subtracting the rest condition from the actual movement of the right hand condition (A) pretreatment (move affected > rest) and (B) posttreatment (move affected > rest). The second row depicts the sites from the subtraction of the rest from imagine moving the right hand condition both (C) pretreatment (imagine move affected > rest) and (D) post-treatment (imagine move affected > rest). Note (B) increased contralateral cortical activation and (D) increased bilateral cortical activation postintervention. Shown are all activations that passed a criterion of P <.001 uncorrected for multiple comparisons with an extent threshold of 0.
It is known that the cerebellar cortex organizes its internal computations so as to regulate the many recurrent pathways in premotor networks. The cerebellum may have transferred some of its motor program knowledge to the premotor network as a result of the intervention. Although immediate functional change was not observed, plasticity may be occurring in the inferior olive, premotor networks, basal ganglia, and cerebral cortex. This activity might interact with the cerebellar cortex to create an advantageous environment for overall motor learning if long-term follow-up had occurred in this patient.80
Motor Recovery After CIMT Plus Mental Practice
We applied a CIMT and mental practice training protocol (dose matched with the other 2 interventions) to determine the feasibility of using mental practice in conjunction with CIMT to improve upper-extremity performance in patients with stroke. As before, the primary outcome measures to assess motor performance were the WMFT and the MAL. A 67-year-old, left-handed man, who suffered a stroke in the left frontoparietal area 14 months previously, was enrolled in the study. At enrollment, he had minimal movement in his wrist and finger extensors and had normal sensation. For this person who completed the mental practice and CIMT, the mean and median WMFT scores remained consistently low for the less impaired arm. For the more affected arm he improved from being able to perform 6 tasks pretreatment to 9 tasks posttreatment (mental practice and CIMT patient 1) (table 1). Considering only the 6 tasks performed during both sessions, WMFT scores were 4.7 seconds for mean pretreatment and 2.9 seconds for mean posttreatment. Mean MAL scores increased from 2.5 to 3.5 on the amount of use score and 3.0 to 2.5 on the how well score. The VMIQ watching somebody else scores were 3.1 for mean pretreatment and 1.9 for mean posttreatment (where lower numbers indicate a more vivid image). The MIQ-R scores improved from a mean pretreatment of 4.8 to a mean posttreatment of 5.8 (where larger numbers indicate ease of imaging). Sirigu’s break test scores decreased for both the imagery and movement conditions with the impaired upper extremity. Mean scores were 26.3 seconds for pretreatment and 9.9 seconds for posttreatment during imagery, and 5.0 seconds for pretreatment and 4.3 seconds for posttreatment when executing the movement (where lower numbers indicate less time to complete the task). These preliminary data suggest that our 2-week CIMT plus mental practice training procedure can favorably impact upper-extremity functional behavior and ability to mentally imagine finger movement.
The second volunteer was a 51-year-old, right-handed woman, who suffered a large hemorrhagic stroke in the left parietal area 16 months previously. As depicted in table 1 (mental practice + CIMT patient 2) this person did not improve her performance scores on WMFT, VMIQ, or MIQ. Although a slight improvement was seen on the MAL scores, no significant change was seen on the FMA score or Sirigu test. The inability to improve on mental imagery scores are in concordance with the idea that the parietal cortex is important for the ability to generate mental movement representations.81 After 2 weeks of CIMT plus mental practice our patient with a lesion restricted to the parietal cortex showed little improvement in upper-extremity function and mental imagery in comparison with patients with damage to nonparietal areas.
Cortical Changes Associated CIMT Plus Mental Practice
The pattern of activation after 2 weeks of CIMT plus mental practice in 1 person led to more focal contralateral activation in M1 when executing the flexion and extension task (fig 3B). Ipsilateral activation in occipital, temporal-occipital, and temporal areas were noticeably concentrated while the patient imagined the flexion and extension task (fig 3D). This pattern of reduced or focused activation is consistent with Ward et al56 who showed a decrease in activation across sessions correlating with recovery in primary motor cortex, premotor and prefrontal cortex, SMAs, cingulate sulcus, temporal lobe, striate cortex, cerebellum, thalamus, and basal ganglia. Furthermore, these activations, part of the ventral processing stream, were probably related to the patients’ “visual imagery” of the task and may be due to the shifting cortical substrates to the healthy, ipsilateral hemisphere.
Fig 3.
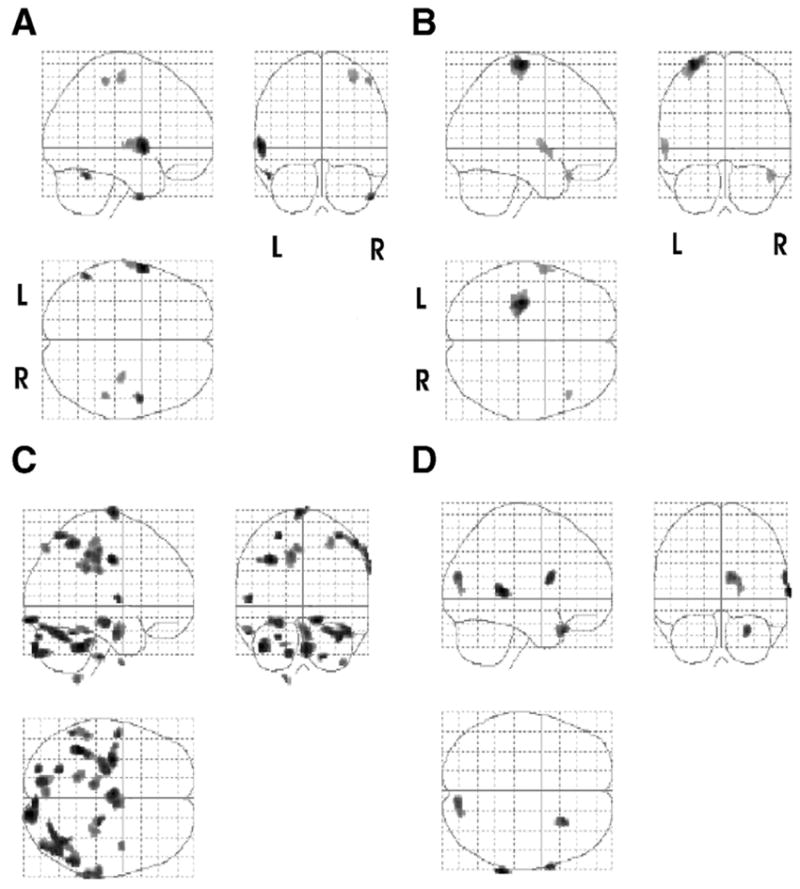
Cortical changes associated CIMT plus mental practice. Images reflecting the activations in 4 subtractions in patient 2. The top row of images depicts the sites of activation by subtracting the rest condition from the actual movement of the affected (right) hand condition (A) pretreatment (move affected > rest) and (B) posttreatment (move affected > rest). The second row depicts the sites from the subtraction of the rest from imagine moving the right hand condition both (C) pretreatment (imagine move affected > rest) and (D) posttreatment (imagine move affected > rest). Note (D) increased ipsilateral cortical activation. Shown are all activations that passed a criterion of P <.05 corrected for multiple comparisons with an extent threshold of 0.
DISCUSSION
The above data suggest that a stroke patient participating in a 2-week regimen of CIMT with mental practice can improve motor function of the affected upper extremity that is associated with massed practice cortical reorganization of the damaged hemisphere during affected hand movement, as shown by fMRI. These data also suggest that a stroke patient can improve the ability to mentally imagine finger movements that take advantage of the previously idle ventral visual processing stream to imagine finger movements. Whereas motor and cortical changes were marked and functional for subjects receiving the combined intervention, and the CIMT-only intervention, the patient receiving mental practice made only slight improvement in certain functional and mental imagery measures that were not clinically meaningful. However, other patient-specific factors not considered in these patients may have ultimately been coupled to the clinical outcomes. Factors such as a person’s ability to imagine, residual sensorimotor capabilities, ability to follow commands, attention, and motivation may be crucial components not considered in these patients.
CONCLUSIONS
Mental practice with motor imagery has emerged as a promising technique to improve motor skill performance. Our preliminary studies with stroke patients using mental practice in conjunction with PT suggest that these combined modes of therapy lead to an improvement in motor performance and change in cortical activation. However, we do not know whether this type of combined therapy yields greater improvements in motor performance than PT alone. Furthermore, our data suggest that these motor changes appear to be associated with increased activation of motor cortices of the undamaged hemisphere (in 1 case) and cortical plasticity of damaged hemisphere (in the other case) during affected hand movement. However, these ideas warrant further exploration as a necessary precursor to gain insights into mechanisms contributing to improved function. Future studies should determine the appropriate delivery, and dosing, of both physical and mental practice, as well as measuring whether mental practice-induced changes positively correlate with distinct patterns of cortical activation as measured using neuroimaging.
Acknowledgments
We thank Veronica Rowe and Vanessa Cavalheiro for the training they provided. We also thank Hui Mao, Chunchun Ni, and Jean Ko for their help with fMRI data acquisition and reduction.
Footnotes
Supported by the National Institutes of Health (grant nos. R21AT002138, R21AT02110-01A1, K01AT02637) and the Retirement Research Foundation (grant no. 2001-037).
No commercial party having a direct financial interest in the results of the research supporting this article has or will confer a benefit upon the author(s) or upon any organization with which the author(s) is/are associated.
References
- 1.Barreca S, Wolf SL, Fasoli S, Bohannon R. Treatment interventions for the paretic upper limb of stroke survivors: a critical review. Neurorehabil Neural Repair. 2003;17:220–6. doi: 10.1177/0888439003259415. [DOI] [PubMed] [Google Scholar]
- 2.Braun SM, Beurskens AJ, Borm PJ, Schack T, Wade DT. The effects of mental practice in stroke rehabilitation: a systematic review. Arch Phys Med Rehabil. 2006;87:842–52. doi: 10.1016/j.apmr.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 3.Sharma N, Pomeroy VM, Baron JC. Motor imagery: a backdoor to the motor system after stroke? Stroke. 2006;37:1941–52. doi: 10.1161/01.STR.0000226902.43357.fc. [DOI] [PubMed] [Google Scholar]
- 4.Feltz D, Landers D. The effects of mental practice on motor skill learning and performance: an article. J Sport Psychol. 1983;5:25–57. [Google Scholar]
- 5.Page SJ, Levine P, Sisto S, Johnston MV. A randomized efficacy and feasibility study of imagery in acute stroke. Clin Rehabil. 2001;15:233–40. doi: 10.1191/026921501672063235. [DOI] [PubMed] [Google Scholar]
- 6.Wittenberg GF, Chen R, Ishii K, et al. Constraint-induced therapy in stroke: magnetic-stimulation motor maps and cerebral activation. Neurorehabil Neural Repair. 2003;17:48–57. doi: 10.1177/0888439002250456. [DOI] [PubMed] [Google Scholar]
- 7.Schaechter JD, Kraft E, Hilliard TS, et al. Motor recovery and cortical reorganization after constraint-induced movement therapy in stroke patients: a preliminary study. Neurorehabil Neural Repair. 2002;16:326–38. doi: 10.1177/154596830201600403. [DOI] [PubMed] [Google Scholar]
- 8.Johansen-Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM. Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain. 2002;125:2731–42. doi: 10.1093/brain/awf282. Pt 12. [DOI] [PubMed] [Google Scholar]
- 9.Hamzei F, Liepert J, Dettmers C, Weiller C, Rijntjes M. Two different reorganization patterns after rehabilitative therapy: an exploratory study with fMRI and TMS. Neuroimage. 2006;31:710–20. doi: 10.1016/j.neuroimage.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 10.Jacobsen E. Electrical measurement of neuromuscular states during mental activities: VI. A note on mental activities concerning an amputated limb. Am J Physiol. 1931;43:122–5. [Google Scholar]
- 11.Hale B. The effects of internal and external imagery on muscular and ocular concomitants. J Sport Psychol. 1982;4:379–87. [Google Scholar]
- 12.Bakker F, Boschker M, Chung J. Changes in muscular activity while imagining weight lifting using stimulus or response propositions. J Sport Exerc Psychol. 1996;18:313–24. [Google Scholar]
- 13.Livesay JR, Samaras MR. Covert neuromuscular activity of the dominant forearm during visualization of a motor task. Percept Mot Skills. 1998;86:371–4. doi: 10.2466/pms.1998.86.2.371. [DOI] [PubMed] [Google Scholar]
- 14.Decety J. Do imagined and executed actions share the same neural substrate? Cogn Brain Res. 1996;3:87–93. doi: 10.1016/0926-6410(95)00033-x. [DOI] [PubMed] [Google Scholar]
- 15.Gandevia SC. Mind, muscles and motoneurones. J Sci Med Sport. 1999;2:167–80. doi: 10.1016/s1440-2440(99)80171-6. [DOI] [PubMed] [Google Scholar]
- 16.Jeannerod M, Frak V. Mental imaging of motor activity in humans. Curr Opin Neurobiol. 1999;9:735–9. doi: 10.1016/s0959-4388(99)00038-0. [DOI] [PubMed] [Google Scholar]
- 17.Fadiga L, Buccino G, Craighero L, Fogassi L, Gallese V, Pavesi G. Corticospinal excitability is specifically modulated by motor imagery: a magnetic stimulation study. Neuropsychologia. 1999;37:147–58. doi: 10.1016/s0028-3932(98)00089-x. [DOI] [PubMed] [Google Scholar]
- 18.Gandevia SC, Macefield VG, Bigland-Ritchie B, Gorman RB, Burke D. Motoneuronal output and gradation of effort in attempts to contract acutely paralysed leg muscles in man. J Physiol. 1993;471:411–27. doi: 10.1113/jphysiol.1993.sp019907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gandevia SC, Wilson LR, Inglis JT, Burke D. Mental rehearsal of motor tasks recruits alpha-motoneurones but fails to recruit human fusimotor neurones selectively. J Physiol. 1997;505:259–66. doi: 10.1111/j.1469-7793.1997.259bc.x. Pt 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonnet M, Decety J, Jeannerod M, Requin J. Mental simulation of an action modulates the excitability of spinal reflex pathways in man. Brain Res Cogn Brain Res. 1997;5:221–8. doi: 10.1016/s0926-6410(96)00072-9. [DOI] [PubMed] [Google Scholar]
- 21.Kiers L, Fernando B, Tomkins D. Facilitatory effect of thinking about movement on magnetic motor-evoked potentials. Electroencephalogr Clin Neurophysiol. 1997;105:262–8. doi: 10.1016/s0921-884x(97)00027-1. [DOI] [PubMed] [Google Scholar]
- 22.Rossini PM, Rossi S, Pasqualetti P, Tecchio F. Corticospinal excitability modulation to hand muscles during movement imagery. Cereb Cortex. 1999;9:161–7. doi: 10.1093/cercor/9.2.161. [DOI] [PubMed] [Google Scholar]
- 23.Facchini S, Muellbacher W, Battaglia F, Boroojerdi B, Hallett M. Focal enhancement of motor cortex excitability during motor imagery: a transcranial magnetic stimulation study. Acta Neurol Scand. 2002;105:146–51. doi: 10.1034/j.1600-0404.2002.1o004.x. [DOI] [PubMed] [Google Scholar]
- 24.Pascual-Leone A, Nguyet D, Cohen LG, Brasil-Neto JP, Cammarota A, Hallett M. Modulation of muscle responses evoked by transcranial magnetic stimulation during the acquisition of new fine motor skills. J Neurophysiol. 1995;74:1037–45. doi: 10.1152/jn.1995.74.3.1037. [DOI] [PubMed] [Google Scholar]
- 25.Pelgrims B, Andres M, Olivier E. Motor imagery while judging object-hand interactions. Neuroreport. 2005;16:1193–6. doi: 10.1097/00001756-200508010-00012. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S, Pasqualetti P, Tecchio F, Pauri F, Rossini PM. Corticospinal excitability modulation during mental simulation of wrist movements in human subjects. Neurosci Lett. 1998;243:147–51. doi: 10.1016/s0304-3940(98)00088-3. [DOI] [PubMed] [Google Scholar]
- 27.Stinear CM, Byblow WD. Motor imagery of phasic thumb abduction temporally and spatially modulates corticospinal excitability. Clin Neurophysiol. 2003;114:909–14. doi: 10.1016/s1388-2457(02)00373-5. [DOI] [PubMed] [Google Scholar]
- 28.Kasai T, Kawai S, Kawanishi M, Yahagi S. Evidence for facilitation of motor evoked potentials (MEPs) induced by motor imagery. Brain Res. 1997;744:147–50. doi: 10.1016/s0006-8993(96)01101-8. [DOI] [PubMed] [Google Scholar]
- 29.Yahagi S, Kasai T. Facilitation of motor evoked potentials (MEPs) in first dorsal interosseous (FDI) muscle is dependent on different motor images. Electroencephalogr Clin Neurophysiol. 1998;109:409–17. doi: 10.1016/s0924-980x(98)00041-1. [DOI] [PubMed] [Google Scholar]
- 30.Hashimoto R, Rothwell JC. Dynamic changes in corticospinal excitability during motor imagery. Exp Brain Res. 1999;125:75–81. doi: 10.1007/s002210050660. [DOI] [PubMed] [Google Scholar]
- 31.Abbruzzese G, Assini A, Buccolieri A, Marchese R, Trompetto C. Changes of intracortical inhibition during motor imagery in human subjects. Neurosci Lett. 1999;263:113–6. doi: 10.1016/s0304-3940(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 32.Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115–23. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brasil-Neto JP. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–25. doi: 10.1093/brain/116.3.511. Pt 3. [DOI] [PubMed] [Google Scholar]
- 34.Porro CA, Francescato MP, Cettolo V, et al. Primary motor and sensory cortex activation during motor performance and motor imagery: a functional magnetic resonance imaging study. J Neurosci. 1996;16:7688–98. doi: 10.1523/JNEUROSCI.16-23-07688.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luft AR, McCombe-Waller S, Whitall J, et al. Repetitive bilateral arm training and motor cortex activation in chronic stroke: a randomized controlled trial [published erratum in: JAMA 2004; 292:2470] JAMA. 2004;292:1853–61. doi: 10.1001/jama.292.15.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szaflarski J, Page S, Kissela B, Levine P, Lee J. Use-dependent cortical reorganization after modified constraint-induced therapy [abstract] Stroke. 2005;36:422. [Google Scholar]
- 37.Nudo RJ, Wise BM, SiFuentes F, Milliken GW. Neural substrates for the effects of rehabilitative training on motor recovery after ischemic infarct. Science. 1996;272:1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 38.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merzenich MM. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1982;8:33–55. doi: 10.1016/0306-4522(83)90024-6. [DOI] [PubMed] [Google Scholar]
- 40.Jenkins WM. Reorganization of neocortical representations after brain injury: a neurophysiological model of the bases of recovery from stroke. Prog Brain Res. 1987;71:249–66. doi: 10.1016/s0079-6123(08)61829-4. [DOI] [PubMed] [Google Scholar]
- 41.Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M. Massive cortical reorganization after sensory deafferentation in adult macaques. Science. 1991;252:1857–60. doi: 10.1126/science.1843843. [DOI] [PubMed] [Google Scholar]
- 42.Nudo RJ, Milliken GW. Reorganization of movement representations in primary motor cortex following focal ischemic infarcts in adult squirrel monkeys. J Neurophysiol. 1996;75:2144–9. doi: 10.1152/jn.1996.75.5.2144. [DOI] [PubMed] [Google Scholar]
- 43.Nudo R, Wise B, SiFuentes F, Milliken G. Neural substrates for the effect of rehabilitative training motor recovery after ischemic infarct. Science. 1996;272:1791–4. doi: 10.1126/science.272.5269.1791. [DOI] [PubMed] [Google Scholar]
- 44.Leonardo M, Fieldman J, Sadato N, et al. A functional magnetic resonance imaging study of cortical regions associated with motor task execution and motor ideation in humans. Hum Brain Mapp. 1995;3:83–92. [Google Scholar]
- 45.Jackson PL, Lafleur MF, Malouin F, Richards CL, Doyon J. Functional cerebral reorganization following motor sequence learning through mental practice with motor imagery. Neuroimage. 2003;20:1171–80. doi: 10.1016/S1053-8119(03)00369-0. [DOI] [PubMed] [Google Scholar]
- 46.Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J. Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp. 2003;19:47–62. doi: 10.1002/hbm.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Luft AR, Skalej M, Stefanou A, Klose U, Voigt K. Comparing motion- and imagery-related activation in the human cerebellum: a functional MRI study. Hum Brain Mapp. 1998;6:105–13. doi: 10.1002/(SICI)1097-0193(1998)6:2<105::AID-HBM3>3.0.CO;2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Johnson SH, Rotte M, Grafton ST, Hinrichs H, Gazzaniga MS, Heinze HJ. Selective activation of a parietofrontal circuit during implicitly imagined prehension. Neuroimage. 2002;17:1693–704. doi: 10.1006/nimg.2002.1265. [DOI] [PubMed] [Google Scholar]
- 49.Roth M, Decety J, Raybaudi M, et al. Possible involvement of primary motor cortex in mentally simulated movement: a functional magnetic resonance imaging study. Neuroreport. 1996;7:1280–4. doi: 10.1097/00001756-199605170-00012. [DOI] [PubMed] [Google Scholar]
- 50.Lotze M, Montoya P, Erb M, et al. Activation of cortical and cerebellar motor areas during executed and imagined hand movements: an fMRI study. J Cogn Neurosci. 1999;11:491–501. doi: 10.1162/089892999563553. [DOI] [PubMed] [Google Scholar]
- 51.Cao Y, D’Olhaberriague L, Vikingstad EM, Levine SR, Welch KM. Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke. 1998;29:112–22. doi: 10.1161/01.str.29.1.112. [DOI] [PubMed] [Google Scholar]
- 52.Weiller C. Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol. 1992;31:463–72. doi: 10.1002/ana.410310502. [DOI] [PubMed] [Google Scholar]
- 53.Weiller C, Ramsay SC, Wise RJ, Friston KJ, Frackowiack RS. Individual Patterns of functional reorganization in the human cerebral cortex after capsular infarction. Ann Neurol. 1993;33:181–9. doi: 10.1002/ana.410330208. [DOI] [PubMed] [Google Scholar]
- 54.Cramer SC, Nelles G, Benson RR, et al. A functional MRI study of subjects recovered from hemiparetic stroke. Stroke. 1997;28:2518–27. doi: 10.1161/01.str.28.12.2518. [DOI] [PubMed] [Google Scholar]
- 55.Binkofski F, Seitz RJ, Hacklander T, Pawelec D, Mau J, Freund HJ. Recovery of motor functions following hemiparetic stroke: a clinical and magnetic resonance-morphometric study. Cerebrovasc Dis. 2001;11:273–81. doi: 10.1159/000047650. [DOI] [PubMed] [Google Scholar]
- 56.Ward NS, Brown MM, Thompson AJ, Frackowiak RS. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain. 2003;126:2476–96. doi: 10.1093/brain/awg245. Pt 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kimberley TJ, Khandekar G, Skraba LL, Spencer JA, Van Gorp EA, Walker SR. Neural substrates for motor imagery in severe hemiparesis. Neurorehabil Neural Repair. 2006;20:268–77. doi: 10.1177/1545968306286958. [DOI] [PubMed] [Google Scholar]
- 58.Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol. 1991;29:63–71. doi: 10.1002/ana.410290112. [DOI] [PubMed] [Google Scholar]
- 59.Nelles G, Spiekermann G, Jueptner M, et al. Reorganization of sensory and motor systems in hemiplegic stroke patients. A positron emission tomography study. Stroke. 1999;30:1510–6. doi: 10.1161/01.str.30.8.1510. [DOI] [PubMed] [Google Scholar]
- 60.Seitz RJ, Hoflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1081–8. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- 61.Carey JR, Kimberley TJ, Lewis SM, et al. Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain. 2002;125:773–88. doi: 10.1093/brain/awf091. Pt 4. [DOI] [PubMed] [Google Scholar]
- 62.Page SJ. Imagery improves upper extremity motor function in chronic stroke patients: a pilot study. Occup Ther J Res. 2000;3:200–15. [Google Scholar]
- 63.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 64.Page SJ, Hewett T, Ford K, Levine P. Mental practice improves reaching kinematics in stroke [abstract] Arch Phys Med Rehabil. 2005;86:E7–8. [Google Scholar]
- 65.Page SJ, Levine P, Sisto SA, Johnston MV. Mental practice combined with physical practice for upper-limb motor deficit in subacute stroke. Phys Ther. 2001;81:1455–62. doi: 10.1093/ptj/81.8.1455. [DOI] [PubMed] [Google Scholar]
- 66.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf motor function test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–9. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 67.Taub E, Miller NE, Novack TA, et al. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–54. [PubMed] [Google Scholar]
- 68.van der Lee JH, Beckerman H, Knol DL, de Vet HC, Bouter LM. Clinimetric properties of the motor activity log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1410–4. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 69.Hall CR, Martin KA. Measuring movement imagery abilities: a revision of the Movement Imagery Questionnaire. J Ment Imagery. 1997;21:143–54. [Google Scholar]
- 70.Isaac AR, Marks DF. Individual differences in mental imagery experience: developmental changes and specialization. Br J Psychol. 1994;85:479–500. doi: 10.1111/j.2044-8295.1994.tb02536.x. Pt 4. [DOI] [PubMed] [Google Scholar]
- 71.Isaac A, Marks DF, Russell DG. An Instrument for assessing imagery of movement: The Vividness of Movement Imagery Questionnaire (VMIQ) J Ment Imagery. 1986;10:23–30. [Google Scholar]
- 72.Sirigu A, Duhamel JR. Motor and visual imagery as two complementary but neurally dissociable mental processes. J Cogn Neurosci. 2001;13:910–9. doi: 10.1162/089892901753165827. [DOI] [PubMed] [Google Scholar]
- 73.Sirigu A, Daprati E, Pradat-Diehl P, Franck N, Jeannerod M. Perception of self-generated movement following left parietal lesion. Brain. 1999;122:1867–74. doi: 10.1093/brain/122.10.1867. [DOI] [PubMed] [Google Scholar]
- 74.Small SL, Hlustik P, Noll DC, Genovese C, Solodkin A. Cerebellar hemispheric activation ipsilateral to the paretic hand correlates with functional recovery after stroke. Brain. 2002;125:1544–57. doi: 10.1093/brain/awf148. Pt 7. [DOI] [PubMed] [Google Scholar]
- 75.Buonomano DV. Cortical plasticity: from synapses to maps. Annu Rev Neurosci. 1998;21:149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 76.Moser E, Moser MB, Andersen P. Synaptic potentiation in the rat dentate gyrus during exploratory learning. Neuroreport. 1993;5:317–20. doi: 10.1097/00001756-199312000-00035. [DOI] [PubMed] [Google Scholar]
- 77.Rogan MT, Staubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–7. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 78.McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–11. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 79.Rioult-Pedotti MS, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science. 2000;290:533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- 80.Houk JC, Keifer J, Barto AG. Distributed motor commands in the limb premotor network. Trends Neurosci. 1993;16:27–33. doi: 10.1016/0166-2236(93)90049-r. [DOI] [PubMed] [Google Scholar]
- 81.Sirigu A, Duhamel JR, Cohen L, Pillon B, Dubois B, Agid Y. The mental representation of hand movements after parietal cortex damage. Science. 1996;273:1564–8. doi: 10.1126/science.273.5281.1564. [DOI] [PubMed] [Google Scholar]


