Abstract
Background
A 24-year-old man presented with previously diagnosed Marfan’s syndrome. Since the age of 9 years, he had undergone eight cardiovascular procedures to treat rapidly progressive aneurysms, dissection and tortuous vascular disease involving the aortic root and arch, the thoracoabdominal aorta, and brachiocephalic, vertebral, internal thoracic and superior mesenteric arteries. Throughout this extensive series of cardiovascular surgical repairs, he recovered without stroke, paraplegia or renal impairment.
Investigations
CT scans, arteriogram, genetic mutation screening of transforming growth factor β receptors 1 and 2.
Diagnosis
Diffuse and rapidly progressing vascular disease in a patient who met the diagnostic criteria for Marfan’s syndrome, but was later rediagnosed with Loeys–Dietz syndrome. Genetic testing also revealed a de novo mutation in transforming growth factor β receptor 2.
Management
Regular cardiovascular surveillance for aneurysms and dissections, and aggressive surgical treatment of vascular disease.
Keywords: aortic disease, fibrillin 1, Loeys-Dietz syndrome, Marfan’s syndrome, transforming growth factor β receptor 2
Medscape Continuing Medical Education online
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit. Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide CME for physicians. Medscape, LLC designates this educational activity for a maximum of 1.0 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To receive credit, please go to http://www.medscape.com/cme/ncp and complete the post-test.
Learning objectives
Upon completion of this activity, participants should be able to:
List characteristics of Marfan’s syndrome.
List characteristics of Loeys–Dietz syndrome.
Identify genetic mutations associated with Marfan’s syndrome and Loeys–Dietz syndrome.
Describe essential elements of management of Loeys–Dietz syndrome.
THE CASE
A 24-year-old man presented with rapidly progressive aneurysms, dissection and tortuous vascular disease involving the aortic root and arch, thoracoabdominal aorta, and the brachiocephalic, vertebral, internal thoracic and superior mesenteric arteries. At 7 years of age, the patient had been diagnosed with Marfan’s syndrome (MFS) following surgical repair of pectus excavatum. During a subsequent clinical assessment, the patient was noted to have an aortic root diameter of 4.0 cm. At the age of 9 years the patient underwent aortic root replacement with a composite valve graft because his aortic root diameter had expanded to 5 cm with associated aortic valve insufficiency and chest pain.
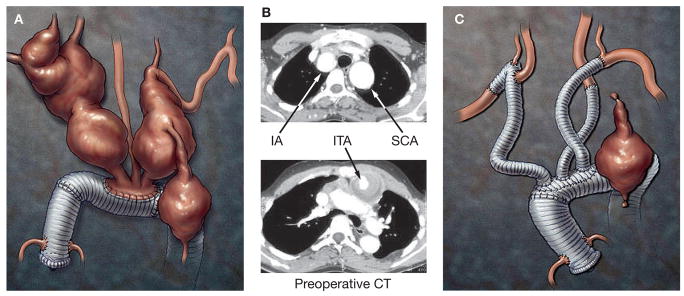
The patient remained clinically well until the age of 17 years, when he presented with an acute dissection involving the entire remaining aorta. He underwent graft replacement of the distal ascending aorta and transverse aortic arch using the elephant trunk technique, with re attachment of the brachiocephalic arteries as a patch. Within 3 months of the initial dissection and repair, the patient’s thoracoabdominal aorta had expanded to a diameter of 5 cm. His entire thoracoabdominal aorta was replaced with a graft from the level of the left subclavian artery to the aortoiliac bifurcation (a Crawford extent II repair) with reattachment of the T11 and L1 segmental arteries, celiac axis, superior mesenteric artery and both renal arteries (Figure 1). He subsequently required surgical repair of symptomatic and rapidly expanding aneurysms in the left internal carotid artery after 2 months and in the superior mesenteric artery after 3 months. When he was 19 years old, the patient underwent surgical repair of aneurysms in the innominate, bilateral subclavian, right vertebral, bilateral common carotid and left internal thoracic arteries; the latter measured 3.6 cm in diameter (Figure 2A–C). He presented with a right internal thoracic artery aneurysm 3.5 years later, which was treated by direct percutaneous injection of fibrin glue.
Figure 1.
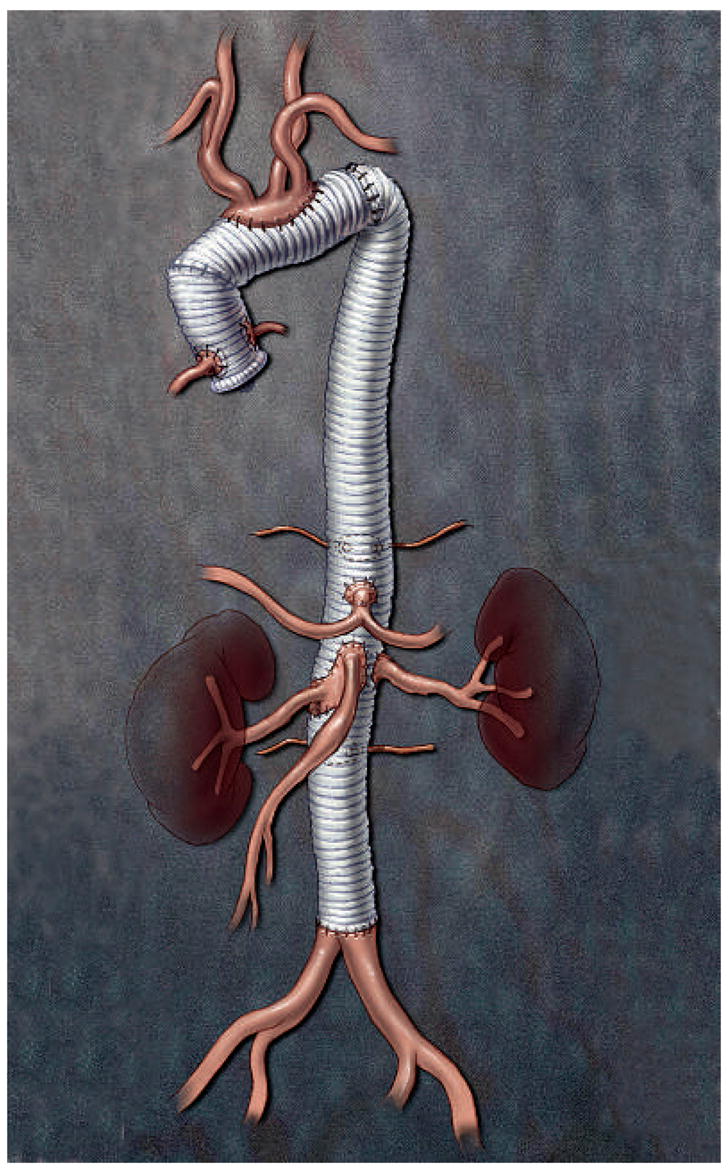
Illustration showing the results of three aortic operations the patient underwent between the ages of 9 and 18 years, involving graft replacement of the aortic root, ascending aorta, transverse aortic arch and entire thoracoabdominal aorta.
Figure 2.
Imaging and reconstruction of aneurysms in arteries originating from the aortic arch. (A) A drawing and (B) preoperative CT scan showing aneurysms identified in the patient at the age of 19 years. (C) Illustration showing graft repair of the superior transverse aortic arch with graft replacement of the brachiocephalic vessels and ligation of the left internal thoracic artery through an upper hemisternotomy. Abbreviations: IA, innominate artery; ITA, internal thoracic arteries; SCA, subclavian artery.
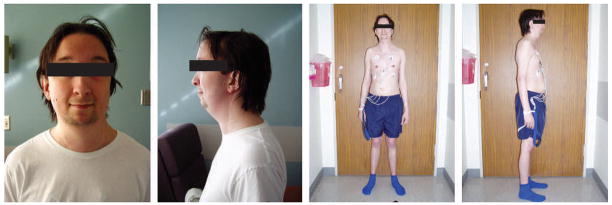
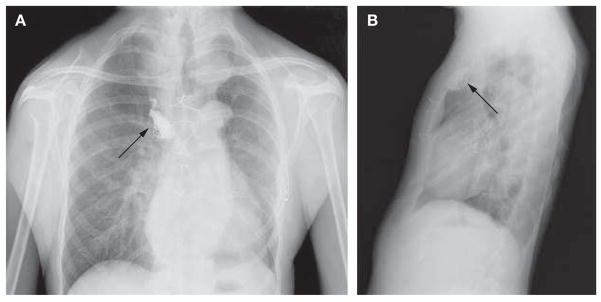
At the age of 24 years, the patient returned to the hospital with a pseudoaneurysm involving the right subclavian artery, and marked tortuosity of the adjacent supraclavicular branch vessels was noted (Figure 3). His aneurysm was treated by percutaneous endovascular obliteration using a combination of platinum coils and hydrocoils. He recovered from the extensive cardiovascular procedures without stroke, paraplegia or renal impairment. Surgical repairs were not complicated by excessively friable tissues or bleeding. The patient has been closely monitored with serial imaging studies of his chest, abdomen and cerebral vasculature. Physical examination at the age of 24 years revealed a thin young man with mildly dysmorphic features with hypertelorism, micrognathia, malar flattening, downslanting palpebral fissures, a long philtrum with a thin upper lip, a highly arched palate and bifid uvula (Figure 4). Skeletal features of MFS included a repaired pectus excavatum, scoliosis, pes planus and joint laxity. Ophthalmologic examination was notable for absence of ectopia lentis. His skin was velvety to the touch and translucent with visible veins. The patient had a history of inguinal hernias, with repairs performed at the age of 3 and 6 weeks, 6 months and 6 years. Imaging studies showed that he had elongated lung fields (Figure 5A,B) and lumbosacral dural ectasia. Features of MFS were absent in the patient’s parents and sister.
Figure 3.
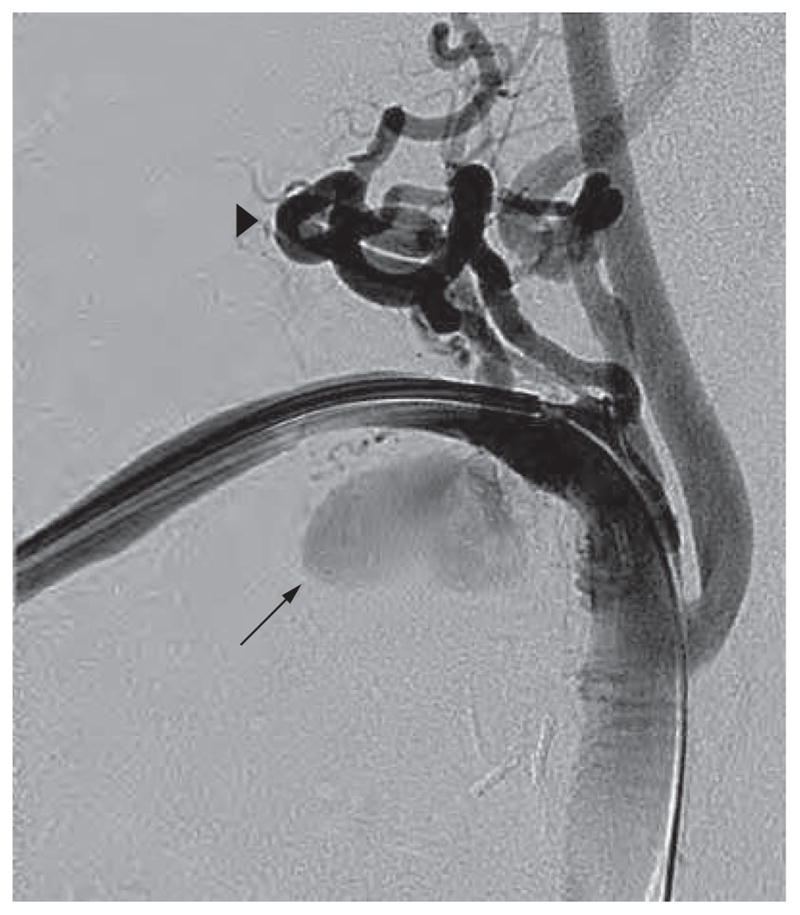
Arteriogram demonstrating marked tortuosity of the supraclavicular branch vessels (arrowhead) and a pseudoaneurysm involving the right subclavian artery (arrow).
Figure 4.
Photographs illustrating the patient’s mildly dysmorphic facial features, including hypertelorism, micrognathia, downslanting palpebral fissures and long philtrum with a thin upper lip, and skeletal features, including a repaired pectus excavatum, scoliosis and pes planus.
Figure 5.
Radiographs demonstrating elongated lung fields of the chest. (A) Posteroanterior and (B) lateral chest radiographs demonstrating elongated lung fields. The radiodense material in the right upper peristernal region (indicated by arrows) is fibrin glue within the thrombosed right internal thoracic artery aneurysm.
The patient presented with features of both MFS and Loeys–Dietz syndrome (LDS), prompting us to carry out DNA sequencing of the genes transforming growth factor β (TGF-β) receptors 1 and 2 (TGFBR1 and TGFBR2). Mutation screening of TGFBR2 revealed a heterozygous missense mutation (C1582>T), resulting in substitution of a cysteine for an arginine at amino acid 528 (Arg528Cys). This mutation has been previously identified in an unrelated patient with LDS.1 Sequencing of DNA obtained from the patient’s parents and unaffected sister did not reveal the TGFBR2 mutation, indicating that the mutation occurred de novo in the patient and that no additional family members were at risk for the disease.
DISCUSSION OF DIAGNOSIS
MFS is an autosomal dominant genetic disorder, and classical features include cardiovascular defects (ascending aortic aneurysms, aortic dissections and mitral valve abnormalities), skeletal manifestations (pectus deformities, scoliosis, dolichostenomelia, arachnodactyly, joint laxity and a highly arched palate) and ocular complications (ectopia lentis, retinal detachment and myopia).2 Diagnostic criteria for MFS—currently known as the Ghent criteria—emphasize the aortic aneurysms and dissections, a constellation of skeletal findings, ectopia lentis, dural ectasia and the affected patient’s family history.3 Since the patient presented here had aortic aneurysm and dissection, skeletal features and dural ectasia, he met the Ghent diagnostic criteria for MFS.
In the early 1990s, causative mutations for MFS were identified in the gene encoding the extracellular matrix protein fibrillin 1 (FBN1), and initially there was no evidence of genetic heterogeneity.4,5 In 1994, MFS was described in a large French kindred, in which the phenotype could not be linked to markers in close proximity to FBN1, providing the first evidence that MFS is genetically heterogeneous.6 The disease-causing locus in this family was mapped to chromosome 3p24–25 and the diagnosis of MFS, as well as the validity of the linkage analysis, was debated in the literature.7 Subsequent studies, however, confirmed that the 3p locus was associated with aortic disease through linkage mapping in a family with thoracic aortic aneurysms and dissections (TAAD), and mapped the causative gene to the same chromosomal locus.8 In the French kindred, the defective gene at the 3p24–25 locus associated with disease was identified as TGFBR2.9 Mutations in TGFBR2 were subsequently identified in three additional, unrelated, MFS families and have also been identified in four unrelated families with familial TAAD who did not meet the diagnostic criteria for MFS.9,10 In these families with TAAD and TGFBR2 mutations, the primary vascular disease was progressive enlargement of the ascending aorta leading to dissection. Affected individuals also developed aneurysms and dissections involving the descending thoracic aorta and other arteries.10
Heterozygous mutations in either TGFBR1 or TGFBR2, including de novo mutations, have also been associated with LDS—a disorder that was initially described by Furlong and delineated more completely by Loeys and colleagues.1,11,12 Clinical features of LDS include vascular disease, craniosynostosis, cleft palate/bifid uvula, hypertelorism, congenital heart defects (patent ductus arteriosus and atrial septal defect) and mental retardation. Patients with LDS present with aneurysms or dissections of the ascending aorta, similar to those observed in patients with MFS. In LDS, however, these defects are more likely to manifest at a young age, and death from aortic disease can occur when patients are as young as 3 years. In contrast to MFS, generalized arterial tortuosity and aneurysms of other arteries have been noted in patients with LDS. Mutations in TGFBR2 are, therefore, associated with a spectrum of aortic disease syndromes, including LDS, MFS and familial TAAD, and the age of onset of aortic disease can range from 3–82 years.1,10 Interestingly, mutations in TGFBR1 or TGFBR2 have not been reported in patients with ectopia lentis, a clinical feature that seems to be specific for MFS owing to mutations in FBN1. Although the patient described here met the Ghent diagnostic criteria for MFS, LDS was considered a more accurate diagnosis because he also had features of LDS, such as bifid uvula, hypertelorism and arterial tortuosity. Current reports indicate that TGFBR1 and TGFBR2 mutations are rare in patients who meet the Ghent diagnostic criteria, but the present case indicates that the diagnostic criteria for MFS need to be revised and those for LDS established, so that the correct diagnosis can be made and vascular disease managed properly.9,13,14
FBN1 mutational analysis is not routinely recommended for diagnosis of MFS because of the complexity of the gene and identification of FBN1 mutations causing related phenotypes.15 We would recommend TGFBR1/2 mutational analysis in patients with features of LDS and familial TAAD. In addition, the literature and our current clinical experience suggest that TGFBR1/2 mutational analysis should be considered in patients with MFS who do not have ectopia lentis. Recommendations for genetic testing of individuals with TAAD need to be addressed as part of the revised diagnostic criteria for these disorders.
TREATMENT AND MANAGEMENT
As this case illustrates, arterial disease associated with TGFBR2 mutations is diffuse and progresses more rapidly than in classic MFS resulting from FBN1 mutations where the vascular disease is mainly limited to the aorta.16 Although the optimal treatment strategy for patients with aortic aneurysm syndromes associated with TGFBR2 mutations has not yet been delineated, initial data indicate that vascular disease should be aggressively managed, whether the associated diagnosis is LDS, MFS or familial TAAD. A noteworthy case is that of an adult patient with a TGFBR2 mutation who presented at our institution with acute aortic dissection a few months after a maximal aortic dimension of only 4.2 cm had been documented (DM Milewicz, unpublished data). To prevent life-threatening rupture or dissection, we recommend, therefore, that patients with TGFBR2 mutations undergo cardiovascular surveillance for aneurysms and dissection using imaging modalities such as CT scans and magnetic resonance angiography, followed by aggressive surgical treatment of identified vascular disease.
CONCLUSION
Compared with FBN1 mutations causing classic MFS, TGFBR2 mutations are associated with more extensive and progressive aortic and peripheral vascular disease. Furthermore, patients with TGFBR2 mutations might have features usually absent in classic cases of MFS, including hypertelorism, bifid uvula and marked arterial tortuosity. We recommend that patients with TGFBR2 mutations receive diligent lifelong cardiovascular surveillance and aggressive surgical management, whether the diagnosis is LDS, MFS or familial TAAD.
Acknowledgments
The authors wish to thank the patient and his family for participation in their studies, and Scott Weldon for his outstanding illustrations. This work was supported in part by grant numbers R01 HL62594 (DMM), P50HL08379-01 (DMM), and the Doris Duke Charitable Foundation (DMM), and grant K08 HL080085 (SL) and the Thoracic Surgery Foundation for Research and Education (SL).
Footnotes
Competing interests
The authors declared they have no competing interests.
References
- 1.Loeys BL, et al. A syndrome of altered cardiovascular, craniofacial, neurocognitive and skeletal development caused by mutations in TGFBR1 or TGFBR2. Nat Genet. 2005;37:275–281. doi: 10.1038/ng1511. [DOI] [PubMed] [Google Scholar]
- 2.McKusick VA. Heritable Disorders of Connective Tissue. Saint Louis, MO: The CV Mosby Company; 1972. [Google Scholar]
- 3.De Paepe A, et al. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet. 1996;62:417–426. doi: 10.1002/(SICI)1096-8628(19960424)62:4<417::AID-AJMG15>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 4.Kainulainen K, et al. Marfan syndrome: no evidence for heterogeneity in different populations, and more precise mapping of the gene. Am J Hum Genet. 1991;49:662–667. [PMC free article] [PubMed] [Google Scholar]
- 5.Dietz HC, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- 6.Collod G, et al. A second locus for Marfan syndrome maps to chromosome 3p24.2-p25. Nat Genet. 1994;8:264–268. doi: 10.1038/ng1194-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dietz H, et al. The question of heterogeneity in Marfan syndrome. Nat Genet. 1995;9:228–231. doi: 10.1038/ng0395-228. [DOI] [PubMed] [Google Scholar]
- 8.Hasham SN, et al. Mapping a locus for familial thoracic aortic aneurysms and dissections (TAAD2) to 3p24–25. Circulation. 2003;107:3184–3190. doi: 10.1161/01.CIR.0000078634.33124.95. [DOI] [PubMed] [Google Scholar]
- 9.Mizuguchi T, et al. Heterozygous TGFBR2 mutations in Marfan syndrome. Nat Genet. 2004;36:855–860. doi: 10.1038/ng1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pannu H, et al. Mutations in transforming growth factor-beta receptor type II cause familial thoracic aortic aneurysms and dissections. Circulation. 2005;112:513–520. doi: 10.1161/CIRCULATIONAHA.105.537340. [DOI] [PubMed] [Google Scholar]
- 11.Furlong J, et al. New Marfanoid syndrome with craniosynostosis. Am J Med Genet. 1987;26:599–604. doi: 10.1002/ajmg.1320260314. [DOI] [PubMed] [Google Scholar]
- 12.Ades LC, et al. FBN1, TGFBR1, and the Marfan-craniosynostosis/mental retardation disorders revisited. Am J Med Genet A. 2006;140:1047–1058. doi: 10.1002/ajmg.a.31202. [DOI] [PubMed] [Google Scholar]
- 13.Ki CS, et al. Identification of a novel TGFBR2 gene mutation in a Korean patient with Loeys-Dietz aortic aneurysm syndrome; no mutation in TGFBR2 gene in 30 patients with classic Marfan’s syndrome. Clin Genet. 2005;68:561–563. doi: 10.1111/j.1399-0004.2005.00535.x. [DOI] [PubMed] [Google Scholar]
- 14.Disabella E, et al. Two novel and one known mutation of the TGFBR2 gene in Marfan syndrome not associated with FBN1 gene defects. Eur J Hum Genet. 2006;14:34–38. doi: 10.1038/sj.ejhg.5201502. [DOI] [PubMed] [Google Scholar]
- 15.Loeys B, et al. Genotype and phenotype analysis of 171 patients referred for molecular study of the fibrillin-1 gene FBN1 because of suspected Marfan syndrome. Arch Intern Med. 2001;161:2447–2454. doi: 10.1001/archinte.161.20.2447. [DOI] [PubMed] [Google Scholar]
- 16.Milewicz DM, et al. Treatment of aortic disease in patients with Marfan syndrome. Circulation. 2005;111:e150–e157. doi: 10.1161/01.CIR.0000155243.70456.F4. [DOI] [PubMed] [Google Scholar]