Abstract
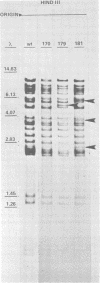
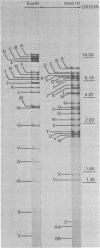
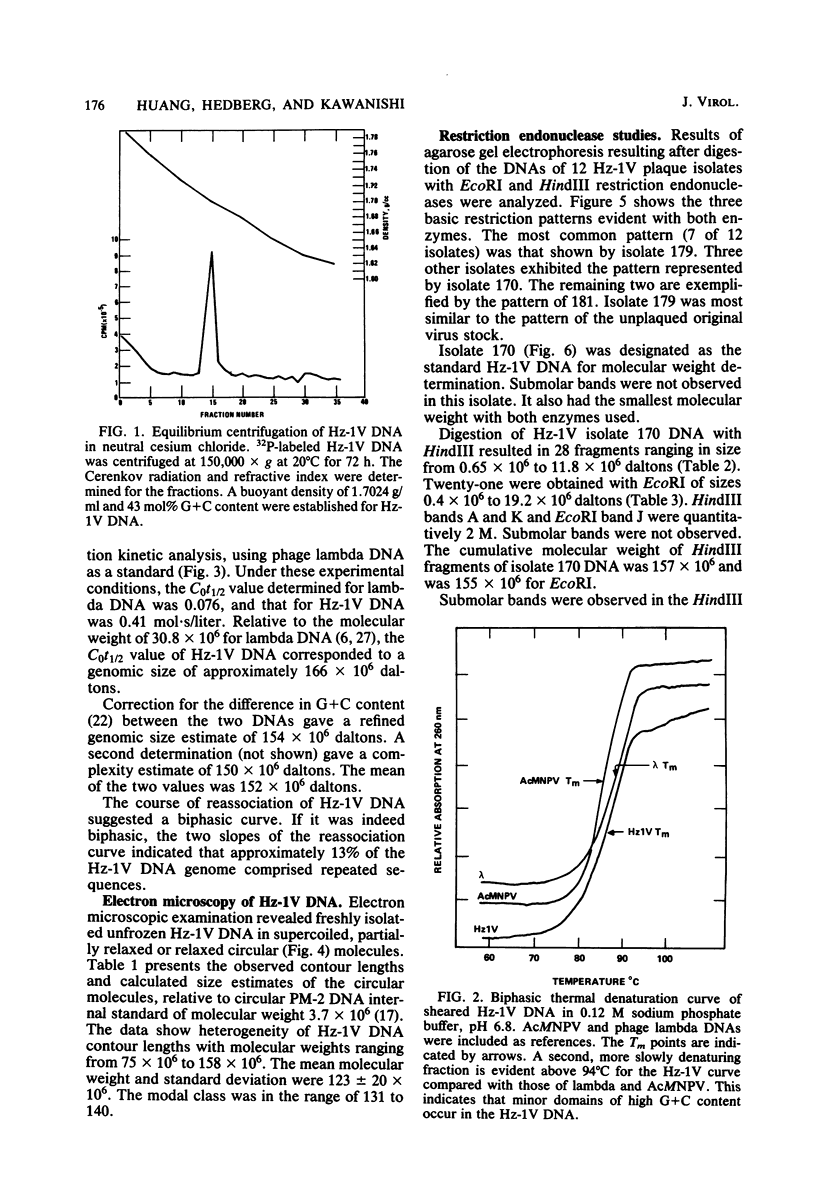
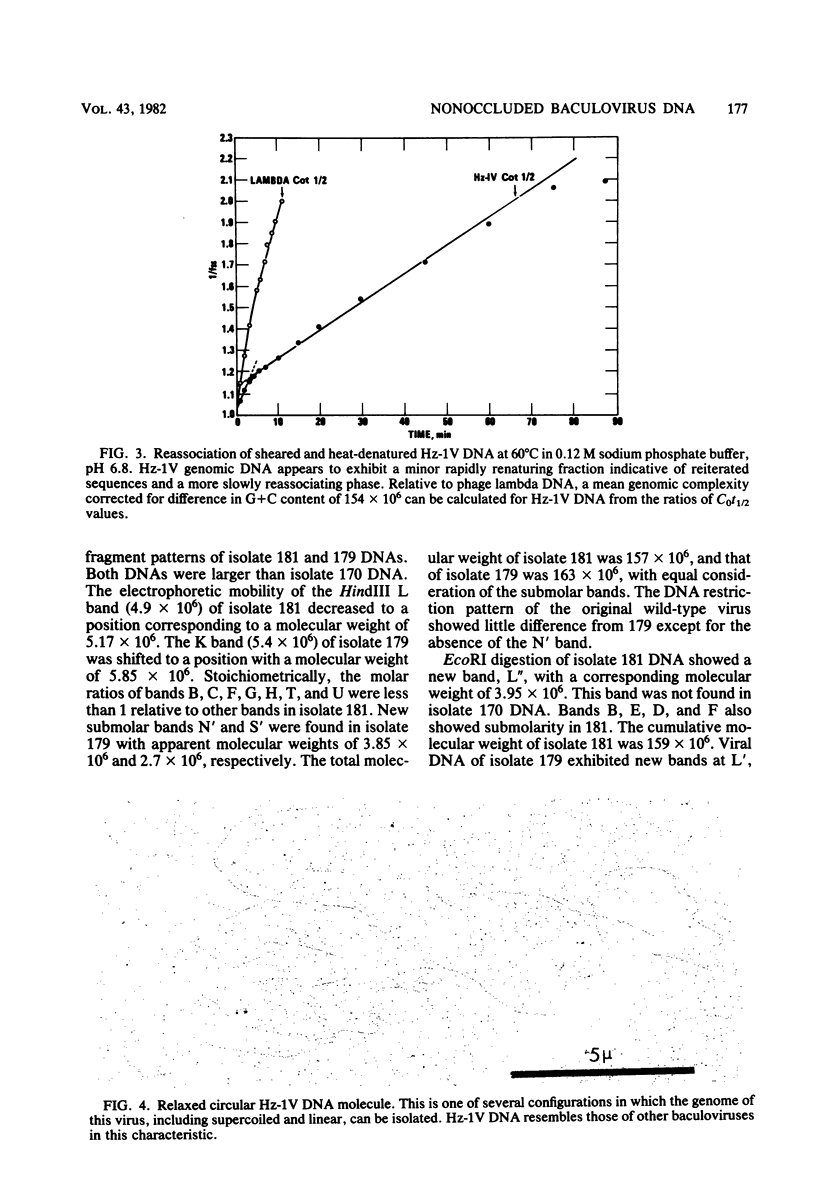
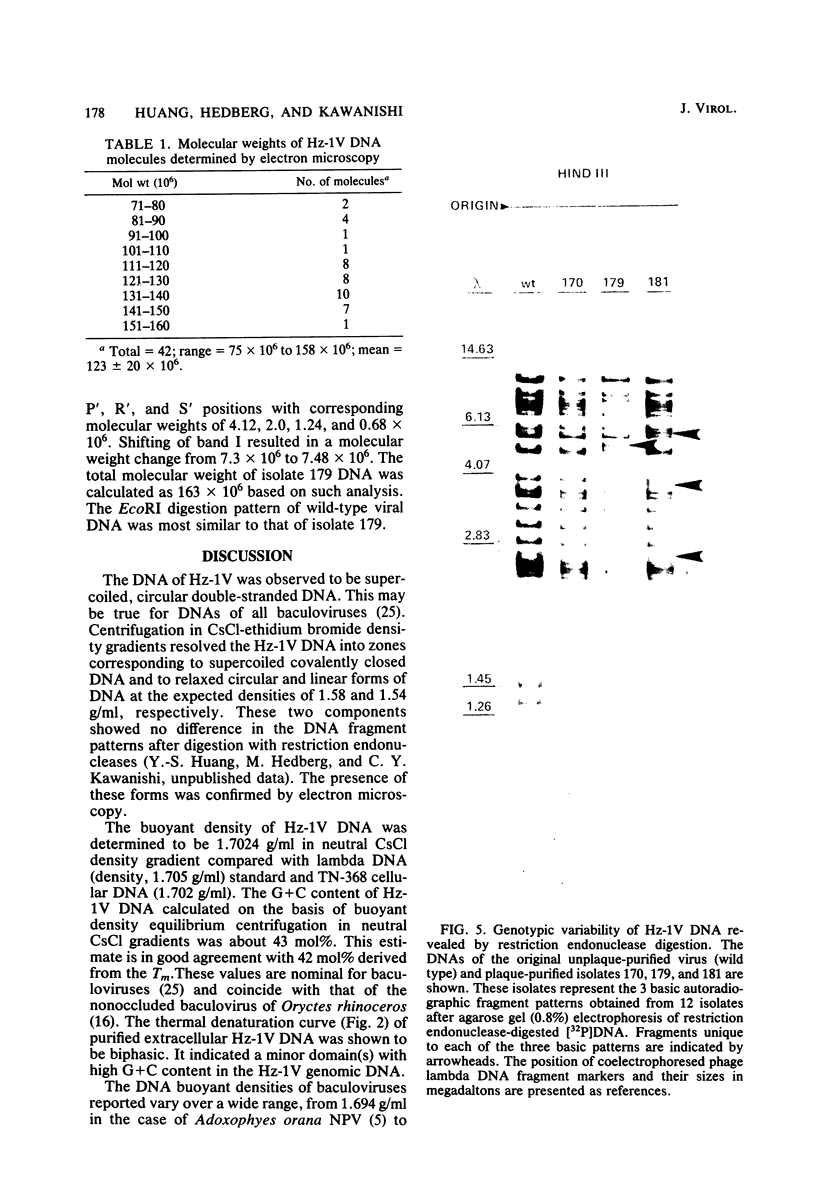
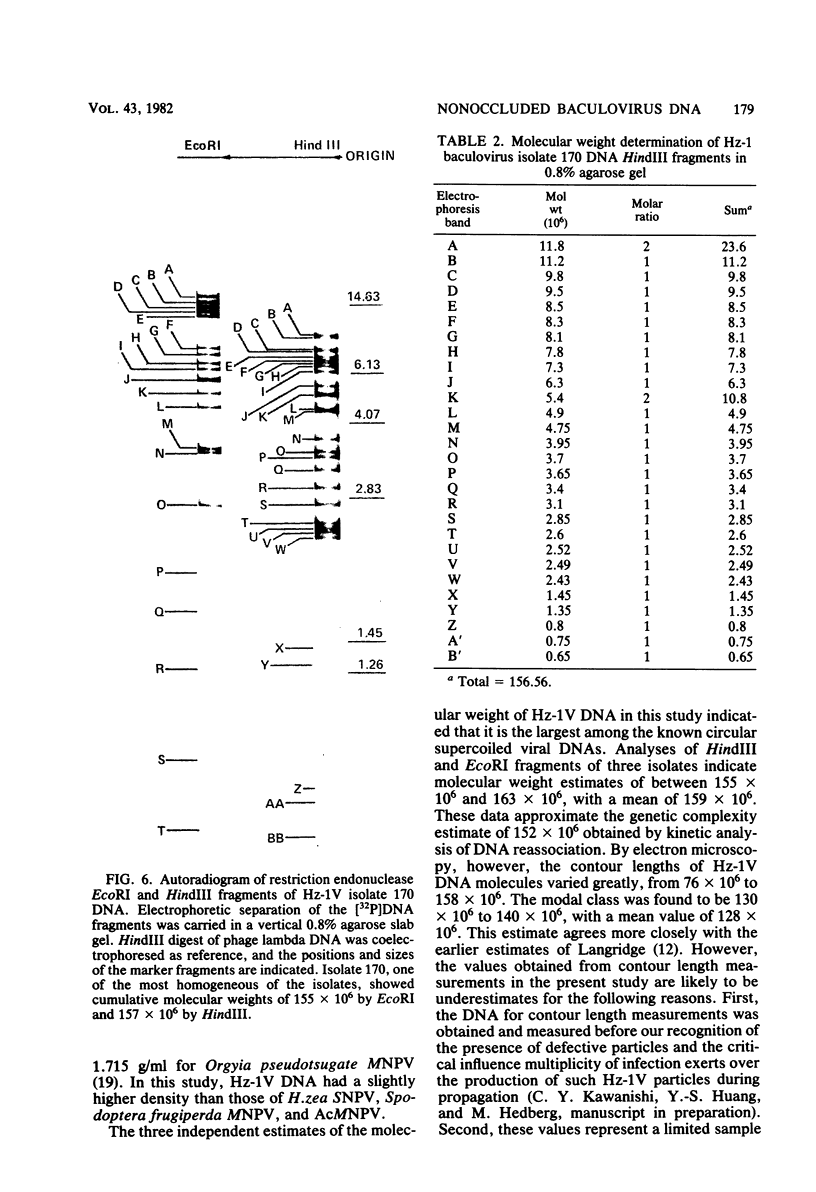
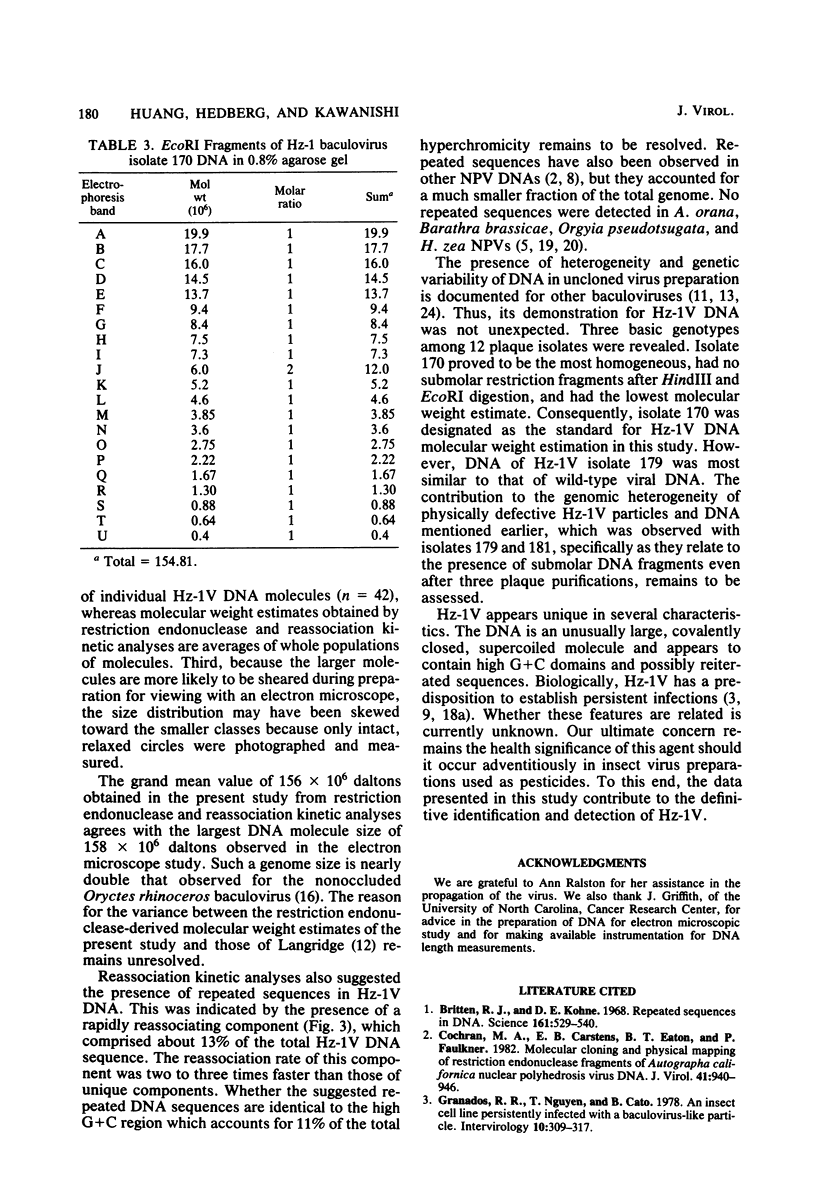
The DNA of the nonoccluded baculovirus (Hz-1V) obtained from the IMC-Hz-1 cell line was characterized by physicochemical and restriction endonuclease techniques. Hz-1V DNA isolated from purified virus had buoyant densities of 1.58 and 1.54 g/ml in CsCl-ethidium bromide density gradients, which corresponded to supercoiled and to relaxed circular and linear DNA, respectively. Neutral CsCl equilibrium centrifugation indicated that the Hz-1V DNA had a buoyant density of 1.7024 g/ml, which corresponded to a guanine-plus-cytosine (G+C) content of 43%. Thermal denaturation indicated a high G+C domain(s) in the Hz-1V genomic DNA. The domain(s), which included about 11% of the total genomic DNA, exhibited a Tm of 97°C. The remaining portion (89%) of the DNA had a Tm of 86.5°C. The Tms corresponded to G+C contents of 42 and 67%, respectively. The mean genetic complexity of Hz-1V DNA determined by DNA reassociation kinetic analysis was found to be 152 × 106. A possible rapidly reassociating component comprising approximately 13% of the genome was observed. The mean molecular weights from restriction endonuclease digests were 159 × 106 for both HindIII and EcoRI. Genomic heterogeneity was found in both the wild-type Hz-1V stock and in two plaque isolates. Of 12 single-plaque isolates, 3 basic restriction endonuclease DNA fragment patterns were observed. The molecular size estimates from electron microscopic contour lengths of uncloned viral DNA ranged from 70 to 158 megadaltons, and the mode was the 130- to 140-megadalton class.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Carstens E. B., Eaton B. T., Faulkner P. Molecular Cloning and Physical Mapping of Restriction Endonuclease Fragments of Autographa californica Nuclear Polyhedrosis Virus DNA. J Virol. 1982 Mar;41(3):940–946. doi: 10.1128/jvi.41.3.940-946.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granados R. R., Nguyen T., Cato B. An insect cell line persistently infected with a baculovirus-like particle. Intervirology. 1978;10(5):309–317. doi: 10.1159/000148993. [DOI] [PubMed] [Google Scholar]
- Hink W. F. Established insect cell line from the cabbage looper, Trichoplusia ni. Nature. 1970 May 2;226(5244):466–467. doi: 10.1038/226466b0. [DOI] [PubMed] [Google Scholar]
- Jurkovícová M., van Touw J. H., Sussenbach J. S., Ter Schegget J. Characterization of the nuclear polyhedrosis virus DNA of Adoxophyes orana and of Barathra brassicae. Virology. 1979 Feb;93(1):8–19. doi: 10.1016/0042-6822(79)90271-x. [DOI] [PubMed] [Google Scholar]
- KAISER A. D., HOGNESS D. S. The transformation of Escherichia coli with deoxyribonucleic acid isolated from bacteriophage lambda-dg. J Mol Biol. 1960 Dec;2:392–415. doi: 10.1016/s0022-2836(60)80050-2. [DOI] [PubMed] [Google Scholar]
- KLEINSCHMIDT A. K., LANG D., JACHERTS D., ZAHN R. K. [Preparation and length measurements of the total desoxyribonucleic acid content of T2 bacteriophages]. Biochim Biophys Acta. 1962 Dec 31;61:857–864. [PubMed] [Google Scholar]
- Kelly D. C. The DNA contained by nuclear polyhedrosis viruses isolated from four Spodoptera sp. (Lepidoptera, Noctuidae): genome size and homology assessed by DNA reassociation kinetics. Virology. 1977 Jan;76(1):468–471. doi: 10.1016/0042-6822(77)90325-7. [DOI] [PubMed] [Google Scholar]
- Lee H. H., Miller L. K. Isolation of genotypic variants of Autographa californica nuclear polyhedrosis virus. J Virol. 1978 Sep;27(3):754–767. doi: 10.1128/jvi.27.3.754-767.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Morris T. J., Hess R. T., Pinnock D. E. Physicochemical characterization of a small RNA virus associated with baculovirus infection in Trichoplusia ni. Intervirology. 1979;11(4):238–247. doi: 10.1159/000149040. [DOI] [PubMed] [Google Scholar]
- Payne C. C. The isolation and characterization of a virus from Oryctes rhinoceros. J Gen Virol. 1974 Oct;25(1):105–116. doi: 10.1099/0022-1317-25-1-105. [DOI] [PubMed] [Google Scholar]
- Pettersson U., Mulder C., Deluis H., Sharp P. A. Cleavage of adenovirus type 2 DNA into six unique fragments by endonuclease R-RI. Proc Natl Acad Sci U S A. 1973 Jan;70(1):200–204. doi: 10.1073/pnas.70.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrmann G. F., Carnegie J. W., Martignoni M. E., Beaudreau G. S. Characterization of the genome of the nucleopolyhedrosis bundle virus pathogenic for Orgyia pseudotsugata. Virology. 1977 Jul 15;80(2):421–425. doi: 10.1016/s0042-6822(77)80017-2. [DOI] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Scharnhorst D. W., Saving K. L., Vuturo S. B., Cooke P. H., Weaver R. F. Structural studies on the polyhedral inclusion bodies, virions, and DNA of the nuclear polyhedrosis virus of the cotton bollworm Heliothis zea. J Virol. 1977 Jan;21(1):292–300. doi: 10.1128/jvi.21.1.292-300.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler R. J., Mandel M. Quantitative aspects of deoxyribonucleic acid renaturation: base composition, state of chromosome replication, and polynucleotide homologies. J Bacteriol. 1971 May;106(2):608–614. doi: 10.1128/jb.106.2.608-614.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A., Pettersson U., Sambrook J. Viral DNA in transformed cells. I. A study of the sequences of adenovirus 2 DNA in a line of transformed rat cells using specific fragments of the viral genome. J Mol Biol. 1974 Jul 15;86(4):709–726. doi: 10.1016/0022-2836(74)90348-9. [DOI] [PubMed] [Google Scholar]
- Summers M. D., Anderson D. L. Characterization of nuclear polyhedrosis virus DNAs. J Virol. 1973 Dec;12(6):1336–1346. doi: 10.1128/jvi.12.6.1336-1346.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Wetmur J. G., Davidson N. Kinetics of renaturation of DNA. J Mol Biol. 1968 Feb 14;31(3):349–370. doi: 10.1016/0022-2836(68)90414-2. [DOI] [PubMed] [Google Scholar]