Abstract
Human thymic stromal lymphopoietin (TSLP) promotes CD4+ T-cell proliferation both directly and indirectly through dendritic cell (DC) activation. Although human TSLP-activated DCs induce CD8+ T-cell proliferation, it is not clear whether TSLP acts directly on CD8+ T cells. In this study, we show that human CD8+ T cells activated by T-cell receptor stimulation expressed TSLP receptor (TSLPR), and that TSLP directly enhanced proliferation of activated CD8+ T cells. Although non-stimulated human CD8+ T cells from peripheral blood did not express TSLPR, CD8+ T cells activated by anti-CD3 plus anti-CD28 did express TSLPR. After T-cell receptor stimulation, TSLP directly enhanced the expansion of activated CD8+ T cells. Interestingly, using monocyte-derived DCs pulsed with a cytomegalovirus (CMV)-specific pp65 peptide, we found that although interleukin-2 allowed expansion of both CMV-specific and non-specific CD8+ T cells, TSLP induced expansion of only CMV-specific CD8+ T cells. These results suggest that human TSLP directly enhances expansion of CD8+ T cells and that the direct and indirect action of TSLP on expansion of target antigen-specific CD8+ T cells may be beneficial to adoptive cell transfer immunotherapy.
Keywords: CD8+ T cells, direct action, human, proliferation, TSLP
Introduction
Human thymic stromal lymphopoietin (TSLP), an interleukin (IL)-7-like cytokine, activates CD11c+ blood myeloid dendritic cells (DCs); subsequently, the primed DCs induce strong T-cell proliferation [1–4]. Immature myeloid DCs in blood highly express the functional receptor for TSLP, IL-7Rα chain and TSLP receptor (TSLPR) [1]. TSLP strongly up-regulates surface expression of costimulatory molecules on DCs, prolongs DC survival, and enhances DC-T cell conjugate formation, resulting in the strong proliferation of not only CD4+ but also CD8+ T cells [1–5]. Although TSLP-activated DCs strongly induce the proliferation of T cells, these DCs do not produce detectable amounts of proinflammatory cytokines, such as IL-1β, IL-6, IL-12p70, and tumour necrosis factor-α[2,3]. In physiological conditions, human TSLP is preferentially expressed by epithelial cells within the thymus and the mucosal surface in lymphoid tissues [3,6], suggesting that TSLP is involved in the proliferation and differentiation of T cells through DC activation in vivo[7,8].
In addition to indirect action through DC activation, the direct action of TSLP on CD4+ T cell proliferation has recently been found in humans [9]. Freshly isolated peripheral blood CD4+ T cells do not express the functional receptor for TSLP and do not respond to TSLP. However, CD4+ T cells activated by anti-CD3 plus anti-CD28 express the functional receptor for TSLP, and directly respond to TSLP, resulting in enhanced proliferation [9]. Although human TSLP promotes CD4+ T-cell proliferation both directly and indirectly through DC activation, whether it can directly act on CD8+ T cells has yet to be clarified.
In this study, we show that human TSLP directly influenced activation of CD8+ T cells. Non-stimulated human CD8+ T cells from peripheral blood did not express TSLPR. However, stimulation of anti-CD3 plus anti-CD28 induced TSLPR expression in CD8+ T cells. After T-cell receptor (TCR) stimulation, TSLP directly enhanced the expansion of activated CD8+ T cells.
Materials and methods
CD8+ T cell isolation and culture
This study was approved by the Institutional Review Board for Human Research in the Graduate School of Medicine, Kyoto University. Peripheral blood mononuclear cells (PBMCs) were obtained from adult buffy coat of healthy donors (kindly provided by Kyoto Red Cross Blood Center, Kyoto, Japan), were frozen in a cell freezing medium without serum, Cellbanker™ (Nippon Zenyaku, Fukushima, Japan), and were kept in liquid N2 until use. CD8+ T cells were purified from PBMCs by positive selection using anti-CD8-coated magnetic beads (Miltenyi Biotec, Gladbach, Germany) to reach >99% purity. Isolated cells were cultured in RPMI 1640 (Gibco BRL, Grand Island, NY, USA) supplemented with 10% heat inactivated fetal calf serum (FCS) or 5% AB human serum (Sigma, St Louis, MO, USA), penicillin G and streptomycin (Gibco), 10 mM HEPES (Gibco), 1 mM sodium pyruvate (Gibco), and 20 ng/ml IL-7 (R&D Systems, Minneapolis, MN, USA) (referred to as complete medium). The cells were seeded at a density of 2·5 × 105 cells/ml in flat-bottomed 96-well plates in triplicate.
T cell proliferation and expansion assay
Purified CD8+ T cells were cultured with FCS supplemented complete medium and stimulated with 5 µg/ml plate-bound anti-CD3 (UCHT1, eBioscience, San Diego, CA, USA) and soluble 1 µg/ml anti-CD28 (CD28·1, eBioscience). For some experiments, 3 × 10−2–10 µg/ml plate-bound anti-CD3 were used, and 20 ng/ml recombinant human TSLP (R&D systems) was added on day 5 of culture. Viable cells were counted by trypan blue exclusion of dead cells. Purified CD8+ T cells were labelled with carboxyfluorescein diacetate succinimidyl diester (CFSE, Molecular Probes, Invitrogen, Carlsbad, CA, USA) as described [3,6].
Flow cytometry
The following monoclonal antibodies (mAbs) were used for surface staining: fluorescein isothiocyanate (FITC)-conjugated anti-CD3, FITC and phycoerythrin-conjugated anti-CD8, all purchased from BD Bioscience (San Jose, CA, USA), and FITC-conjugated anti-CD80, phycoerythrin cyanine chrome 5-conjugated anti-CD8, biotinylated mouse IgG1 isotype control Ab, and allophycocyanin-conjugated streptavidin all from eBioscience. For detection of TSLPR, cells were stained with biotinylated anti-human TSLPR mAb as described [9]. Stained cells were analysed with a FACS Calibur™ (BD Bioscience).
T cell cytokine production
After 7 days of culture of CD8+ T cells, intracellular cytokine production was assessed. Cultured CD8+ T cells were collected, washed twice, and restimulated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma) plus 2 µg/ml ionomycin (Sigma) in flat-bottomed 96-well plates at a concentration of 1 × 106 cell/ml. After 3·5 h, brefeldin A (Sigma) was added at 10 µg/ml. After 2·5 h, cells were collected and stained with phycoerythrin cyanine chrome 5-conjugated anti-CD8. Cells were fixed and permeabilized using Fix & Perm Cell Permeabilization Kit (Caltag Laboratories, An Der Grub, Austria), and stained with FITC-conjugated anti-interferon (IFN)-γ (eBioscience). Stained cells were analyzed with a FACS Calibur™.
Real-time quantitative reverse transcription-polymerase chain reaction (RT-PCR)
CD4+CD25- T cells and CD4+CD25+ regulatory T (Treg) cells were isolated from PBMCs by cell sorting. Total RNA was extracted using an RNeasy mini kit (Qiagen, Valencia, CA, USA) and treated with DNase I (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed with SuperScript™ II (Invitrogen). Real-time quantitative reactions were performed with a LightCycler™ 480 Instrument (Roche Diagnostics Gmbh, Mannheim, Germany) according to the manufacturer's instructions. Values are expressed as arbitrary units relative to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers were used as previously described [6].
Monocyte-derived DC (MoDC) generation and blood myeloid DC purification
MoDCs were generated as described [10–12]. Briefly, CD14+ monocytes were purified from PBMCs by positive selection with anti-CD14-coated magnetic beads (Miltenyi Biotec) to reach >97% purity and cultured in 12-well plates in FCS supplemented complete medium in the presence of 10 ng/ml IL-4 (Pepro Tech, Rocky Hill, NJ, USA) and granulocyte-macrophage colony-stimulating factor (Pepro Tech) for 5 days. On day 5, immature MoDCs were washed and resuspended in the same medium with 10 ng/ml of IL-6, tumour necrosis factor-α, IL-1β (all from Pepro Tech), and 1 µg/ml of prostaglandin-E2 (Sigma), and cultured for 2 days. On day 7, matured MoDCs were harvested. Viable DCs were counted by trypan blue exclusion of dead cells. CD11c+ blood myeloid DCs were isolated from PBMCs as described previously [3,6]. CD11c+lineage- cells were isolated by a FACS Aria™ (BD Biosciences) to reach >99% purity. Blood myeloid DCs were cultured immediately after being sorted in FCS supplemented complete medium. In experiments as shown in Fig. 4, DCs were seeded at a density of 1 × 106 cells/ml in 96-well plates with or without 20 ng/ml of TSLP and cultured for 24 h.
Fig. 4.
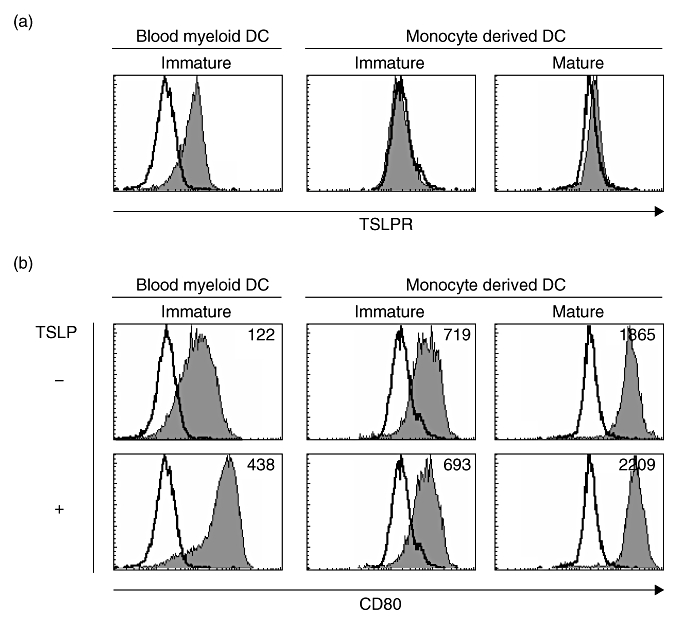
TSLPR and CD80 expression on blood myeloid DCs and monocyte-derived DCs. (a) Purified immature CD11c+ blood myeloid DCs and monocyte-derived DCs with or without maturation with IL-6, tumour necrosis factor-α, IL-1β and prostaglandin-E2, were stained with mAb to TSLPR or isotype control. Cell surface marker phenotypes were determined by flow cytometry. Filled histograms represent staining of DCs with TSLPR; open histograms represent isotype controls. Data represent one of three experiments. (b) Indicated DCs were cultured for 24 h with or without TSLP and stained with mAb to CD80 or isotype control. Filled histograms represent staining of DCs with CD80; open histograms represent isotype controls. Numbers in histograms indicate the mean fluorescence intensity. Data represent one of three experiments.
Synthetic peptide and human leukocyte antigen (HLA)-A2402-cytomegalovirus (CMV)pp65 tetramer
HLA-A24-restricted CMVpp65 peptide (QYDPVAALF aa 341-349), which is reported to be HLA-A24-restricted CMV-specific CD8+ T cell epitope [13], was purchased from OPERON Biotechnologies (Tokyo, Japan). HLA-A2402-CMVpp65 peptide tetramer conjugated with phycoerythrin were used for CMV-specific CD8+ T cell staining as previously described [13,14].
DC-T cell co-culture
In experiments of allogeneic MoDC-CD8+ T cell co-culture, T cells (2·5 × 105 cells/ml) were cultured with DCs at 1:2 DC : T cell ratio in round-bottomed 96-well plates in FCS supplemented complete medium. For analysis of CMVpp65-specific CD8+ T cells, cells were isolated from HLA-A2402-positive CMV-seropositive healthy donors and cultured with autologous MoDCs. MoDCs were cultured with 1 µM HLA-A24-restricted CMVpp65 peptide for the last 4 h and washed three times to remove any cytokines before co-culture. The cells were co-cultured at the same ratio and density as used in the allogeneic condition in human serum supplemented complete medium with either 20 ng/ml of IL-2 (R&D Systems) or TSLP. The number of viable cells was determined by trypan blue exclusion.
Statistical analysis
Statistical significance (P < 0·05) between groups was determined by paired t-test.
Results
TCR stimulation induces TSLPR expression on activated human CD8+ T cells
Freshly isolated human CD4+ T cells express IL-7Rα chain but not TSLPR, while CD4+ T cells stimulated by anti-CD3 plus anti-CD28 induce the cell surface expression of TSLPR [9]. To examine whether TCR stimulation induces the cell surface expression of TSLPR on activated human CD8+ T cells, purified peripheral blood CD8+ T cells were cultured with the stimulation of anti-CD3 plus anti-CD28. Before purification from PBMCs, human CD8+ T cells expressed an IL-7Rα chain, but they did not show any detectable level of TSLPR expression, as described previously [1,9] (Fig. 1a and data not shown). After 5 days culture of purified human CD8+ T cells with TCR stimulation, activated CD8+ T cells showed TSLPR expression of various degrees (3·6–13·9% of total CD8+ T cells, Fig. 1b).
Fig. 1.
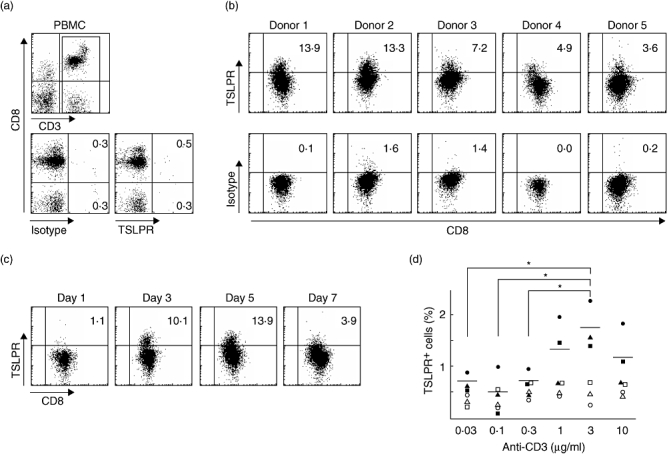
TCR stimulation induces TSLPR expression on human CD8+ T cells. (a) PBMCs were stained with mAb to CD3, CD8 and TSLPR or isotype control. Cell surface marker phenotypes were determined by flow cytometry. Lower panels represent staining of CD3+ cells with TSLPR or isotype control. Numbers in quadrants indicate the percentages of cells for each given phenotype in CD3+ cells. Data represent one of five independent experiments. (b, c) Purified human CD8+ T cells were stimulated for 5 days (b) or indicated days (c) with immobilized anti-CD3 plus soluble anti-CD28, and numbers in quadrants indicate the percentages of cells for each given phenotype in CD3+CD8+ cells. Data shown from five individuals (b) and one of five independent experiments (c). (d) CD8+ T cells were stimulated with anti-CD28 and various concentrations of anti-CD3. Data shown are the percentages of TSLPR+ cells in CD3+CD8+ cells in three individual experiments (closed symbols). Horizontal bars indicate the mean of percentages of TSLPR+ cells and open symbols show isotype control from individuals. P-values as determined by the paired t-test. *P < 0·05.
TCR stimulation induces brief expression of TSLPR on CD8+ T cells
Next, we monitored TSLPR-expressing cells in CD8+ T cells from 1 day to 7 days of culture with anti-CD3 plus anti-CD28 by flow cytometry. The induction of TSLPR on CD8+ T cell surface by TCR stimulation was detectable by 3 days and reached a maximal level at 3–5 days after TCR stimulation (Fig. 1c). However, the percentages of TSLPR+ cells in CD8+ T cells decreased after 7 days of culture, suggesting that TCR stimulation induces the brief expression of TSLPR on CD8+ T cells.
TSLPR induction of CD8+ T cells depends on the strength of TCR stimulation
To further analyse the characteristics of TSLPR induction of CD8+ T cells, we examined TSLPR induction on CD8+ T cells in different concentrations of immobilized anti-CD3 in the presence of anti-CD28. The induction of TSLPR on CD8+ T cell surface by TCR stimulation was detectable when we used 1 µg/ml of immobilized anti-CD3; it reached a maximal level at 3 µg/ml of anti-CD3 and was reduced at 10 µg/ml (Fig. 1d). These data suggest that the induction of TSLPR on CD8+ T cells depends on the strength of TCR stimulation and may occur only within a narrow ‘window’ of the strength of TCR stimulation.
CD8+ T cells express TSLPR on their surface in mixed lymphocyte reaction (MLR)
We showed that human CD8+ T cells expressed TSLPR on their surface when we used anti-CD3 and anti-CD28 for polyclonal TCR stimulation. Next, we assessed whether human CD8+ T cells express TSLPR on their surface in MLR in which CD8+ T cells were co-cultured with activated allogeneic MoDCs. Purified human CD8+ T cells were stimulated for 7 days with allogeneic MoDCs at a DC : T cell ratio of 1:2. After 7 days of co-culture, 99·6% of remaining viable cells showed CD3+CD8+, and these CD8+ T cells contained 11·2% of TSLPR+ cells (Fig. 2). These data suggest that human CD8+ T cells express TSLPR on their surface in MLR.
Fig. 2.

CD8+ T cells express TSLPR on their surface in mixed lymphocyte reaction. Purified human CD8+ T cells were cultured with allogeneic MoDCs for 7 days at a DC : T cell ratio of 1:2. Cell surface marker phenotypes were determined by flow cytometry as in Fig. 1b. Data represent one of five independent experiments.
TSLP directly enhances CD8+ T-cell expansion induced by TCR stimulation
Human TSLP directly enhances the expansion of activated CD4+ T cells expressing TSLPR [9]. To examine whether human TSLP directly enhances the expansion of activated CD8+ T cells, purified CD8+ T cells stimulated with anti-CD3 plus anti-CD28 were traced for cell division by using the CFSE dilution method. Without TSLP, TCR stimulation resulted in cell division of input CD8+ T cells after 6 days of culture; these divided T cells underwent further divisions in the following 2 days of culture (Fig. 3a, upper panels). In contrast, although addition of TSLP on day 5 did not affect the cell division of CD8+ T cells on day 6, TSLP induced a larger fraction of dividing cells in the following 2 days of culture (Fig. 3a, lower panels). In addition, fold expansion of activated CD8+ T cells cultured with TSLP was significantly greater than that without TSLP (Fig. 3b). These data indicate that human TSLP directly enhances the expansion of CD8+ T cells activated with TCR stimulation.
Fig. 3.
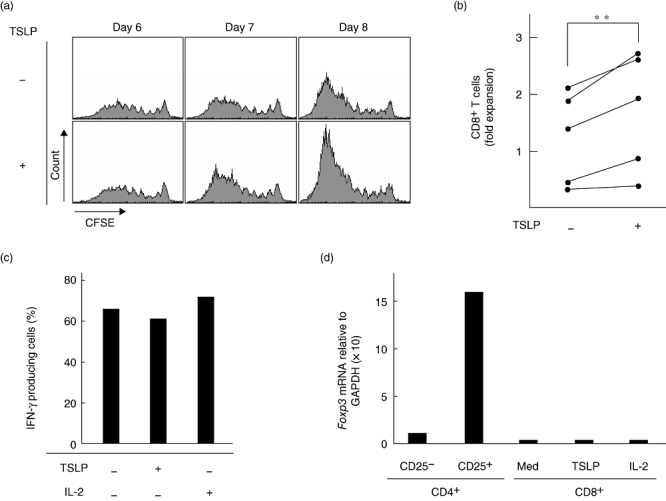
TSLP directly enhances expansion of functional CD8+ T cells induced by TCR stimulation. (a) Cell divisions of CFSE-labelled purified human CD8+ T cells stimulated with anti-CD3 plus anti-CD28 with or without TSLP for indicated days. Filled histograms represent CFSE dilution in CD8+ T cells. Data represent one of five individual experiments. (b) Closed circles indicate fold expansion of CD8+ T cells stimulated with anti-CD3 plus anti-CD28 with or without TSLP compared with the initial CD8+ T-cell number in five individual experiments. The lines indicate CD8+ T cells from the same individual. (**P < 0·05). (c) The percentages of IFN-γ producing cells in CD8+ T cells using intracellular cytokine staining. CD8+ T cells were stimulated with anti-CD3 plus anti-CD28 with or without TSLP or IL-2 and restimulated with PMA plus ionomycin. Data represent one of three independent experiments. (d) Expression levels of mRNA encoding Foxp3 were measured using the real-time quantitative RT-PCR. CD8+ T cells were stimulated with anti-CD3 plus anti-CD28 with or without TSLP or IL-2. Data represent one of three independent experiments.
CD8+ T cells expanded by TCR plus TSLP stimulation produce IFN-γ, but do not express forkhead box P3(Foxp3) mRNA
Because of the indirect effect of TSLP on differentiation of CD4+Foxp3+ Treg cells in humans [6], we examined whether CD8+ T cells directly expanded by TCR plus TSLP stimulation are functional. We cultured CD8+ T cells under the stimulation of anti-CD3 plus anti-CD28 with or without TSLP or IL-2 and evaluated cytokine production capacity using intracellular cytokine staining of expanded T cells restimulated with PMA plus ionomycin and the expression level of Foxp3 mRNA using real-time quantitative RT-PCR. Intracellular cytokine staining of CD8+ T cells demonstrated that the percentages of IFN-γ producing cells in CD8+ T cells cultured with TSLP is similar to those of CD8+ T cells cultured with IL-2 or medium alone (Fig. 3c). In addition, CD8+ T cells expanded by TSLP did not express Foxp3 mRNA (Fig. 3d). These data suggest that CD8+ T cells expanded by TCR plus TSLP stimulation have cytotoxic activity, but not regulatory function.
TSLP enhances expansion of CMV-specific CD8+ T cells after TCR engagement
Both CD11c+ blood myeloid DCs and mature MoDCs expressed TSLPR and responded to TSLP, resulting in enhanced CD80 expression (Fig. 4). Although percentages of CD11c+ blood myeloid DCs in PBMCs are less than 1·0%, MoDCs are easily generated from PBMCs and widely used for clinical application of antigen (Ag)-specific T-cell expansion.
To assess whether human TSLP can enhance expansion of Ag-specific CD8+ T cells after TCR engagement in the clinical setting, we purified CD8+ T cells from HLA-A24-positive healthy donors and cultured these T cells with autologous MoDCs pulsed with HLA-A24-restricted CMVpp65 peptide in the presence or absence of TSLP. After 7 days of culture, tetramer positive CMV-specific CD8+ T cells cultured with TSLP expanded more greatly than cells cultured without TSLP (Fig. 5a and b and Table 1). In contrast, TSLP did not affect expansion of tetramer negative non-specific CD8+ T cells (Fig. 5b and Table 1). These data indicate that human TSLP enhances expansion of Ag-specific CD8+ T cells in co-culture with MoDCs which are widely used for clinical application of Ag-specific T-cell expansion.
Fig. 5.
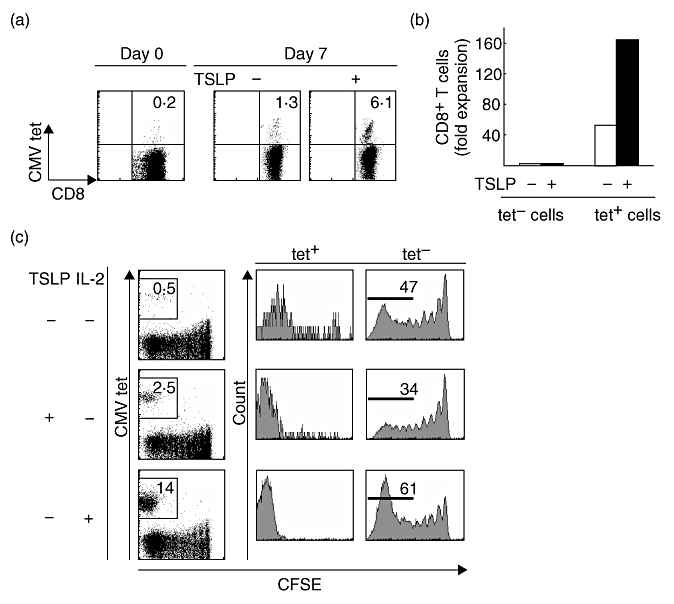
Human TSLP induces predominant expansion of CMV-specific CD8+ T cells. (a) Purified human CD8+ T cells were stimulated with autologous MoDCs pulsed with HLA-A24-restricted CMVpp65 peptide for 7 days with or without TSLP at a DC : T cell ratio of 1:2. Cells were stained with mAb to cell surface marker phenotypes and HLA-A2402-CMVpp65 tetramer. (b) Fold expansion of tetramer-CD8+ T cells and tetramer+CD8+ T cells after 7 days of culture with (filled bars) and without (open bars) TSLP. Data represent one of 12 independent experiments. (c) Cell divisions of CFSE-labelled purified human CD8+ T cells stimulated for 7 days with autologous MoDCs pulsed with CMVpp65 peptide (upper panels) or with either TSLP (middle panels) or IL-2 (lower panels). Cells were stained with anti-CD8 and CMVpp65 tetramer. Numbers indicate the percentages of proliferated tetramer+CD8+ T cells (left panels) and proliferated tetramer-CD8+ T cells (right panels) in CD8+ cells. Data represent one of five independent experiments.
Table 1.
The effect of TSLP on fold expansion of CMV-specific CD8+ T cells.
| CD8+ T cells | TSLP stimulation | Fold expansion |
|---|---|---|
| Tetramer−, n = 12 | − | 1·6 ± 0·8 |
| + | 1·7 ± 0·9* | |
| Tetramer+, n = 12 | − | 60·6 ± 83·3 |
| + | 101·5 ± 111·5** |
Fold expansions of HLA-A2402-CMVpp65 tetramer- and tetramer+CD8+ T cells from 12 individuals after 7 days of culture with or without TSLP under the same conditions as Fig. 5a and b. The data shown are the mean ± SD. P-values versus fold expansion of the cells cultured without TSLP as determined by the paired t-test.
P = 0·39,
P < 0·05.
TSLP predominantly enhances expansion of Ag-stimulated CD8+ T cells
To further test whether TSLP predominantly enhances the expansion of Ag-specific CD8+ T cells after TCR engagement, we traced CD8+ T cell division by using the CFSE dilution method for 7 days. TSLP enhanced cell division of tetramer positive CMV-specific CD8+ T cells (Fig. 5c, left and middle panels), but reduced the population of divided tetramer negative non-specific CD8+ T cells (Fig. 5c, right panels). In contrast, IL-2 enhanced cell division of both tetramer-positive and -negative CD8+ T cells (Fig. 5c, lower panels). These data suggest that in comparison with IL-2, TSLP induces predominant expansion of Ag-specific CD8+ T cells after TCR engagement.
Discussion
In the present study, we showed that human TSLP directly acts on activated CD8+ T cells. TCR stimulation induced expression of the functional receptor for TSLP in CD8+ T cells, and the activated CD8+ T cells directly responded to TSLP, resulting in their enhanced expansion. The direct action of TSLP on CD4+ T cell proliferation has recently been found in humans [9]. Moreover, TSLP-activated DCs induce the proliferation of both CD4+ and CD8+ T cells [1–5]. Therefore, human TSLP enhances proliferation of not only CD4+ but also CD8+ T cells, both directly and indirectly through DC activation. Although the species difference had appeared to exist in the effect of TSLP on DCs in humans and on DCs, T cells and mast cells in mice, the direct action of TSLP on T cells has become evident in humans, implying that human TSLP might additionally act on other unknown cells in the immune system.
IL-7, IL-15 and IL-2 bind to multimeric receptors that share the common γ chain (γc) and directly act on human CD8+ T cells [15–17]. IL-7 binds to heterodimeric receptors composed of IL-7Rα and γc, whereas IL-15 binds to heterotrimeric receptors composed of IL-15Rα, IL-2/15Rβ and γc. All these subunits of receptors are expressed on resting human CD8+ T cells. IL-7 and IL-15 can induce the proliferation of human CD8+ T cells in the absence of TCR stimulation in vitro[17,18]. In contrast, IL-2Rα, which comprises the functional IL-2 receptor, is not expressed at significant levels in resting T cells; instead, expression of IL-2Rα is induced in T cells after TCR engagement. After activation by TCR engagement, IL-2 can efficiently enhance proliferation of human CD8+ T cells in vitro[17,18]. In this study, we showed that although TSLPR was not expressed in resting human CD8+ T cells, it was induced by TCR stimulation. Xu et al. reported that TSLPR is up-regulated by TSLP on MoDCs [19]. However, even in the presence of TSLP, IL-7 and IL-15, human CD8+ T cells cultured without anti-CD3 plus anti-CD28 stimulation did not express TSLPR (data not shown). In addition, the stimulation of anti-CD3 plus anti-CD28 induced TSLPR, even in the absence of TSLP, IL-7 and IL-15; moreover, percentages of TSLPR+CD8+ T cells did not differ in the presence or absence of those cytokines (data not shown). These data suggest that TCR stimulation is sufficient to induce cell surface expression of TSLPR in human CD8+ T cells.
Lee et al. reported that in mice, TSLP promotes the differentiation of Treg cells from CD4+CD8-CD25- single-positive thymocytes in a DC-independent manner [20]. In addition, we previously showed that the effect of human TSLP on Treg cell differentiation is restricted to the CD4+CD8-CD25- single-positive thymocyte stage in the human thymus [6]. However, CD8+ T cells expanded with TSLP produced IFN-γ (Fig. 3c) and did not express Foxp3 mRNA (Fig. 3d). Thus, it is not likely that the direct action of human TSLP on CD8+ T cells induces Treg cell differentiation.
In this study, we showed that in comparison to IL-2, TSLP induced exclusive expansion of CMV-specific CD8+ T cells after TCR engagement. One possibility is that indirect action of TSLP through MoDC activation affects the exclusive expansion of CMV-specific CD8+ T cells. There is also another possible explanation for our results. Although both IL-2Rα and TSLPR are induced after TCR engagement, the induction of TSLPR on CD8+ T cells may occur only within a narrow ‘window’ related to the strength of TCR stimulation (Fig. 1d). In addition, most naive CD8+ T cells down-regulate IL-7Rα after TCR activation, while memory and effector CD8+ T cells can selectively retain IL-7Rα expression [21,22]. Because tetramer positive CMV-specific CD8+ T cells show the effector function and memory phenotype [13,14,23,24], after TCR engagement, CMV-specific CD8+ T cells may retain IL-7Rα. Taken together, TCR engagement by CMVpp65-peptide–MHC complexes may induce efficient TSLPR expression and retain IL-7Rα in CMV-specific CD8+ T cells, resulting in TSLP mediated selective proliferation of these cells.
Recent studies have shown the efficacy of Ag-specific CD8+ T-cell transfer therapies for treatment of patients with selected metastatic cancers [25,26]. The procedure of adoptive cell transfer includes the isolation of Ag-specific CD8+ T cells, their ex vivo expansion and activation, and subsequent autologous administration. IL-2 is useful for ex vivo expansion and activation of Ag-specific CD8+ T cells [25,26]. However, for therapeutic application in a variety of tumours, recent procedures using IL-2 appear not to be sufficient to generate large numbers of tumour-specific CD8+ T cells [27–29]. In this study, we showed that in comparison to IL-2, TSLP induced predominant expansion of Ag-specific CD8+ T cells after TCR engagement, suggesting that human TSLP may contribute to efficient expansion of tumour-specific CD8+ T cells ex vivo for potential therapeutic application.
In conclusion, we demonstrated that TCR stimulation induced TSLPR expression in human CD8+ T cells and that TSLP then directly enhanced proliferation of the activated CD8+ T cells. Because TSLP is expressed in epithelial cells of mucosal lymphoid tissues in physiological conditions [3], the direct action of TSLP on TCR-stimulated CD8+ T cells might contribute to enhancement of protective immune responses in the mucosa against invading microbes, leading to their eradication from the host. In addition, the direct and indirect action of human TSLP on expansion of target Ag-specific CD8+ T cells after TCR engagement suggests that TSLP may be a useful tool for efficient adoptive cell transfer of immunotherapy.
Acknowledgments
We thank Ms Yoshimi Yamakawa for assistance in preparation of the manuscript. This work is supported by Grants-in-aid for Scientific Research, 17659212, 18012029, 18015028, 18209027 and 18590679 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, Grant-in-Aid for Research on Measures for Intractable Diseases, and Research on Advanced Medical Technology from the Ministry of Health, Labor and Welfare, Japan, and Grant-in-Aid by the Uehara Memorial Foundation, Takeda Science Foundation, Mochida Memorial Foundation for Medical and Pharmaceutical Research, Novartis Foundation for the Promotion of Science, Astellas Foundation for Research on Metabolic Disorders, Yakult Bioscience Research Foundation, and Japan Leukemia Research Fund. T. Akamatsu is a Research Fellow of the Japan Society for the Promotion of Science and supported by Research Fellowships of the JSPS for Young Scientists.
References
- 1.Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43. doi: 10.4049/jimmunol.167.1.336. [DOI] [PubMed] [Google Scholar]
- 2.Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe N, Hanabuchi S, Soumelis V, et al. Human thymic stromal lymphopoietin promotes dendritic cell-mediated CD4+ T cell homeostatic expantion. Nat Immunol. 2004;5:426–34. doi: 10.1038/ni1048. [DOI] [PubMed] [Google Scholar]
- 4.Wang YH, Ito T, Wang YH, et al. Maintenance and polarization of human Th2 central memory T cells by thymic stromal lymphopoietin-activated dendritic cells. Immunity. 2006;24:827–38. doi: 10.1016/j.immuni.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 5.Gilliet M, Soumelis V, Watanabe N, et al. Human dendritic cells activated by TSLP and CD40L induce proallergic cytotoxic T cells. J Exp Med. 2003;197:1059–63. doi: 10.1084/jem.20030240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watanabe N, Wang YH, Lee HK, et al. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 7.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–14. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 8.Liu YJ, Soumelis V, Watanabe N, et al. TSLP: an epithelial cell cytokine that regulates T cell differentiation by conditioning dendritic cell maturation. Annu Rev Immunol. 2007;25:193–219. doi: 10.1146/annurev.immunol.25.022106.141718. [DOI] [PubMed] [Google Scholar]
- 9.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–4. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 10.Pickl WF, Majdic O, Kohl P, et al. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996;157:3850–9. [PubMed] [Google Scholar]
- 11.Kadowaki N, Antonenko S, Ho S, et al. Distinct cytokine profiles of neonatal natural killer T cells after expansion with subsets of dendritic cells. J Exp Med. 2001;193:1221–6. doi: 10.1084/jem.193.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different Toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–9. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuzushima K, Hayashi N, Kimura H, Tsurumi T. Efficient identification of HLA-A*2402-restricted cytomegalovirus-specific CD8+ T-cell epitopes by a computer algorithm and an enzyme-linked immunospot assay. Blood. 2001;98:1872–81. doi: 10.1182/blood.v98.6.1872. [DOI] [PubMed] [Google Scholar]
- 14.Akiyama Y, Kuzushima K, Tsurumi T, Yamaguchi K. Analysis of HLA-A24-restricted CMVpp65 peptide-specific CTL with HLA-A*2402-CMVpp65 tetramer. Immunol Lett. 2004;95:199–205. doi: 10.1016/j.imlet.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–79. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- 16.Kovanen PE, Leonard WJ. Cytokines and immunodeficiency disease: critical roles of the γc-dependent cytokines interleukins 2, 4, 7, 9, 15, and 21, and their signaling pathways. Immunol Rev. 2004;202:67–83. doi: 10.1111/j.0105-2896.2004.00203.x. [DOI] [PubMed] [Google Scholar]
- 17.Ma A, Koka R, Burkett P. Diverse functions of IL-2, IL-15, and IL-7 in lymphoid homeostasis. Annu Rev Immunol. 2006;24:657–79. doi: 10.1146/annurev.immunol.24.021605.090727. [DOI] [PubMed] [Google Scholar]
- 18.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–6. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 19.Xu W, He B, Chiu A, et al. Epithelial cells trigger frontline immunoglobulin class switching through a pathway regulated by the inhibitor SLPI. Nat Immunol. 2007;8:294–303. doi: 10.1038/ni1434. [DOI] [PubMed] [Google Scholar]
- 20.Lee JY, Lim YM, Park MJ, et al. Murine thymic stromal lymphopoietin promotes the differentiation of regulatory T cells from thymic CD4+CD8-CD25- naïve cells in a dendritic cell-independent manner. Immunol Cell Biol. 2008;86:206–13. doi: 10.1038/sj.icb.7100127. [DOI] [PubMed] [Google Scholar]
- 21.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 22.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 23.Chen G, Shankar P, Lange C, et al. CD8 T cells specific for human immunodeficiency virus, Epstein–Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–64. doi: 10.1182/blood.v98.1.156. [DOI] [PubMed] [Google Scholar]
- 24.Pittet MJ, Zippelius A, Speiser DE, et al. Ex vivo IFN-γ secretion by circulating CD8 T lymphocytes: implications of a novel approach for T cell monitoring in infectious and malignant diseases. J Immunol. 2001;166:7634–40. doi: 10.4049/jimmunol.166.12.7634. [DOI] [PubMed] [Google Scholar]
- 25.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gattinoni L, Powell DJ Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S, Riley J, Rosenberg S, Parkhurst M. Comparison of common γ-chain cytokines, interleukin-2, interleukin-7, and interleukin-15 for the in vitro generation of human tumor-reactive T lymphocytes for adoptive cell transfer therapy. J Immunother. 2006;29:284–93. doi: 10.1097/01.cji.0000190168.53793.6b. [DOI] [PubMed] [Google Scholar]
- 28.Leen AM, Rooney CM, Foster AE. Improving T cell therapy for cancer. Annu Rev Immunol. 2007;25:243–65. doi: 10.1146/annurev.immunol.25.022106.141527. [DOI] [PubMed] [Google Scholar]
- 29.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8:299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]


