Abstract
Maternal autoantibodies to the p200-epitope of Ro52 have been suggested to correlate with development of congenital heart block. The aim of the present study was to evaluate the clinical relevance and predictive value of p200-antibodies in high-risk pregnancies. Sera from 515 Finnish, Swedish and American women were included in the study. Sera originated from 202 mothers with an infant affected by second- or third-degree atrioventricular block (AVB), 177 mothers with rheumatic disease having infants with normal heart rate and female blood donors (n = 136). A novel serological assay for Ro52 p200-antibodies with intra- and inter-assay variability of 3% and 3·8% respectively was developed. Mothers of children affected by AVB II-III had significantly higher p200-antibody levels than mothers with rheumatic disease having children with normal heart rate (P < 0·001). In the Swedish cohort, a distinction between foetuses with normal conduction, AVB I, AVB II and III was possible. A significant difference in anti-p200 levels between AVB I and AVB II-III groups compared with foetuses with normal conduction (P < 0·05 and P < 0·01) was observed. Using p200-antibodies as a second step analysis in Ro52-positive pregnancies increased the positive predictive value for foetal cardiac involvement (AVB I, II or III) from 0·39 (0·27–0·51) to 0·53 (0·37–0·68). In conclusion, Ro52 p200-antibodies may occur in women with unaffected children, but levels are significantly higher in mothers of children with congenital heart block and are suggested as a relevant marker in evaluating the risk for foetal AV block.
Keywords: autoantibodies, congenital heart block, p200, Ro52
Introduction
Congenital heart block is a potentially lethal condition, affecting foetuses of women with Ro/SSA autoantibodies. During pregnancy, autoantibodies from the mother are transported across the placenta and may affect the developing child. Congenital heart block is a rare disease with an incidence of 1/15 000–20 000 in the general population [1], but the prevalence of complete congenital heart block in women with anti-Ro/SSA antibodies is about 2% [2], and higher in subpopulations of Ro/SSA-positive mothers defined by their autoantibody profiles [3–7]. The mortality of affected infants is 15–30% [8,9], and the majority of live born children will need life-long pacemaker implants [9–12]. Congenital heart block usually develops during the 18–24th week of gestation. It may be initiated as a first-degree atrioventricular (AV) block [4], and case reports suggest that introduction of steroid treatment before a complete block has developed can inhibit progression or even revert the block [4,13–18], stressing the importance of identifying the high-risk pregnancies and of close monitoring during susceptibility weeks.
Maternal antibodies to the amino acid (aa) 200–239 (p200) of the Ro52 protein have been suggested as a serological marker for an increased risk of having a child with congenital heart block [19]. To investigate the clinical relevance and predictive value of Ro52-p200 antibodies, we developed a novel highly reproducible assay, taking into account the structural features and constraints of the alpha-helical p200 peptide. Congenital heart block is a rare disease, and to investigate a substantial number of cases we used the developed assay to analyse biobanks of sera from mothers of foetuses with congenital heart block in Finland, Sweden and the United States, including a total of 515 sera with 202 cases of AVB II-III.
Materials and methods
Patients and controls
The study included sera from 515 women (Table 1). These women were from Helsinki University Hospital in Finland (n = 194) and Karolinska University Hospital in Sweden (n = 169) and the US Registry for Neonatal Lupus (n = 152). A total of 202 cases of AVB II-III were included. One hundred and seventy-seven sera originated from mothers with rheumatic disease and/or Ro52 antibodies giving birth to infants without AVB II-III. These were classified as normal heart rate.
Table 1.
Patients included in the study. Number of Finnish, Swedish and American mothers in the study, their diagnoses, presence of Ro52 autoantibodies and pregnancy outcome.
| Country of origin | Total no. of patients and controls | Foetal diagnoses | Maternal diagnoses (AVB II/III, NHR)† | Ro52+ mothers (AVB II/III, NHR) | Blood donors | ||||
|---|---|---|---|---|---|---|---|---|---|
| AVB II/III | NHR‡ | pSS | SLE | Other§ | No Info. | ||||
| Finland | 194 | 67 | 96 | 62 (31, 31) | 59 (7, 52) | 41 (28, 13) | 1 (1, 0) | 94 (60, 34) | 31 |
| Sweden | 169 | 10 | 54¶ | 17 (5, 12) | 30 (2, 28) | 17 (3, 14) | 0 (0, 0) | 58 (10, 48) | 105 |
| USA | 152 | 125 | 27 | 34 (27, 7) | 36 (27, 9) | 56 (46, 10) | 26 (25, 1) | 122 (101, 21) | 0 |
| Total | 515 | 202 | 177 | 113 (63, 50) | 125 (36, 89) | 114 (77, 37) | 27 (26, 1) | 274 (171, 103) | 136 |
Figures in parenthesis refer to the diagnoses of the foetus of the mothers.
NHR: normal heart rate. Infants without second- or third-degree heart block. The group includes both foetuses with AVB I and normal conduction.
Other refers to other rheumatic diseases (RA, MCTD, UCTD, scleroderma) or the presence of Ro52 antibodies without a defined autoimmune disease.
Thirteen cases with signs of first-degree heart block and 41 with normal conduction as mid-gestational foetuses.
The details of the Finnish [20] and US [21] patients and collection of corresponding samples have been described previously. All Swedish patients were systematically followed with foetal Doppler echocardiography during mid-trimester pregnancy, and the group with normal heart rate was further divided into two groups based on the foetal findings; AVB I was defined as at least two examinations where the Doppler atrioventricular time intervals exceeded the 95% reference range based on recordings from 284 women with normal pregnancies [4,22], and those with normal atrioventricular conduction (NC). Twenty-five of the Swedish patients have been previously described [4,23].
Sera were sampled from the mothers during or after pregnancy. Sera from 136 female Finnish and Swedish blood donors between 18 and 54 years of age were used as normal control sera (Table 1). Human ethical review boards in the respective countries approved the investigations, and informed consent was given by the mothers.
Peptide synthesis
A synthetic peptide representing aa 200–239 of Ro52 was synthesized by Thermo Biosciences, Ulm, Germany, with biotin conjugated at the N-terminal end. Peptide purity was confirmed by high performance liquid chromatography (HPLC) and mass spectrometry.
Enzyme-linked immunosorbent assay for antibodies binding the p200 peptide
High-binding 96-well plates (Nunc) were coated with 100 µl of 3 µg/ml streptavidin diluted in water. Plates were incubated at +4°C for 2 days, and then dried at 37°C and stored at +4°C until use. Plates were washed four times with wash buffer (0·15 M NaCl, ×0·006 M NaH2PO4·H2O, 20% NaN3/0·05% Tween-20/2% BSA) and unspecific binding blocked with 200 µl 4% BSA in PBS. Plates were washed once with PBS and coated for at least 6 h at room temperature with 100 µl of 3 µg/ml biotin-p200 peptide in coating buffer (0·03 M Na2CO3, 0·07 M NaHCO3, 0·1% NaN3). Plates were washed four times with wash buffer. One hundred µl serum was added per well at a dilution of 1:300 and plates were incubated by shaking at room temperature for 2 h. Plates were washed four times and affinity-purified alkaline phosphatase (AP)-conjugated, rabbit anti-human IgG antibodies (Dakopatts, Glostrup, Denmark) were added at a dilution of 1:1000. Plates were washed four times with wash buffer. As substrate, phosphatase substrate tablets (Sigma, St Louise, MO, USA) were dissolved in diethanolamine pH 9·8, and 100 µl incubated in the wells for 2 h at room temperature for detection of IgG. The absorbance was measured at 405 nm using a Sunrise absorbance reader (Tecan) and the Magellan V 3·11 software. A p200 index was calculated based on a ratio with one high-titre patient selected as standard where the p200 index = [(OD sample − OD negative control)/(OD positive control − OD negative control)]*100. All p200 assays in this study were run in the same laboratory, at the Department of Clinical Immunology, Karolinska Institute.
Ro52 autoantibodies were detected in routine serology at the respective hospital, or by ELISA as previously described [3].
Statistical analysis
Statistical analysis was performed using Statistica 7·0 (Statsoft, Tulsa, OK, USA). Shapiro–Wilk's W test demonstrated that our data did not fit a normal distribution, and therefore the Mann–Whitney U-test or Kruskal–Wallis anova was used for statistical analysis. The level of significance was set at P < 0·05.
The University of British Columbia calculator (http://www.healthcare.ubc.ca) was used for Bayesian calculations. Receiver operating characteristics (ROC) curve analysis was performed to calculate sensitivity and specificity of the ELISA for p200 antibodies using the Med Calc program.
Results
Detecting Ro52-p200 antibodies with low intra- and inter-assay variability
A novel, highly reproducible assay for detection of Ro52-p200 specific antibodies was developed. To allow free folding of the alpha helical p200 sequence and to give a set orientation to the peptide during assay performance, biotin was conjugated at the N-terminal end during synthesis before coating to streptavidin-plates and subsequent ELISA. This was of importance for the Ro52-derived p200 peptide, as the epitope formation and antigenicity of aa 200–239 of Ro52 is dependent on correct folding and structure [24]. Patients with high, intermediate or low p200 antibody levels were selected for determining intra- and inter-assay variability, which were established at 3% and 3·8%, respectively.
Ro52-p200 antibodies correlate with AVB II-III
Investigating the sera from the Finnish, Swedish and American cohorts, we first performed an analysis of all 515 samples from the different populations together (Table 1). Sera were grouped as originating from: mothers of children with AVB II-III, mothers with an autoimmune rheumatic disease and/or Ro52-positive mothers who had children with normal heart rate (NHR), and healthy blood donors. We found a significantly higher level of p200 specific antibodies in sera from mothers of children with AVB II-III, both compared with mothers of children with NHR (P < 0·0001) and healthy blood donors (P < 0·00001) (Fig. 1).
Fig. 1.
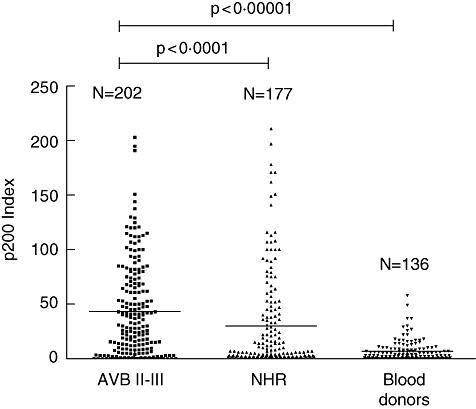
p200 levels in mothers of foetuses with or without AVB II-III. Antibodies to the p200 peptide in maternal sera were measured by ELISA. Sera from all mothers included in the study (n = 515) were tested and p200 antibody levels compared between mothers of foetuses with AVB II-III and mothers with rheumatic disease and foetuses with normal heart rate (NHR), P < 0·0001. The study population includes mothers from Finland, Sweden and the USA. Female blood donors were from Finland (n = 31) and Sweden (n = 105).
p200 antibodies in Ro52-positive mothers and in the Finnish, Swedish and American cohorts
While the difference in maternal p200 antibody levels between the groups with children affected by, and not affected by, AVB II-III was statistically significant, a central question is whether p200 antibodies are a more sensitive and specific marker of congenital heart block than Ro52 antibodies. We therefore performed an analysis of p200 antibody levels exclusively in Ro52-positive sera. In the analysis we noted substantial differences in p200 levels between the three populations (Fig. 2), therefore Ro52-positive sera from each cohort were analysed separately. We found significantly higher levels of p200 antibodies in sera from mothers of foetuses with AVB II-III compared with mothers whose foetuses had NHR in the Swedish and American cohorts (P < 0·05 and P < 0·05, respectively), but not in the Finnish cohort (Fig. 2).
Fig. 2.
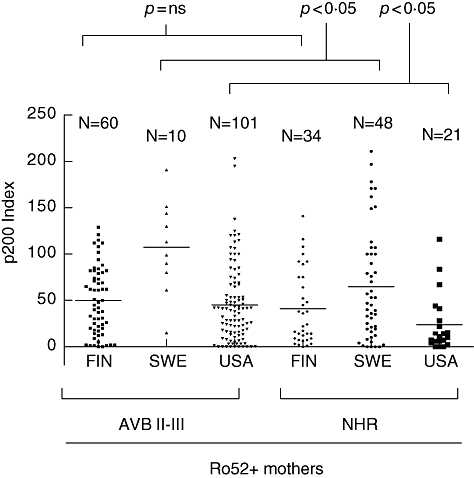
Levels of p200 antibodies in Ro52-positive Finnish, Swedish and American mothers of foetuses with or without AVB II-III. The cohorts were separated to allow comparisons between the three nationalities. Only Ro52-positive women are included. Separating the populations, the p200 levels are significantly different between mothers of foetuses with AVB II-III and mothers of foetuses with normal heart rate (NHR) in the Swedish and American populations, but not in the Finnish population.
Maternal diagnosis and p200 levels
We further analysed the p200 antibody levels in Ro52-positive women with foetuses affected by AVB II-III and foetuses with NHR in relation to the diagnosis of the mothers (Table 1 and Fig. 3). While a graphic plot of the p200 values indicated a trend toward differences (Fig. 3a), anova analysis of p200 levels did not reveal significant differences in the AVB II-III versus NHR pregnancies in the Finnish, Swedish or US cohorts when subdivided according to maternal diagnoses of Sjögren's syndrome, SLE or other rheumatic disease (denoted ‘Other’, and including rheumatoid arthritis, myositis, undifferentiated connective tissue disease, scleroderma or asymptomatic women with Ro52 antibodies). However, by using the Mann–Whitney U-test a significant difference was observed in p200 antibody levels in the diagnostic group ‘Other’ in mothers of foetuses with AVB II-III compared with foetuses with NHR (P < 0·005).
Fig. 3.
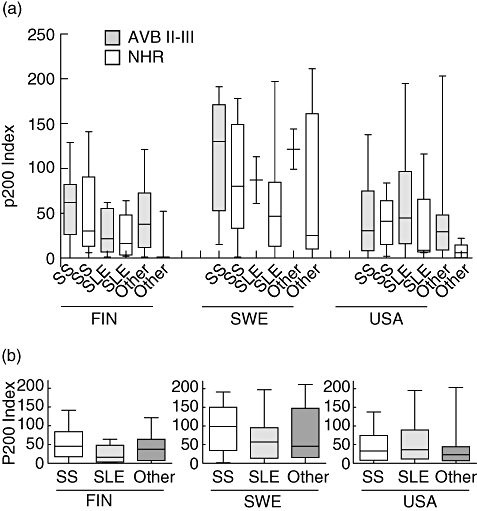
Maternal diagnosis and p200 levels in Finnish, Swedish and American patients. (a) The nationally grouped Ro52-positive mothers were stratified also by their diagnosis of SS, SLE or other rheumatic disease (including RA, MCTD, UCTD, scleroderma or asymptomatic women with Ro52 antibodies). (b) Analysing the Ro52-positive mothers, disregarding the infant diagnosis, the Finnish mothers with SS have significantly higher levels of p200 levels than mothers with SLE (P < 0·05).
When p200 antibody levels were analysed in relation to maternal diagnosis in the Finnish, Swedish and US cohorts, a difference was noted between mothers with Sjögren's syndrome and mothers with SLE in the Finnish material (P < 0·05) (Fig. 3b). The difference was not significant in patients from the other countries.
Increased levels of antibodies specific for p200 are found in AVB I, II and III
Congenital heart block may be initiated or present as a first degree heart block [4]. In the present study, all Swedish patients (n = 64) were systematically followed by serial Doppler echocardiographic recordings during pregnancy to detect signs of both first, second and third degree heart block. This made it possible to identify foetuses with signs of AVB I in the group of foetuses with NHR. By using anova it was demonstrated that the degree of foetal cardiac involvement was a highly significant factor of variance for the maternal p200 antibody levels (P < 0·005). Mothers of both foetuses with AVB II-III and those with signs of AVB I had higher p200 levels than those with a foetus without cardiac involvement (Fig. 4). No difference was found between mothers where the foetus had signs of AVB II-III or AVB I.
Fig. 4.
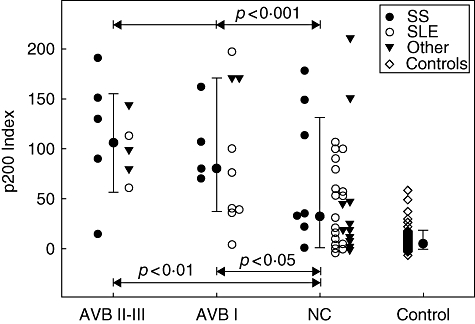
p200 antibody levels are equally high in AVB I and AVB II-III. In the Swedish cohort all pregnancies were systematically followed by foetal Doppler echocardiography, allowing further distinction of foetal diagnosis into AVB II-III, AVB I and normal conduction (NC). p200 antibody levels in mothers with AVB II-III as well as AVB I foetuses were significantly higher than p200 antibody levels in mothers of foetuses with NC. There was not a significant difference in p200 antibody levels between mothers of foetuses with AVB I and AVB II-III.
Determination of the p200 assay performance and predictive value of p200 antibodies
To evaluate the ability of the assay to discriminate AVB cases (AVB I, II, III) from cases without AVB, including normal controls, a receiver operating characteristics (ROC) curve analysis was performed in the Swedish cohort. The cut-off point of a p200 index = 30 gave the optimal assay performance with a sensitivity of 90·6% and a specificity of 96% (Fig. 5). The positive likelihood ratio defined as sensitivity/(1-specificity) was 23, indicating that a positive assay result is likely to identify presence of disease in the form of AV-conduction abnormality.
Fig. 5.
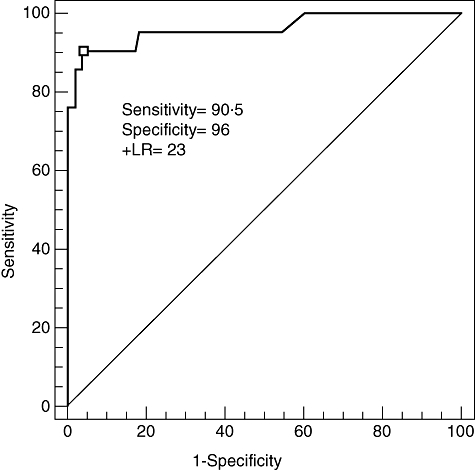
Discrimination performance of the p200 antibody ELISA. ROC analysis of the Swedish cohort including healthy controls optimizes the cut-off at the p200 index = 30, which results in a sensitivity of 90·5% and a specificity 96% and a positive likelihood ratio (+LR) of 23 of cardiac involvement (AVB I, II or III).
A potential clinical use of analysing p200 antibody levels would be to guide the clinician as to the risk of congenital heart block in an individual pregnancy. By using the p200 assay as a second step analysis in Ro52-positive women, the positive predictive value for detecting foetuses with AVB II-III increased from 0·17 (0·07–0·27, 95% confidence range) to 0·23 (0·10–0·35). For detecting foetuses with AVB I-III the corresponding increase in probabilities was from 0·39 (0·27–0·51) to 0·53 (0·37–0·68). The odds of obtaining a positive p200 antibody test result in a Ro52-positive AVB II-III and AVB I-III pregnancy compared with a woman with a NC foetus (+ LR) were 1·42 (1·06–1·91) and 1·73 (1·24–2·42), respectively. The negative likelihood ratios, the odds of obtaining a negative p200 antibody test result in Ro52-positive women with a AVB II-III or AVB I-III foetus compared with a NC pregnancy, were 0·27 (0·04–1·81) and 0·18 (0·05–0·72), respectively.
Discussion
In the present collaborative study we examined the relevance of specific Ro52 antibodies as maternal serological indicators of high risk for congenital heart block in the foetus during pregnancy. Assay development confirmed that stable conditions for testing can be established, and low intra- and interassay variability was obtained. Using the assay for analysis of the combined serological material from Finland, Sweden and the USA, there was a significant difference in p200 antibody levels between mothers of children affected and not affected by AVB II-III, but also an overlap in p200 levels between the two groups of mothers. The material was therefore stratified to identify potential groups of patients where p200 antibodies could be of practical clinical value. Further, we limited analysis of p200 levels to Ro52-positive sera, as the main objective of the study was to relate the value of p200 antibody analysis to Ro52 antibody analysis. In stratification, a difference in p200 levels between the three national cohorts became evident, where Finnish and American sera had generally lower p200 antibody levels than Swedish sera, and also when only Ro52-positive sera were included in analysis. In Swedish and American Ro52-positive sera, the p200 antibody levels were significantly higher in mothers of infants with AVB II-III than in mothers of infants with normal heart rate. However, another recent report analysing the levels of Ro52-p200 antibodies in sera from the Research Registry for Neonatal Lupus did not identify p200-binding antibodies as specific for sera of mothers of children with complete congenital heart block [25]. A potential reason for the discrepancy between the studies is the difference in assay format. Different conditions were used for peptide coating; either attaching p200 peptide directly to the plastic ELISA well surface [25], or by a biotin–streptavidin interaction via a biotin molecule conjugated to the p200 peptide during synthesis (the present study). The latter allows free folding of the peptide and creates a set orientation during assay performance, which is of importance as the epitope formation and antigenicity of aa 200–239 of Ro52 is strongly dependent on correct folding and structure [24]. A further difference, besides serum dilution and incubation times, is that the previous study [25] used reagents to detect p200 specific antibodies that may bind to several Ig-isotypes, and recorded the collective signal generated [26]. The present study focused detection at p200 antibodies of the IgG isotype only, as these are the immunoglobulins that are transferred across the placenta during pregnancy.
In Finnish sera the difference in p200 antibody levels between mothers of children with AVB II-III and with NHR was not significant, although p200 antibodies were detected in the sera from mothers of children with AVB II-III. The explanation for this could be that in the Finnish series mothers with children having non-diagnosed AVB I may be present in the NHR group, as these pregnancies were not monitored by Doppler echocardiography. Another factor that might explain why a difference between the groups was not observed in the Finnish cohort is that most of the sera from control pregnancies originated from women with primary Sjögren's syndrome. Analysis revealed that in the total Finnish cohort mothers with Sjögren's syndrome as a group had higher p200 antibody levels, which is in line with previous reports where higher Ro and La antibody levels have been observed [27].
The general difference in antibody levels between the whole national cohorts could be due to factors such as the time of sampling of sera in relation to the affected pregnancy or storage conditions, and ethnic or genetic differences. Studies of congenital heart block and collection of sera have been running for close to two decades in the Finnish and American groups, while investigations were more recently initiated by the Swedish researchers. Serum storage time will therefore naturally vary between the biobanks, and potentially also the number of times each individual sample has been thawed and re-frozen, factors both known to affect IgG stability [28]. The time of serum sampling in relation to the affected pregnancy also differs between the three national materials. The majority of Finnish mothers of children with congenital heart block were identified by register-based investigations, and blood samples taken after pregnancies, in some cases several decades after the affected pregnancy. Also the American material includes sera sampled years after birth of an affected child. While levels of Ro and La autoantibodies rarely vary to the extent of making a positive individual test negative, substantial variations over time have been demonstrated [29–31], and the timing of serum sampling may accordingly influence the result. Thus sera may not be directly comparable between the cohorts. Congenital heart block is, however, a rare disease and to analyse any substantial number of patients, collaborative efforts and pooling of existing clinical materials is needed. While differences may exist between the included biobanks and need to be kept in mind when interpreting the results, it is important to evaluate novel potential biomarkers in different materials to understand their applicability. The analysed serum banks constitute the, or some of the, largest collections worldwide and have taken decades to collect, and until prospective multicentre studies are initiated remain the best alternative for analysis.
Levels of p200 antibodies have been claimed to correlate with prolongation of the PR-interval [4], while a subsequent study did not confirm this observation [25]. The present study allowed an analysis of p200 antibody levels in an extended number of mothers of foetuses with AVB I, II and III, as well as in foetuses without cardiac involvement. Levels of p200 antibodies were equally high in mothers of children affected by AVB II-III and AVB I, and in Ro52-positive mothers an additional positive predictive value of p200 antibody analysis in identifying pregnancies complicated by foetal AV block was observed. The clinical long-term value of detecting AVB I in Ro52-positive pregnancies other than for excluding AVB II-III in the foetus remains an open question. This prolongation of the PR interval in utero appears spontaneously reversible at birth or shortly after, and in our opinion in the face of present data and collective knowledge does not call for any treatment. However, progression of AV-block postnatally in children of autoantibody positive women has been described, although it is rare [20,32]. It is possible that foetal AVB I is an indication of later conduction pathologies in life, but no data presently support this possibility. Prospective longitudinal follow-up studies will be required in order to understand whether foetuses with signs of AVB I in utero are at risk for late cardiac complications, and these are underway in our clinic.
Earlier attempts to define a specific antibody profile in mothers of children with CHB have demonstrated a prevalent, but not unique, anti-Ro 52-kDa antibody response [33–38]. Fritsch and colleagues recently presented data from longitudinally collected samples before, during and after pregnancies where congenital heart block occurred [38]. Their results indicate that antibodies to aa 1–13, 277–292 and 365–382 are elevated during week 18–30 when the AV block develops, and antibodies to aa 365–382 are of special interest as they may have a functional impact by cross-reacting with the 5-HT4 receptor. These investigators, however, did not include peptides corresponding to the p200 peptide used in the current study, therefore a direct comparison of results is not feasible. Previous studies have also suggested that antibodies to Ro 60-kDa have a minor role in predicting the clinical outcome in Ro- and La-positive mothers [9,34,39], while La antibodies were recently shown to add to the risk of developing congenital heart block [40]. The present study shows that analysis of p200 antibodies as a second step analysis in Ro52-positive women yields a positive predictive value for detecting foetuses with AVB I-III of 0·53 in the Swedish cohort where this analysis was possible, and may thus be clinically helpful in identifying high-risk pregnancies. However, the recurrence of congenital heart block in subsequent pregnancies is around 20% [8,9], stressing that other factors also influence foetal susceptibility or resistance to disease [41,42].
In conclusion, our data from an international collaborative study of the relevance of p200 specific Ro52 antibodies in congenital heart block demonstrate that p200 antibody levels are significantly higher in mothers who have pregnancies complicated by AVB II-III in the foetus. Also, when analysis was limited to Ro52-positive pregnancies p200 antibody levels of AVB II-III were higher than in pregnancies with normal heart rate in the foetus in the Swedish and American cohorts, but not the Finnish. Our data further show that p200 antibody levels are equally high in mothers of foetuses with signs of AVB I as in those of foetuses with signs of AVB II-III, and that a high likelihood ratio and predictive value for cardiac involvement using p200 antibody levels may be obtained by standardizing the method for analysis. In combination with foetal Doppler echocardiography, determination of Ro52-p200 antibody levels may prove a valuable clinical tool to identify pregnancies where the risk for congenital heart block is high, and allow treatment before the condition has progressed into a complete AV block.
Acknowledgments
This study was supported by the Swedish Research Council, the Swedish Foundation for Strategic Research, Karolinska Institutet, King Gustaf V:s 80-year Foundation, the Heart-Lung Foundation, the Swedish Rheumatism Association, the Stockholm City Council, NIH-NIAMS grants AR42455 and AR46265 (PRIDE study), and by patient data and sera from the Research Registry for Neonatal Lupus (NIAMS contract N01-AR-4-2271).
References
- 1.Michaelsson M, Engle MA. Congenital complete heart block: an international study of the natural history. Cardiovasc Clin. 1972;4:85–101. [PubMed] [Google Scholar]
- 2.Brucato A, Frassi M, Franceschini F, et al. Risk of congenital complete heart block in newborns of mothers with anti-Ro/SSA antibodies detected by counterimmunoelectrophoresis: a prospective study of 100 women. Arthritis Rheum. 2001;44:1832–5. doi: 10.1002/1529-0131(200108)44:8<1832::AID-ART320>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 3.Salomonsson S, Dorner T, Theander E, Bremme K, Larsson P, Wahren-Herlenius M. A serologic marker for fetal risk of congenital heart block. Arthritis Rheum. 2002;46:1233–41. doi: 10.1002/art.10232. [DOI] [PubMed] [Google Scholar]
- 4.Sonesson SE, Salomonsson S, Jacobsson LA, Bremme K, Wahren-Herlenius M. Signs of first-degree heart block occur in one-third of fetuses of pregnant women with anti-SSA/Ro 52-kd antibodies. Arthritis Rheum. 2004;50:1253–61. doi: 10.1002/art.20126. [DOI] [PubMed] [Google Scholar]
- 5.Gordon P, Khamashta MA, Rosenthal E, et al. Anti-52 kDa Ro, anti-60 kDa Ro, and anti-La antibody profiles in neonatal lupus. J Rheumatol. 2004;31:2480–7. [PubMed] [Google Scholar]
- 6.Buyon JP, Winchester RJ, Slade SG, et al. Identification of mothers at risk for congenital heart block and other neonatal lupus syndromes in their children. Comparison of enzyme-linked immunosorbent assay and immunoblot for measurement of anti-SS-A/Ro and anti-SS-B/La antibodies. Arthritis Rheum. 1993;36:1263–73. doi: 10.1002/art.1780360911. [DOI] [PubMed] [Google Scholar]
- 7.Buyon JP, Ben-Chetrit E, Karp S, et al. Acquired congenital heart block. Pattern of maternal antibody response to biochemically defined antigens of the SSA/Ro-SSB/La system in neonatal lupus. J Clin Invest. 1989;84:627–34. doi: 10.1172/JCI114208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eronen M, Siren MK, Ekblad H, Tikanoja T, Julkunen H, Paavilainen T. Short- and long-term outcome of children with congenital complete heart block diagnosed in utero or as a newborn. Pediatrics. 2000;106:86–91. doi: 10.1542/peds.106.1.86. [DOI] [PubMed] [Google Scholar]
- 9.Waltuck J, Buyon JP. Autoantibody-associated congenital heart block: outcome in mothers and children. Ann Intern Med. 1994;120:544–51. doi: 10.7326/0003-4819-120-7-199404010-00003. [DOI] [PubMed] [Google Scholar]
- 10.Buyon JP, Hiebert R, Copel J, et al. Autoimmune-associated congenital heart block: demographics, mortality, morbidity and recurrence rates obtained from a national neonatal lupus registry. J Am Coll Cardiol. 1998;31:1658–66. doi: 10.1016/s0735-1097(98)00161-2. [DOI] [PubMed] [Google Scholar]
- 11.Machado MV, Tynan MJ, Curry PV, Allan LD. Fetal complete heart block. Br Heart J. 1988;60:512–15. doi: 10.1136/hrt.60.6.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Groves AM, Allan LD, Rosenthal E. Outcome of isolated congenital complete heart block diagnosed in utero. Heart. 1996;75:190–4. doi: 10.1136/hrt.75.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Theander EBA, Gudmundsson S, Salomonsson S, Wahren-Herlenius M, Manthorpe R. Primary Sjögren's syndrome – treatment of fetal incomplete atrioventricular block with dexamethasone. J Rheumatol. 2001;28:373–6. [PubMed] [Google Scholar]
- 14.Carreira PE, Gutierrez-Larraya F, Gomez-Reino JJ. Successful intrauterine therapy with dexamethasone for fetal myocarditis and heart block in a woman with systemic lupus erythematosus. J Rheumatol. 1993;20:1204–7. [PubMed] [Google Scholar]
- 15.Buyon JP, Waltuck J, Kleinman C, Copel J. In utero identification and therapy of congenital heart block. Lupus. 1995;4:116–21. doi: 10.1177/096120339500400207. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal D, Druzin M, Chin C, Dubin A. A new therapeutic approach to the fetus with congenital complete heart block: preemptive, targeted therapy with dexamethasone. Obstet Gynecol. 1998;92:689–91. doi: 10.1016/s0029-7844(98)00149-5. [DOI] [PubMed] [Google Scholar]
- 17.Saleeb S, Copel J, Friedman D, Buyon JP. Comparison of treatment with fluorinated glucocorticoids to the natural history of autoantibody-associated congenital heart block: retrospective review of the research registry for neonatal lupus. Arthritis Rheum. 1999;42:2335–45. doi: 10.1002/1529-0131(199911)42:11<2335::AID-ANR12>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Raboisson MJ, Fouron JC, Sonesson SE, Nyman M, Proulx F, Gamache S. Fetal Doppler echocardiographic diagnosis and successful steroid therapy of Luciani-Wenckebach phenomenon and endocardial fibroelastosis related to maternal anti-Ro and anti-La antibodies. J Am Soc Echocardiogr. 2005;18:375–80. doi: 10.1016/j.echo.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 19.Salomonsson S, Larsson P, Tengner P, Mellquist E, Hjelmstrom P, Wahren-Herlenius M. Expression of the B cell-attracting chemokine CXCL13 in the target organ and autoantibody production in ectopic lymphoid tissue in the chronic inflammatory disease Sjogren's syndrome. Scand J Immunol. 2002;55:336–42. doi: 10.1046/j.1365-3083.2002.01058.x. [DOI] [PubMed] [Google Scholar]
- 20.Julkunen H, Miettinen A, Walle TK, Chan EK, Eronen M. Autoimmune response in mothers of children with congenital and postnatally diagnosed isolated heart block: a population based study. J Rheumatol. 2004;31:183–9. [PubMed] [Google Scholar]
- 21.Buyon JP, Clancy RM. Neonatal lupus: review of proposed pathogenesis and clinical data from the US-based Research Registry for Neonatal Lupus. Autoimmunity. 2003;36:41–50. doi: 10.1080/0891693031000067340. [DOI] [PubMed] [Google Scholar]
- 22.Andelfinger G, Fouron JC, Sonesson SE, Proulx F. Reference values for time intervals between atrial and ventricular contractions of the fetal heart measured by two Doppler techniques. Am J Cardiol. 2001;88:1433–6. A8. doi: 10.1016/s0002-9149(01)02130-0. [DOI] [PubMed] [Google Scholar]
- 23.Salomonsson S, Sonesson SE, Ottosson L, et al. Ro/SSA autoantibodies directly bind cardiomyocytes, disturb calcium homeostasis, and mediate congenital heart block. J Exp Med. 2005;201:11–17. doi: 10.1084/jem.20041859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ottosson L, Salomonsson S, Hennig J, et al. Structurally derived mutations define congenital heart block-related epitopes within the 200–239 amino acid stretch of the Ro52 protein. Scand J Immunol. 2005;61:109–18. doi: 10.1111/j.0300-9475.2005.01542.x. [DOI] [PubMed] [Google Scholar]
- 25.Clancy RM, Buyon JP, Ikeda K, et al. Maternal antibody responses to the 52-kd SSA/RO p200 peptide and the development of fetal conduction defects. Arthritis Rheum. 2005;52:3079–86. doi: 10.1002/art.21289. [DOI] [PubMed] [Google Scholar]
- 26.Clancy RM, Buyon JP. Autoimmune-associated congenital heart block: dissecting the cascade from immunologic insult to relentless fibrosis. Anat Rec A Discov Mol Cell Evol Biol. 2004;280:1027–35. doi: 10.1002/ar.a.20072. [DOI] [PubMed] [Google Scholar]
- 27.Harley JB, Reichlin M, Arnett FC, Alexander EL, Bias WB, Provost TT. Gene interaction at HLA-DQ enhances autoantibody production in primary Sjogren's syndrome. Science. 1986;232:1145–7. doi: 10.1126/science.3458307. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida H, Imafuku Y, Nagai T. Matrix effects in clinical immunoassays and the effect of preheating and cooling analytical samples. Clin Chem Lab Med. 2004;42:51–6. doi: 10.1515/CCLM.2004.010. [DOI] [PubMed] [Google Scholar]
- 29.Hassan AB, Lundberg IE, Isenberg D, Wahren-Herlenius M. Serial analysis of Ro/SSA and La/SSB antibody levels and correlation with clinical disease activity in patients with systemic lupus erythematosus. Scand J Rheumatol. 2002;31:133–9. [PubMed] [Google Scholar]
- 30.Praprotnik S, Bozic B, Kveder T, Rozman B. Fluctuation of anti-Ro/SS-A antibody levels in patients with systemic lupus erythematosus and Sjogren's syndrome: a prospective study. Clin Exp Rheumatol. 1999;17:63–8. [PubMed] [Google Scholar]
- 31.Wahren M, Tengner P, Gunnarsson I, et al. Ro/SS-A and La/SS-B antibody level variation in patients with Sjogren's syndrome and systemic lupus erythematosus. J Autoimmun. 1998;11:29–38. doi: 10.1006/jaut.1997.0173. [DOI] [PubMed] [Google Scholar]
- 32.Askanase AD, Friedman DM, Copel J, et al. Spectrum and progression of conduction abnormalities in infants born to mothers with anti-SSA/Ro-SSB/La antibodies. Lupus. 2002;11:145–51. doi: 10.1191/0961203302lu173oa. [DOI] [PubMed] [Google Scholar]
- 33.Julkunen H, Kurki P, Kaaja R, et al. Isolated congenital heart block. Long-term outcome of mothers and characterization of the immune response to SS-A/Ro and to SS-B/La. Arthritis Rheum. 1993;36:1588–98. doi: 10.1002/art.1780361114. [DOI] [PubMed] [Google Scholar]
- 34.Silverman ED, Buyon J, Laxer RM, et al. Autoantibody response to the Ro/La particle may predict outcome in neonatal lupus erythematosus. Clin Exp Immunol. 1995;100:499–505. doi: 10.1111/j.1365-2249.1995.tb03729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buyon JP. Congenital complete heart block. Lupus. 1993;2:291–5. doi: 10.1177/096120339300200503. [DOI] [PubMed] [Google Scholar]
- 36.Meilof JF, Frohn-Mulder IM, Stewart PA, et al. Maternal autoantibodies and congenital heart block: no evidence for the existence of a unique heart block-associated anti-Ro/SS-A autoantibody profile. Lupus. 1993;2:239–46. doi: 10.1177/096120339300200406. [DOI] [PubMed] [Google Scholar]
- 37.Dörner T, Feist E, Wagenmann A, et al. Anti-52 kDa Ro(SSA) autoantibodies in different autoimmune diseases preferentially recognize epitopes on the central region of the antigen. J Rheumatol. 1996;23:462–8. [PubMed] [Google Scholar]
- 38.Fritsch C, Hoebeke J, Dali H, et al. 52-kDa Ro/SSA epitopes preferentially recognized by antibodies from mothers of children with neonatal lupus and congenital heart block. Arthritis Res Ther. 2006;8:R4. doi: 10.1186/ar1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buyon JP. Neonatal lupus and congenital complete heart block: manifestations of passively acquired autoimmunity. Clin Exp Rheumatol. 1989;7:S199–203. [PubMed] [Google Scholar]
- 40.Reed JH, Neufing PJ, Jackson MW, et al. Different temporal expression of immunodominant Ro60/60 kDa-SSA and La/SSB apotopes. Clin Exp Immunol. 2007;148:153–60. doi: 10.1111/j.1365-2249.2007.03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Buyon JP, Clancy RM. Neonatal lupus: basic research and clinical perspectives. Rheum Dis Clin North Am. 2005;31:299–313. vii. doi: 10.1016/j.rdc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Wahren-Herlenius M, Sonesson SE. Specificity and effector mechanisms of autoantibodies in congenital heart block. Curr Opin Immunol. 2006;18:690–6. doi: 10.1016/j.coi.2006.09.012. [DOI] [PubMed] [Google Scholar]


