Abstract
The Z39Ig protein (complement receptor for C3b and iC3b) is expressed on resident tissue macrophages in various tissues. This study was undertaken to examine the distribution of Z39Ig+cells and their phenotypic features in rheumatoid arthritis (RA) synovium, in comparison with those of osteoarthritis (OA) and psoriatic arthritis (PsA) synovium. Monoclonal anti-Z39Ig antibody was produced by immunizing Z39Ig transfected murine pre B cells and used for the identification of Z39Ig+cells. Z39Ig+cells were further stained with antibodies to macrophages, fibroblast-like synoviocytes, complement receptors and dendritic cells by using the double immunostaining method in normal, RA, OA and PsA synovium. RA synovial mononuclear cells were double-stained using anti-Z39Ig and anti-CD11c antibodies and sorted into Z39Ig+CD11c+cells and Z39Ig+CD11c−cells. These cell populations were then analysed by electron microscopy. The expression of the Z39Ig protein was limited to intimal macrophages in normal, RA, OA and PsA synovium. The numbers of Z39Ig+CD11c+cells and the ratios of Z39Ig+CD11c+cells to Z39Ig+cells were increased in the synovial lining layer of RA as compared with those of OA and PsA. The ultrastructural analysis of Z39Ig+CD11c+cells showed the character of macrophages with many secondary lysosomes and swelling of mitochondria. Z39Ig+ cells appeared to be useful for identification of resident tissue macrophages in normal synovium and the corresponding macrophages in the synovial lining layer of inflammatory arthritis. Expansion of Z39Ig+CD11c+cells was characteristic of RA synovial lining layer.
Keywords: immunohistochemistry, macrophage, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease that targets primarily the synovial membrane, leading to progressive cartilage and joint destruction. Recent studies have demonstrated that activated synovial macrophages are predominantly involved in the pathogenesis of RA [1,2]. In RA synovium, the lining layer becomes thickened due to increased numbers of macrophages and lymphocytes, and proliferated fibroblast-like synoviocytes and endothelial cells [3]. In particular, RA intimal macrophages are more highly activated than sublining macrophages and express greater amounts of interleukin (IL)-1 and tumor necrosis factor-α. They are thought to migrate into the expanding pannus that participates in the degradation of articular cartilage and subchondral bone [4].
These synovial intimal macrophages comprise at least two subsets; resident tissue macrophages (corresponding to synovial A cells in normal synovium) and infiltrated inflammatory macrophages which exhibit phenotypes different from sublining macrophages [5–8]. Generally, resident tissue macrophages play a role in maintaining homeostasis by removing dead cells and other debris in those tissues. In addition, they have a central role to play in immune defence [5]. To complicate things further, it has been reported that under inflammatory conditions, infiltrated monocytes can differentiate into resident tissue macrophages [9]. In this regard, infiltrated inflammatory macrophages in the synovial lining layer may undergo local maturation to resident tissue macrophages, similar to CD14+CD16+monocytes that further adapt to the local microenvironment and differentiate into resident tissue macrophages under physiological conditions [10]. Furthermore, resident tissue macrophages activated in the disease process may be inappropriately differentiated or dedifferentiated and they may consequently acquire the functionally distinct phenotype of resident tissue macrophages, leading to aberrant production of cytokines, matrix proteinases and chemical mediators [11]. Thus, the characterization of intimal macrophages in inflammatory arthritis would be important for our understanding of macrophage subsets during the late stages of macrophage differentiation in an inflammatory microenvironment. Moreover, the unique pathophysiology of RA might be elucidated by the comparison of phenotypic features of intimal macrophages between RA and other inflammatory arthropathies. As yet, there is no reliable single marker for the identification of resident tissue macrophages in normal synovium and the corresponding macrophages in inflammatory synovium.
The Z39Ig (CRIg) protein is a type 1 transmembrane protein of the immunoglobulin superfamily member [12–14]. Recently, it has been shown that the Z39Ig protein is a receptor for complement fragments C3b and iC3b on murine kupffer cells and is expressed on subsets of resident tissue macrophages in various tissues such as the lung, the liver and the synovium [15–17]. These expression patterns of the Z39Ig protein suggest that the expression of the Z39Ig protein may be limited to resident tissue macrophages and the expression of this protein may be useful for their identification. Moreover, it has been shown that Z39Ig is not only a phagocytic receptor, but is also a potent inhibitor of the alternative pathway convertases [18]. Indeed, soluble Z39Ig protein suppressed experimental arthritis; however, its role in the pathogenesis of RA remains unknown.
In this study, in order to determine whether the expression of the Z39Ig protein is useful for the identification of resident tissue macrophages in normal synovium and the corresponding macrophages in inflamed synovium, the expression of the Z39Ig protein was examined in relation to that of markers for macrophages and fibroblast-like synoviocytes in normal and inflamed synovium [5–8,19,20]. To further assess phenotypic features of Z39Ig+cells in RA synovial lining layer, we examined the expression of other complement receptors [21,22] and myeloid dendritic cell markers [23,24] on Z39Ig+ cells.
Materials and methods
Tissue samples
Synovial tissue samples were: 4 normal synovium obtained at arthroscopy from trauma/joint derangement, 24 RA synovium, 15 osteoarthritis (OA) synovium and 6 psoriatic arthritis (PsA) synovium from arthroscopic synovial biopsy or total knee replacement surgery. Diagnosis of these diseases was done by the criteria in reference [25] for RA (four cases with a duration of less than 1 year were retrospectively diagnosed as RA), in reference [26] for OA, and in reference [27] for PsA. Clinical and demographic data for patients are summarized in Table 1. Samples of skin (three samples), lung (three samples), liver (two samples), kidney (three samples), intestine (three samples) and cerebrum (three samples) obtained by surgical resection for cancers and placenta (one sample) obtained after delivery were used for the immunohistochemical analysis to detect Z39Ig+cells. Monocytes from five different normal donors were prepared as previously described [28]. Informed consent was obtained from all donors in accordance with the requirements of the Human Investigation Committee of Kagoshima University or the Ethics and Medical Research Committee, St Vincent's University Hospital.
Table 1.
Demographic and clinical data of RA, OA and PsA patients.
| Early RA (n = 4) | RA (n = 20) | OA (n = 15) | PsA (n = 6) | |
|---|---|---|---|---|
| Demographic features | ||||
| Age, mean (range) years | 63·0 (39–74) | 61·5 (48–78) | 72·5 (60–84) | 42·3 (22–60) |
| Sex: female/male | 4/0 | 19/1 | 12/3 | 3/3 |
| Disease duration, mean (range) years | < 1·0 | 22·1 (5–61) | 11·6 (3–30) | 8 (2–13) |
| Medications (Yes/No) | ||||
| NSAIDs | 4/0 | 4/16 | 11/4 | 3/3 |
| Corticosteroids | 0/4 | 10/10 | 0/15 | 0/6 |
| DMARDs | 0/4 | 17/3 | 0/15 | 1/5 |
| TNF-α blockade | 0/4 | 1/19 | 0/15 | 0/6 |
| Joint synovium studied (no.) | ||||
| Knee | 4 | 20 | 15 | 6 |
Production of monoclonal antibody against the Z39Ig protein
cDNAs from RA synovium were amplified by using the following sense (5′-gcacaggtctagaggatcctgtgatggggatcttactgggcctg-3′) and anti-sense primers (5′-cgacgctctagattttaacagacacttttgccct-3′). Anti-Z39Ig monoclonal antibodies (mAbs) were produced as previously reported [28].
Phenotypic analysis, cell sorting and electron microscopic analysis
Monocytes were stimulated with IL1-β (100 units/ml) (Pierce, Rockford, IL), lipopolysaccharide (1 µg/ml) (Sigma-Aldrich, St Louis, MO), IFN-γ (1000 units/ml) (Pierce), M-CSF (100 ng/ml) (Pierce), GM-CSF (100 ng/ml) (Peprotech EC, London, UK), vitamin D3 (10−7 M) (Sigma-Aldrich) or dexamethasone (10−7 M) (Sigma-Aldrich) as previously described [28]. After cultures of monocytes/macrophages with various reagents, cells were stained with anti-Z39Ig mAb, followed by FITC conjugated anti-mouse Ig antibody (Southern Biotech, Birmingham, AL), and the expression of Z39Ig protein was examined by the flow cytometry as described below.
Rheumatoid arthritis synovial mononuclear cells were prepared as previously described [28]. Cells were incubated with one of the following monoclonal antibodies: biotinylated anti-human Z39Ig (IgG2a), FITC anti-human CD11c (IgG1) (Southern Biotech), or isotype-matched controls for 15 min at 4°C. Then, cells were labelled with streptavidin-allophycocyanin (Becton Dickinson, San Jose, CA) for 20 min at 4°C. Cell sorting was performed on a BD FACSAria Cell Sorter (Becton Dickinson) with data acquisition using BD FACSDiva software (Becton Dickinson). The purity of sorted Z39Ig+CD11c+cells and Z39Ig+CD11c−cells was more than 93% and 95% in each experiment respectively. Sorted cells were immediately prepared and viewed as described previously [29].
Immumnofluorescence analysis
Five micrometre-thick synovial sections were cut with a cryostat, mounted on silanized slides, and fixed in acetone. Sections were blocked with PBS-1% BSA for 10 min at room temperature. Sections were incubated with biotinylated anti- Z39Ig mAb (IgG2a) or isotype-matched control (IgG2a) for 45 min at room temperature. The excess mAb was removed by washing in the PBS. Sections were incubated with Alexa fluor 488 conjugated anti-CD163 [28], Alexa Fluor 647-conjugated anti-CD1a (AbD Serotec, Oxford, UK), Alexa Fluor 647-conjugated anti-CD83 (AbD Serotec), anti-CD11c (IgG1) (Southern Biotech), anti-CD16 (Chemicon Europe, Hampshire, UK), anti-27E10 (Dakocytomation, Kyoto, Japan), anti-25F9 (Dakocytomation ), anti-VCAM-1 [30], anti-cadherin11 (Zymed, South San Francisco, CA), anti-CD11b (IgM) (Beckman Coulter, Tokyo, Japan) or isotype-matched control (IgG1) for 45 min at room temperature. The excess mAb was removed by washing in the PBS. Then, Rhodamine avidin D (Vector Laboratories, Burlingame, CA), FITC conjugated goat F(ab')2 anti-mouse IgG1 (Southern Biotech) and FITC conjugated goat F(ab')2 anti-mouse IgM (Americanqualex, San Clemente, CA) were added for biotinylated anti-Z39Ig mAb, for anti-CD11c, anti-CD16, anti-25F9, anti-27E10, anti-VCAM-1 or anti-cadherin 11 mAb and for anti-CD11b mAb respectively. After incubation for 45 min, sections were extensively washed by the PBS. Then, sections were incubated with DAPI (2 ng/µl) for 5 min. Sections were extensively washed by the PBS and analysed by fluorescent microscopy (BZ-8000, KEYENCE, Osaka, Japan). The 12 synovial lining areas were randomly selected in each specimen from inflamed synovium for the quantitative analysis of Z39Ig+cells expressing the CD11c antigen. Images were acquired by using appropriate filters of the fluorescent microscopy (magnification, ×40). Using values of the ratio of Z39Ig+CD11c+cells to Z39Ig+cells, the sensitivity and specificity of Z39Ig+CD11c+cells in differentiating between RA and other types of arthritis were determined for all available values. They were then applied to plot a receiver operating characteristic curve (ROC). The pathological activity of RA synovial sections was calculated as previously described [31].
Statistical analysis
The nonparametric Mann–Whitney U-test was used for differences among experimental groups. The relationship between the number of Z39Ig+cells and the disease activity in RA synovium was presented as the relation coefficiency. P values less than 0·05 were considered statistically significant. In the case of ROC analysis, the area under the curve (AUC) was calculated with STATA version 8·5.
Results
(1)Expression of the Z39Ig protein in differentiated resident tissue macrophages
Antibody 15-b (IgG2a) reacted with B300-19 cells transfected with the long Z39Ig gene but not with B300-19 cells and B300-19 cells transfected with the short Z39Ig gene (Fig. 1a). While CD163+macrophages (or in cerebrum HLA-DR+) was detected in various tissues, Z39Ig protein expression was confined to the lung, liver, synovium and placenta tissues, and not in the cerebrum, kidney, skin, spleen, or intestine tissues (data not shown). Interestingly, we did not find the expression of the Z39Ig protein in cerebral microglia cells. Z39Ig+cells were included in CD163+ macrophages of lung, liver, synovium and placenta in serial sections that were stained by anti-Z39Ig and anti-CD163 (a macrophage specific marker) mAbs. When anti-Z39Ig mAb was used for the staining of peripheral blood T cells, B cells, neutrophils and platelets, the expression of Z39Ig was not observed. The expression of the Z39Ig protein was observed, however, on macrophages differentiated with M-CSF, GM-CSF, dexamethazone or vitamine D3 (Fig. 1b). There was little or no expression on peripheral blood monocytes, monocytes activated with IL1-β, IFN-γ and lipopolysaccharide, myeloid dendritic cells and osteoclasts differentiated from monocytes (data not shown).
Fig. 1.
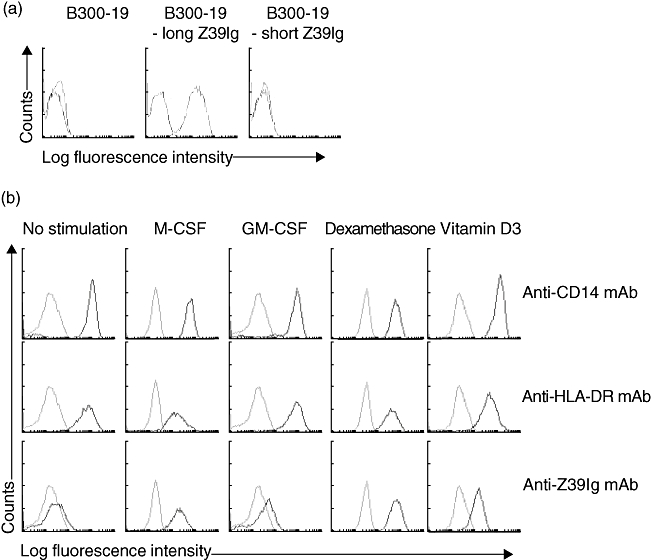
(a) The reactivity of anti-Z39Ig mAb with the long form of the Z39Ig protein. B300-19 cells and B300-19 cells transfected with the long Z39Ig or short Z39Ig gene were stained with anti-Z39Ig mAb, and analysed by flow cytometry. Light and dark lines show isotype-matched mAb and anti-Z39Ig mAb respectively. (b) The expression of the Z39Ig protein on differentiated macrophages. Monocytes were cultured with the indicated reagents for 60 h (M-CSF at 100 ng/ml or GM-CSF at 100 ng/ml) or 120 h (dexamethasone at 10−7 M or vitamin D3 at 10−7 M). Cells were stained with mAbs and analysed by the flow cytometry. Light and dark lines show isotype-matched mAb and anti-CD14, anti-HLA-DR or anti-Z39Ig mAb respectively. The data are representative of independent experiments from four different healthy donors.
(2)Expansion of Z39Ig+CD11c+ cells in RA synovial lining layer
It has been shown that there are increased numbers of macrophages in RA synovial lining layer [3], but the relationship of these macrophages to resident tissue macrophages in normal synovium remains unknown. Accordingly, we examined the distribution of Z39Ig+cells in RA synovium. As shown in Fig. 2a, the distribution of Z39Ig+cells was limited mainly to synovial lining layer. The phenotypes of Z39Ig+cells were CD163, 25F9 (a mature macrophage marker) and CD16 (a marker of synovial lining macrophages in both OA and RA) positive but 27E10 (an early acute inflammatory macrophage marker) negative. In line with resident tissue macrophages in normal synovium [18,19], Z39Ig+cells were present in contact with cadherin 11 or VCAM-1 expressing cells (Fig. 2b). Z39Ig+cells in RA synovium were also examined in relation to those of other complement receptors, CD11b (CR3) and CD11c (CR4) (Fig. 3). The Z39Ig+CD11b+cells were observed in RA synovial lining layer and Z39Ig-CD11b+cells were observed in RA synovial lining and sublining layer but were absent in normal, OA and PsA synovial lining layer. A small number of these cells were observed in OA and PsA synovial sublining layer (Fig. 3a). Surprisingly, many Z39Ig+CD11c+cells were observed in RA synovial lining layer while these cells were very few in normal, OA or PsA synovial lining layer (Fig. 3b). The number of Z39Ig+cells was increased in the synovial lining layer of RA as compared with that of OA, though not significantly (Fig. 4). When the number of Z39+CD11c+cells and the ratio of Z39Ig+CD11c+cells to Z39+cells were compared among RA, OA and PsA synovial lining layer, they were predominantly increased in RA synovial lining layer (Fig. 4). Interestingly, this finding was also observed in the synovial lining layer obtained from early RA. In addition, the ROC analysis revealed that the ratio of Z39Ig+CD11c+cells to Z39Ig+cells in synovium clearly separated other arthritis from RA with the AUC of 1. The ratio of Z39Ig+CD11c+cells to Z39Ig+cells of RA synovial lining layer was found not to be related to disease duration, synovial lining layer hyperplasia, the pathological activity, or the effects of treatment (data not shown). As the CD11c antigen is also a dendritic cell marker, we examined whether Z39Ig+CD11c+cells express other dendritic markers; CD1a and CD83. The co-expression of the Z39Ig protein and these dendritic cell markers was rarely observed in RA synovial lining layer, supporting the concept that most of Z39Ig+CD11c+cells belong to a macrophage subset (data not shown).
Fig. 2.
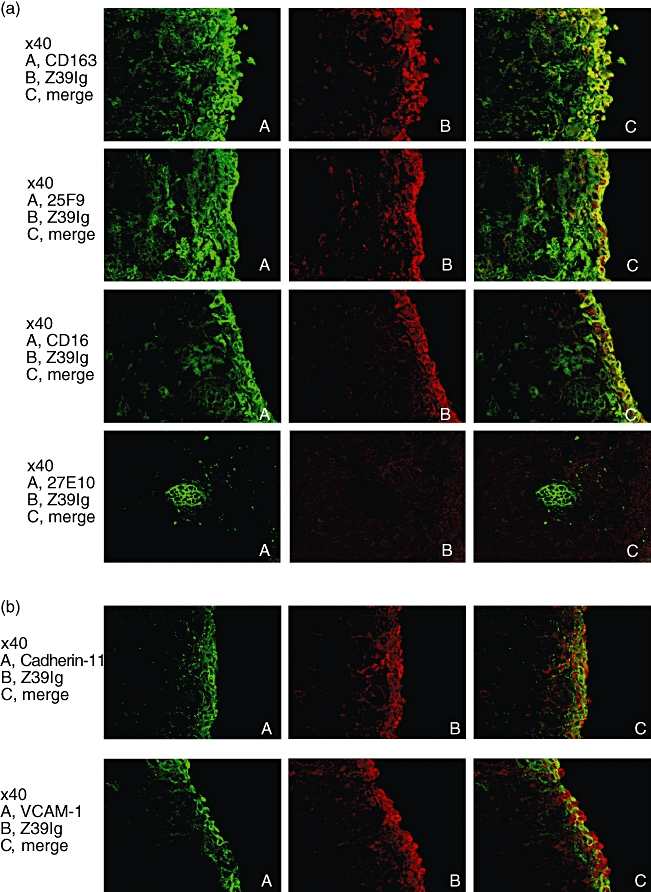
(a) Double-immunofluorescence staining for the Z39Ig antigen and the CD163, 25F9 or CD16 in RA synovium, and for the Z39Ig antigen and 27E10 antigen in the synovial sublining layer of RA synovium. Frozen sections of RA synovium were double-stained with anti-Z39Ig mAb and indicated mAbs, and analysed by the fluorescent microscopy as described in Materials and Methods. The data are representative of independent experiments from four different RA synovium. (b) Double-immunofluorescence staining for the Z39Ig antigen and the cadherin-11 or VCAM-1 antigen in RA synovium. Frozen sections of RA synovium were stained with anti-Z39Ig mAb and anti-cadherin-11 or anti-VCAM-1 mAb, and analysed by fluorescent microscopy as described in Materials and Methods. The data are representative of independent experiments from four different RA synovium.
Fig. 3.
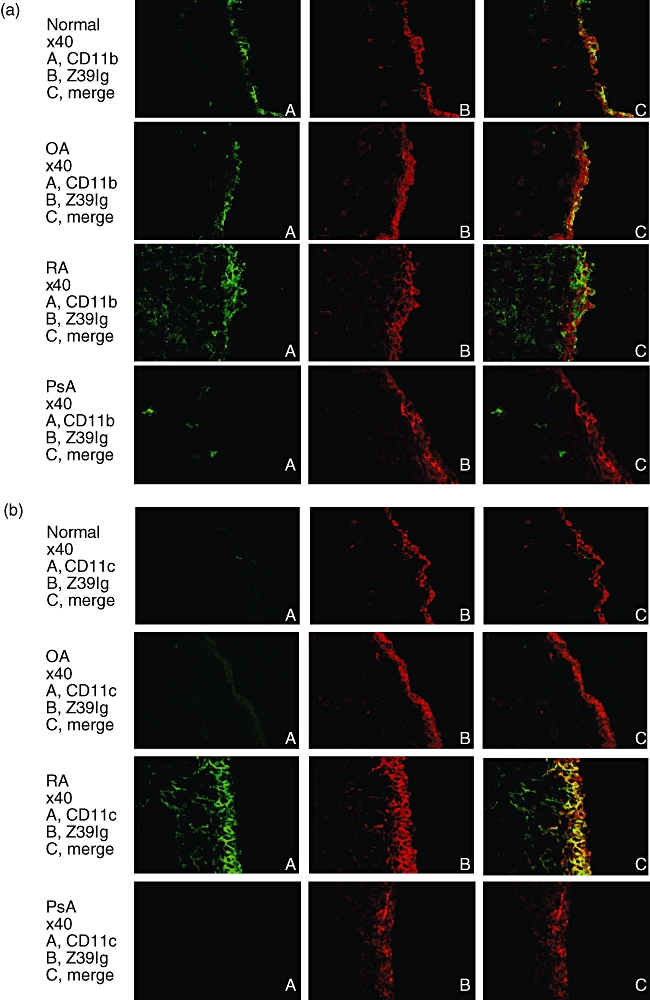
Double-immunofluorescence staining for the Z39Ig antigen and the CD11b or CD11c antigen in normal and inflamed synovium. (a) Frozen sections of normal, OA, PsA and RA synovium were double-stained with anti-Z39Ig and anti-CD11b mAbs, and analysed by fluorescent microscopy as described in Materials and Methods. The data are representative of independent experiments from four different normal, OA, RA, and PsA synovium. (b) Frozen sections of normal, OA, PsA and RA synovium were double-stained with anti-Z39Ig and anti-CD11c mAbs, and analysed by fluorescent microscopy as described in Materials and Methods. The data are representative of independent experiments from four different normal, OA, RA, and PsA synovium.
Fig. 4.
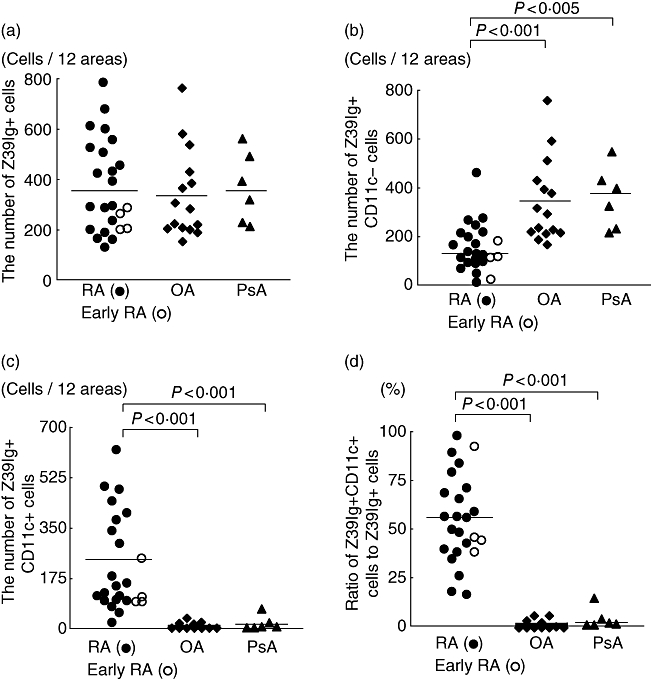
The quantitative analysis of Z39Ig+ cells expressing the CD11c antigen in synovial lining layer of inflammatory arthritis. Figure 4a shows the number of synovial lining Z39Ig+ cells of RA (•), early RA (○), OA (♦), and PsA (▴). Figure 4b shows the number of synovial lining Z39Ig+CD11c−cells of RA (•), early RA (○), OA (♦), and PsA (▴). Figure 4c shows the number of synovial lining Z39Ig+CD11c+ cells of RA (•), early RA (○), OA (♦), and PsA (▴). Figure 4d shows the ratio of Z39Ig+CD11c+ cells in synovial lining Z39Ig+ cells of RA (•), early RA (○), OA (♦), and PsA (▴). The mean values in the figures are indicated by horizontal lines. Statistical significance was P < 0·005 or P < 0·001.
(3)Comparison of the structure of Z39Ig+CD11c+cells and Z39Ig+CD11c−cells in electron microscopic analysis
Figure 5b and b shows that Z39Ig+CD11c+cells are larger in size and more complex in their cytoplasm than Z39Ig+CD11c−cells, judging from the forward scatter and the side scatter in the gating area of these cells. On electron microscopic examination, freshly sorted Z39Ig+CD11c+cells exhibited the following characteristics: many secondary lysosomes with the irregular shapes, swollen mitochondria and less membrane ruffling as compared with Z39Ig+CD11c−cells (Fig. 5c). These morphological characteristics suggest that Z39Ig+CD11c+cells are activated macrophages with disruption of mitochondria, as has been described previously in RA synovium [32]. In addition, some of the Z39Ig+CD11c−cells had the characteristics of dendritic cells, including thin and short cytoplasmic processes and less phagosomes and lysosomes. The nucleus of both cells was non-lobulated, abundant in euchromatin and both contained a distinct nucleolus.
Fig. 5.
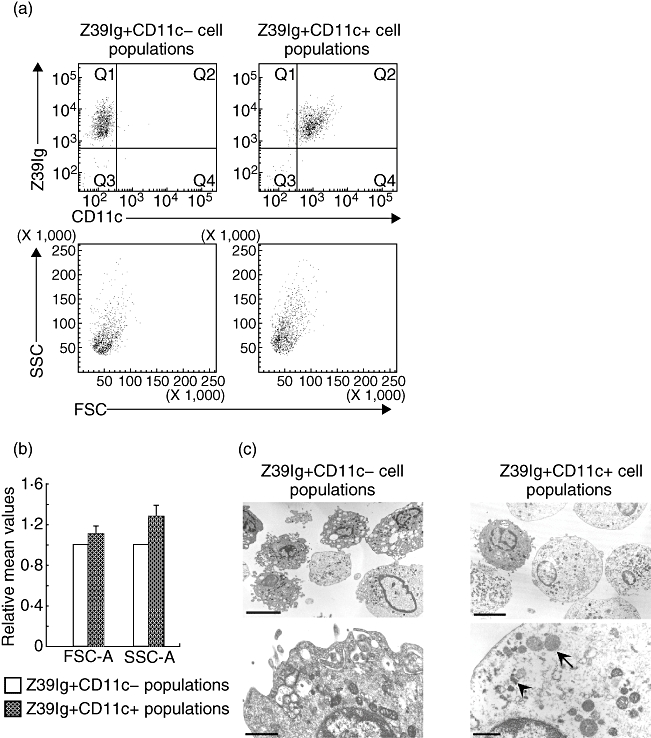
Analysis of Z39Ig+CD11c− and Z39Ig+CD11c+cells from RA synovial mononuclear cells. (a) RA synovial mononuclear cells were stained for Z39Ig+CD11c− cells and Z39Ig+CD11c+ cells, and analysed by the flow cytometry. Upper left and right panels show the analysis of sorted Z39Ig+CD11c− and Z39Ig+CD11c+ cell populations respectively. Lower left and right panels show two-dimensional dotplots showing FSC and SSC characteristics, corresponding to upper left and right panels respectively. The data are representative of independent experiments from five different RA synovial mononuclear cells. (b) The figure shows values of forward scatter-area (FSC-A) and side scatter-area (SSC-A) of Z39Ig+CD11c− or Z39Ig+CD11c+ cell populations from five different RA synovial mononuclear cells. Values of FSC-A and SSC-A of Z39Ig+CD11c− cells were arbitrarily set at 1·0 and relative values of FSC-A and SSC-A of Z39Ig+CD11c+cells to those of Z39Ig+CD11c−cells were presented as the mean ± SD. (c) Representative electron microscopic images of sorted cells in independent electron microscopic analysis from five different RA synovial mononuclear cells. *Cells in upper left and right panels were magnified in lower left and right panels respectively. Arrows and arrowheads indicate swollen mitochondrias and lysosomes respectively. Bars represent 6 µm in upper panels and 1 µm in lower panels.
Discussion
Using an antibody to the long form of the Z39Ig protein, the expression was found to be limited to resident tissue macrophage such as Kupffer cells, bronchial alveolar macrophages, synovial A cells in normal tissues and Hofbeuer cells, consistent with findings of Helmy et al.[15] but not with those of Lee KY et al., where their anti-Z39Ig antibody reacted with peripheral blood monocytes and synovial sublining macrophages in inflammatory arthritis [17,33]. The expression of the Z39Ig protein was induced on peripheral blood monocytes with reagents that promote differentiation of macrophages but not by those which promote activation. Collectively, the Z39Ig protein appears to be expressed uniquely on macrophages at more advanced maturation and consistent with resident tissue macrophages.
In this study, Z39Ig+cells are present mainly in the synovial lining layer and they are in contact with cadherin11 and VCAM-1 expressing cells in inflamed synovium. Intimal macrophages in inflamed synovium do not necessarily represent tissue macrophages. It is conceivable that the intimal macrophages may be trapped from the synovial fluid. However, we did not find Z39Ig+cells in RA blood monocytes and synovial fluid macrophages (data not shown). Thus, Z39Ig+cells appear to maintain the character of resident tissue macrophages in inflamed synovium. Z39Ig+macrophages co-expressed other macrophage markers such as CD16, CD163 and 25F9 in the inflamed synovial lining layer. Interestingly, the ratios of Z39Ig+CD11c+cells to Z39Ig+cells were increased in RA synovial lining layer, as compared with OA and PsA.
A wide range of variation in this ratio was observed among RA individual patients; however, there was no association between these ratios and synovial lining layer thickness, total pathological activity, and the duration and the treatment of RA. Accordingly, this variation may be due to heterogeneity of RA, the effects of long-term DMARD treatment, and/or secondary OA, which may further complicate RA synovium [34]. The finding that the ratio of Z39Ig+CD11c+cells to Z39Ig+cells was independent of the degree of total pathological activity further emphasizes the central importance of Z39Ig+CD11c+ cells in RA. As a significant number of Z39Ig+CD11c+cells were observed in some case of OA and PsA, it is supposed that Z39Ig+CD11c+cells in RA synovium are expanded from a minor population but not newly developed. At present, we do not understand if there is a relationship between differentiation of Z39Ig+CD11c+cells and Z39Ig+CD11c−cells; however, a relationship seems likely because we found a reciprocal change in numbers of these subsets in RA synovial lining layer. The increased number of 27E10 positive acute macrophages around RA synovial vessels and infiltrated Z39Ig−CD11b+cells in RA synovial lining layer support the presence of a large number of infiltrated inflammatory macrophages in RA synovium, consistent with previous reports [9,35,36]. In considering the differentiation of resident tissue macrophages and RA synovial inflammatory macrophages, RA synovial inflammatory macrophages could differentiate to Z39Ig+CD11c+cells but not to Z39Ig+CD11c−cells. Additionally, the physiological differentiation of resident tissue macrophages to Z39Ig+CD11c−cells might be prevented in RA synovial lining layer. Results from this study show that Z39Ig+CD11c+cells do not express dendritic markers such as CD1a and CD83. In addition, Z39Ig+CD11c+cells do not show morphological characteristics of dendritic cells on electron microscopy. Thus, Z39Ig+CD11c+cells appear to be a subset of resident tissue macrophages. Recently, it has been reported that DC-Sign positive and CD163 negative macrophages are remarkable as a subset of inflammatory macrophages in RA synovium [37]. In considering the expression of CD163, Z39Ig+CD11c+cells are different from DC-Sign positive cells. To our knowledge, Z39Ig+CD11c+cells are a unique subset of resident tissue macrophages not yet reported.
How might the expansion of Z39Ig+CD11c+cells in the synovial lining layer contribute to the pathogenesis of RA? Recently, it has been shown that the alternative pathway of complement is not only required for disease induction, but is also required for disease progression in experimental arthritis. This occurs through the inhibition of C3 and C5 convertases by soluble Z39Ig-Fc protein, although it is not yet determined whether its membrane form acts as an intrinsic inhibitor of complement receptor [18]. Additionally, the CD11c antigen is able to bind multiple ligands such iC3b, ICAM-1, ICAM-2 and VCAM-1 [38]. Thus, macrophages with the Z39Ig and CD11c antigens may be more active in antigen uptake via iC3b than those with the Z39Ig antigen alone. Furthermore, the binding of Z39Ig+CD11c+cells to VCAM-1 on VCAM-1 expressing fibroblast-like synoviocytes via the CD11c antigen may lead to the activation of both cells. It remains to be shown whether Z39Ig+CD11c+cells act to accelerate or to prevent RA synovitis.
While there are many proposals for the pathological diagnosis of RA synovitis, based on the grading of inflammatory activities [31,39], a major problem is the heterogeneity of the inflammatory reaction. In this study, we demonstrated a unique immunohistologic feature of RA intimal macrophages, which is independent of the inflammatory reaction. Further accumulating evidence supports the hypothesis that the increased numbers of Z39Ig+CD11c+cells are valuable for the identification of RA in early arthritis. The expansion of Z39Ig+CD11c+cells suggests that alternation of macrophage differentiation during the late stages of disease may be present in RA synovitis.
Furthermore, in relation to RA pathogenesis, it will be useful to define what cellular interactions and/or cellular mediators promote the expansion of Z39Ig+CD11c+cells and how this subset contributes to the inflammation and joint destruction of RA.
Acknowledgments
We thank Dr Chihaya Koriyama for the statistical analysis.
References
- 1.Smeets TJ, Kraan MC, van Loon ME, Tak PP. Tumor necrosis factor alpha blockade reduces the synovial cell infiltrate early after initiation of treatment, but apparently not by induction of apoptosis in synovial tissue. Arthritis Rheum. 2003;48:2155–62. doi: 10.1002/art.11098. [DOI] [PubMed] [Google Scholar]
- 2.Szekanecz Z, Koch AE. Macrophages and their products in rheumatoid arthritis. Curr Opin Rheumatol. 2007;19:289–95. doi: 10.1097/BOR.0b013e32805e87ae. [DOI] [PubMed] [Google Scholar]
- 3.Veale D, Yanni G, Rogers S, Barnes L, Bresnihan B, Fitzgerald O. Reduced synovial membrane macrophage numbers, ELAM-1 expression, and lining layer hyperplasia in psoriatic arthritis as compared with rheumatoid arthritis. Arthritis Rheum. 1993;36:893–900. doi: 10.1002/art.1780360705. [DOI] [PubMed] [Google Scholar]
- 4.Bresnihan B, Youssef P. Macrophages in rheumatoid arthritis. In: Burke B, Lewis CE, editors. The macrophages. Oxford: Oxford University Press; 2002. pp. 391–433. [Google Scholar]
- 5.Revell PA. Synovial lining cells. Rheumatol Int. 1989;9:49–51. doi: 10.1007/BF00270244. [DOI] [PubMed] [Google Scholar]
- 6.Athanasou NA, Quinn J. Immunocytochemical analysis of human synovial lining cells: phenotypic relation to other marrow derived cells. Ann Rheum Dis. 1991;50:311–15. doi: 10.1136/ard.50.5.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Edwards JC, Blades S, Cambridge G. Restricted expression of Fc gammaRIII (CD16) in synovium and dermis: implications for tissue targeting in rheumatoid arthritis (RA) Clin Exp Immunol. 1997;108:401–6. doi: 10.1046/j.1365-2249.1997.3941286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamann J, Wishaupt JO, van Lier RA, Smeets TJ, Breedveld FC, Tak PP. Expression of the activation antigen CD97 and its ligand CD55 in rheumatoid synovial tissue. Arthritis Rheum. 1999;42:650–8. doi: 10.1002/1529-0131(199904)42:4<650::AID-ANR7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 9.Xu H, Manivannan A, Dawson R, et al. Differentiation to the CCR2+ inflammatory phenotype in vivo is a constitutive, time-limited property of blood monocytes and is independent of local inflammatory mediators. J Immunol. 2005;175:6915–23. doi: 10.4049/jimmunol.175.10.6915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ziegler-Heitbrock HW, Fingerle G, Strobel M, et al. The novel subset of CD14+/CD16+ blood monocytes exhibits features of tissue macrophages. Eur J Immunol. 1993;23:2053–8. doi: 10.1002/eji.1830230902. [DOI] [PubMed] [Google Scholar]
- 11.Mantovani A, Sica A, Locati M. New vistas on macrophage differentiation and activation. Eur J Immunol. 2007;37:14–16. doi: 10.1002/eji.200636910. [DOI] [PubMed] [Google Scholar]
- 12.Langnaese K, Colleaux L, Kloos DU, Fontes M, Wieacker P. Cloning of Z39Ig, a novel gene with immunoglobulin-like domains located on human chromosome X. Biochim Biophys Acta. 2000;1492:522–5. doi: 10.1016/s0167-4781(00)00131-7. [DOI] [PubMed] [Google Scholar]
- 13.Walker MG. Z39Ig is co-expressed with activated macrophage genes. Biochim Biophys Acta. 2002;1574:387–90. doi: 10.1016/s0167-4781(01)00358-x. [DOI] [PubMed] [Google Scholar]
- 14.Ahn JH, Lee Y, Jeon C, et al. Identification of the genes differentially expressed in human dendritic cell subsets by cDNA subtraction and microarray analysis. Blood. 2002;100:1742–54. [PubMed] [Google Scholar]
- 15.Helmy KY, Katschke KJ, Jr, Gorgani NN, et al. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–27. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 16.Wiesmann C, Katschke KJ, Yin J, et al. Structure of C3b in complex with CRIg gives insights into regulation of complement activation. Nature. 2006;444:217–20. doi: 10.1038/nature05263. [DOI] [PubMed] [Google Scholar]
- 17.Lee MY, Kim WJ, Kang YJ, et al. Z39Ig is expressed on macrophages and may mediate inflammatory reactions in arthritis and atherosclerosis. J Leukoc Biol. 2006;80:922–8. doi: 10.1189/jlb.0306160. [DOI] [PubMed] [Google Scholar]
- 18.Katschke KJ, Jr, Helmy KY, Steffek M, et al. A novel inhibitor of the alternative pathway of complement reverses inflammation and bone destruction in experimental arthritis. J Exp Med. 2007;204:1319–25. doi: 10.1084/jem.20070432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson LS, Edwards JC, Poston RN, Haskard DO. Expression of vascular cell adhesion molecule-1 in normal and inflamed synovium. Lab Invest. 1993;68:82–8. [PubMed] [Google Scholar]
- 20.Valencia X, Higgins JM, Kiener HP, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J Exp Med. 2004;200:1673–9. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer DG, Hogg N, Revell PA. Lymphocytes, polymorphonuclear leukocytes, macrophages and platelets in synovium involved by rheumatoid arthritis. A study with monoclonal antibodies. Pathology. 1986;18:431–7. doi: 10.3109/00313028609087564. [DOI] [PubMed] [Google Scholar]
- 22.Allen CA, Highton J, Palmer DG. Increased expression of p150,95 and CR3 leukocyte adhesion molecules by mononuclear phagocytes in rheumatoid synovial membranes. Comparison with osteoarthritic and normal synovial membranes. Arthritis Rheum. 1989;32:947–54. doi: 10.1002/anr.1780320803. [DOI] [PubMed] [Google Scholar]
- 23.Page G, Miossec P. Paired synovium and lymph nodes from rheumatoid arthritis patients differ in dendritic cell and chemokine expression. J Pathol. 2004;204:28–38. doi: 10.1002/path.1607. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar S, Fox DA. Dendritic cells in rheumatoid arthritis. Front Biosci. 2005;10:656–65. doi: 10.2741/1560. [DOI] [PubMed] [Google Scholar]
- 25.Arnett FC, Edworthy SM, Bloch DA, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 26.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–49. doi: 10.1002/art.1780290816. [DOI] [PubMed] [Google Scholar]
- 27.Moll JMH, Wright V. Psoriatic arthritis. Semin Arthritis Rheum. 1973;3:55–78. doi: 10.1016/0049-0172(73)90035-8. [DOI] [PubMed] [Google Scholar]
- 28.Nagayoshi R, Nagai T, Matsushita K, et al. Effectiveness of anti-folate receptor beta antibody conjugated with truncated Pseudomonas exotoxin in the targeting of rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2005;52:2666–75. doi: 10.1002/art.21228. [DOI] [PubMed] [Google Scholar]
- 29.Gushi A, Tanaka M, Tsuyama S, et al. The 3G5 antigen is expressed in dermal mast cells but not pericytes. J Cutan Pathol. 2008;35:278–84. doi: 10.1111/j.1600-0560.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 30.Kitani A, Nakashima N, Matsuda T, et al. T cells bound by vascular cell adhesion molecule-1/CD106 in synovial fluid in rheumatoid arthritis: inhibitory role of soluble vascular cell adhesion molecule-1 in T cell activation. J Immunol. 1996;156:2300–8. [PubMed] [Google Scholar]
- 31.Krenn V, Morawietz L, Burmester GR, et al. Synovitis score: discrimination between chronic low-grade and high-grade synovitis. Histopathology. 2006;49:358–64. doi: 10.1111/j.1365-2559.2006.02508.x. [DOI] [PubMed] [Google Scholar]
- 32.Barland P, Novikoff AB, Hamerman D. Fine structure and cytochemistry of the rheumatoid synovial membrane, with special reference to lysosomes. Am J Pathol. 1964;44:853–66. [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JK, Choi EM, Shin HI, et al. Characterization of monoclonal antibody specific to the Z39Ig protein, a member of the immunoglobulin superfamily. Immunol Lett. 2005;99:153–61. doi: 10.1016/j.imlet.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Rau R, Herborn G. Healing phenomena of erosive changes in rheumatoid arthritis patients undergoing disease-modifying antirheumatic drug therapy. Arthritis Rheum. 1996;39:162–8. doi: 10.1002/art.1780390123. [DOI] [PubMed] [Google Scholar]
- 35.Youssef P, Roth J, Frosch M, et al. Expression of myeloid related proteins (MRP) 8 and 14 and the MRP8/14 heterodimer in rheumatoid arthritis synovial membrane. J Rheumatol. 1999;26:2523–8. [PubMed] [Google Scholar]
- 36.el-Gabalawy H, Canvin J, Ma GM, et al. Synovial distribution of alpha d/CD18, a novel leukointegrin. Comparison with other integrins and their ligands. Arthritis Rheum. 1996;39:1913–21. doi: 10.1002/art.1780391119. [DOI] [PubMed] [Google Scholar]
- 37.Page G, Lebecque S, Miossec P. Anatomic localization of immature and mature dendritic cells in an ectopic lymphoid organ: correlation with selective chemokine expression in rheumatoid synovium. J Immunol. 2002;168:5333–41. doi: 10.4049/jimmunol.168.10.5333. [DOI] [PubMed] [Google Scholar]
- 38.Sadhu C, Ting HJ, Lipsky B, et al. CD11c/CD18: novel ligands and a role in delayed-type hypersensitivity. J Leukoc Biol. 2007;81:1395–403. doi: 10.1189/jlb.1106680. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca JE, Canhao H, Resende C, et al. Histology of the synovial tissue: value of semiquantitative analysis for the prediction of joint erosions in rheumatoid arthritis. Clin Exp Rheumatol. 2000;18:559–64. [PubMed] [Google Scholar]


