Abstract
The role of the phagocytic function of monocytes and neutrophils in sepsis has been poorly investigated. The present study evaluated the impact of the phagocytic activity of neutrophils and monocytes on the outcome of patients with severe sepsis. Thirty-one patients and 30 healthy individuals were enrolled in the study. The phagocytic activity of monocytes and neutrophils was evaluated during 24 h after admission and the results were correlated to the expression of CD64 on neutrophils and monocytes, CD14 antigen on monocytes, the Simplified Acute Physiology Score II and the patients' survival. A reduced phagocytic activity of neutrophils during the first 24 h after admission was a negative predictor for survival. Increased expression of CD64 antigen on polymorphonuclear cells (PMNs) and monocytes was favourably correlated to the patients' survival. In multivariate analysis the phagocytic activity of PMNs was the only independent predictor factor for survival. Patients with PMN phagocytic activity <37% had lower expression of CD64 on monocytes and PMNs and worse outcome, while those with phagocytic activity >37% had higher expression of CD64 on monocytes and PMNs and better outcome. Reduced phagocytic activity of neutrophils may represent a state of neutrophil inactivation similar to that previously described for monocytes during the compensatory anti-inflammatory response.
Keywords: flow cytometry/FACS, infections, neutrophils, phagocytosis, phenotype/cell markers
Introduction
Sepsis is a systemic inflammatory syndrome triggered by viral or bacterial infections classified to the following entities: sepsis, severe sepsis and septic shock [1].
Recent literature demonstrates that sepsis is characterized by an initial type-1 cytokine response (systemic inflammatory response syndrome, SIRS) followed by a phase characterized by the production of a mixture of type-1/type 2 cytokines (mixed anti-inflammatory syndrome) and finally by a phase characterized by the production of type-2 cytokines (compensatory anti-inflammatory response, CARS) [2]. The systemic release of cytokines is responsible for many of the symptoms noted in these patients but also for the function of phagocytic cells [3]. The production of inflammatory cytokines during SIRS has been associated with organ dysfunction and hypoperfusion [4].
The anti-inflammatory response leads to a state of immunoparesis considered responsible for life-threatening secondary infections and increased mortality [5].
The immune changes during CARS are partially mediated by interleukin (IL)-10 and include functional deactivation of monocytes that become unable to produce inflammatory cytokines and also diminished expression of human leucocyte antigen D-related (HLA-DR) on their surface, resulting in reduced antigen presentation on lymphocytes and impaired immune responses [4,6,7].
Cytokines representing the phases described for septic progression have been evaluated for their ability to predict the outcome of individual patients. It seems that the ratio of IL-10/tumour necrosis factor (TNF)-α that can distinguish SIRS from CARS is a better predictor of the final outcome of patients with sepsis than the measurement of individual cytokines [8–13].
Cytokine measurements, however, are not rapidly available, making them unsuitable in the clinical setting for diagnostic purposes. In addition, data from animal models of sepsis show that administration of anti-TNF-α might be harmful, while clinical studies show that the use of anti-TNF-α and anti-IL-1 has not improved the clinical outcome of septic patients [14,15]. Besides, carriers of the TNFB2 allele are at risk for lethal septic shock after administration of anti-TNF-α[16]. These data prove that these cytokines are pleiotropic and their correlation to the sub-group of patients defined by clinical criteria unclear.
Global markers of systemic inflammation induced by cytokines, such as C-reactive protein (CRP) and procalcitonin (PCT), have been extensively evaluated for their ability to differentiate sepsis from non-infectious SIRS as well as to predict outcome [17,18]. Both markers are cheap, easily available, with high diagnostic sensitivity. Although PCT has been shown to predict clinical outcome in septic patients, its expression is cytokine dependent and bound to the limitations applied for the cytokines [18,19].
There are suggestions that biomarkers reflecting directly the ability of the host to eliminate bacteria alone or in combination with markers of systemic inflammation might be more relevant to the clinical outcome of patients [3].
Recent evidence indicates that during the pathophysiologic acute inflammatory response there is a transient increase of CD64 (the high affinity receptor to Fc fragment of IgG) on polymorphonuclear cells (PMNs) [20,21]. This expression is induced by binding of bacterial membrane components such as lipopolysaccharide (LPS) or peptidoglycan to the monocyte membrane receptor CD14 [22–26], by cytokines such as IFN-γ, granulocyte colony-stimulating factor (G-CSF) and by IgG and complement opsonized particles [27–31]. In vitro activation of normal PMNs through the CD64 receptor results in increased oxidative burst and phagocytic ability of cells [27,32–34]. Up-regulation of CD64 receptor on PMNs from non-septic patients receiving G-CSF is associated with increased phagocytic function, while in sickle cell patients it enhances adherence to vascular endothelium [20,35].
It is unclear whether up-regulation of CD64 expression on PMNs and monocytes in septic patients is associated with increased phagocytic function. Spitler et al. have shown that in septic patients' monocytes with increased CD64 expression demonstrate increased phagocytic activity [11] while Hirsh et al. have shown that CD64+ monocytes had reduced phagocytic activity compared with CD64− cells [36]. Decreased phagocytic activity of PMNs with increased CD64 expression may interpret the clinical data showing that high expression of CD64 is associated with adverse clinical outcome in septic patients [37].
The aim of the present study was to investigate globally the phagocytic activity in patients with sepsis and evaluate its prognostic significance for the final outcome. During the first 24 h patients with severe sepsis were evaluated for: (i) the phagocytic activity of monocytes and neutrophils by measuring the ability of phagocytes to ingest Escherichia coli; (ii) the expression of CD64 antigen on neutrophils and monocytes; and (iii) the expression of CD14 antigen on monocytes. Measurements were repeated on the day of discharge. The findings were correlated to the expression of CD64 on both neutrophils and monocytes, the CD14 antigen on monocytes and the survival of patients.
Materials and methods
Study populations
The study was performed at Patras University Hospital, in the Achaia region, southwestern Greece. A longitudinal analysis of 41 patients with severe sepsis or septic shock was conducted over a 6-month period of time in the intermediate care unit of the department of medicine. Patients with trauma, human immunodeficiency virus disease, renal disease, neutropenia or end stage hepatic disease, and subjects receiving immunosuppressive agents, were excluded.
All patients were enrolled within 24 h of the onset of the sepsis.Thirty healthy individuals similar in age (median age 70·6 years, range 64–77), matched for sex (16 males, 14 females), of the same ethnic background, were also included as controls. All of them were relatives or friends of hospitalized patients.
The study protocol was approved by the University Hospital Ethics Committee and written informed consent was obtained from participating patients (or relatives) and healthy volunteers on enrollment.
The definition of sepsis was based on the presence or presumed presence of an infection accompanied by a SIRS. SIRS is defined as the presence of at least two of the following criteria:
Temperature greater than 38°C or less than 36°C.
Respiratory rate ≥20 breath/min or PaCO2 less than 32 Torr.
Pulse rate ≥90 beats/min.
WBC count ≥12 000 or ≤4000/mm3or >10% immature band forms.
Additional requirements for severe sepsis were taken into account, as it represents a homeostatic imbalance with evidence of organ dysfunction (at least one of the following):
Hypotension: systolic blood pressure ≤90 mmHg; decrease of 40 mmHg in SBP compared with baseline, or mean arterial pressure less than 65 mmHg responding to fluid resuscitation within 1 h.
Arterial hypoxemia: partial pressure of arterial oxygen ≤75 mmHg without evidence of primary lung disease.
Metabolic acidosis: pH ≤7·3 or base deficit ≥5 meq/lt [38].
Renal impairement: urine output ≤30 ml/h for at least 2 h, unresponsive to fluid replacement or creatinine concentration of 3·5 mg/dl or hemodialysis.
Altered mental status evaluated through the Glascow Coma Score [1,38].
Disseminated intravascular coagulation: coagulation abnormalities of recent onset, prothrombin time or aPTT of 1·2 times the upper normal limit plus D-dimers at ≥500 or platelets at ≤100 000/µl.
Respiratory failure: ratio of partial pressure of arterial oxygen to forced inspiratory oxygen fraction of inspired oxygen <300.
Septic shock was defined as the presence of sepsis and refractory hypotension that lasts >1 h despite adequate fluid resuscitation and pharmacologic intervention with vasopressor agents.
Evidence of illness severity was assessed using the Simplified Acute Physiology Score II (SAPS II score) on the same day as blood samples were taken.
To confirm the diagnosis of sepsis, blood or other site cultures were done for all the patients on the day of admission, while all patients were screened on a daily basis for the presence of pathogens by blood and urine cultures and total peripheral blood leukocyte counts during their hospitalization. Furthermore, specific diagnostic procedures (ultrasound, computed tomography scan or gallium scan) were performed to identify the infection site.
Critically ill patients with septic shock were transferred to the intensive care unit.
Phagocytic assay
Five ml of peripheral blood were collected in heparinized tubes for investigating the phagocytic activity of neutrophils and monocytes. Another EDTA sample was collected for WBC and flow cytometric analysis of CD14 and CD64 antigens. Samples were collected from all patients on the day of their admission. A second sample was collected from patients who survived to the day of discharge.
In vitro phagocytic activity was determined using the Phagotest kit (Opregen Pharma, Heidelberg, Germany). The principle of the test is that whole blood is incubated with opsonised (by complement and immunoglobulin) E. coli that are labelled by fluorescein (fluorescein isothiocyanate, FITC). Bacteria are ingested by phagocytes generating a green fluorescence signal that can be quantified by flow cytometry [39–41]. The test was performed according to the manufacturer's instructions.
Briefly, 100 µl of heparinized whole blood were incubated for 10 min in an ice bath. Subsequently, 20 µl of pre-cooled E. coli were added to each tube. After mixing, the control samples remained in an ice bath and the test samples were incubated for 10 min at 37°C. Precisely at the end of the incubation time all samples on one rack were taken out of the water bath and placed on ice in order to stop phagocytosis. One hundred µl of ice-cold quenching solution was added to each sample and mixed well. Samples were washed twice with 3 ml of washing solution. Subsequently, whole blood was lysed and fixed by the addition of 2 ml of lysis solution. After 20 min incubation in 37°C, samples were washed once and finally incubated for 10 min with 200 µl of DNA staining solution.
Flow cytometric analysis
Samples were analysed by flow cytometry using a Coulter EPICS-XL-MCL cytometer, and the data were processed using the XL-2 software (Coulter, Miami, Florida, USA).
During data acquisition a ‘live’ gate was set in the red fluorescence histogram on those events that had at least the same DNA content as a human diploid cell.
The phagocytic ability was evaluated in neutrophils and monocytes. For that purpose the above population was gated in the software program in the scatter diagram (FCS versus SSC) and its green fluorescence histogram (FL1) was analysed. We collected 10 000–15 000 leucocytes per sample. The results were expressed as percentage of fluorescent cells in the total population studied.
Surface immunophenotyping
The percentages and absolute numbers of the peripheral blood lymphocytes subpopulations were determined by a dual-colour direct immunofluorescence technique in a whole blood method, using the following directly conjugated mouse-antihuman monoclonal antibodies (MoAbs): CD14-FITC (clone RMO52) and CD-64phycoerythrin(PE) (clone22) from Immunotech (Marseille, France). After 30-min incubation at 40°C, the red blood cells were lysed and the white blood cells were fixed with the Multi-Q-Prepsystem (Coulter, Miami, Florida, USA).The appropriate isotypic controls were used. At least 10 000 cells from each sample were analysed by flow-cytometry using a Coulter-EPICS-XL-MCL cytometer and the data were processed using the XL-2 software. Results were expressed as percentage and mean intense fluorescence (MIF) (Fig. 1).
Fig. 1.
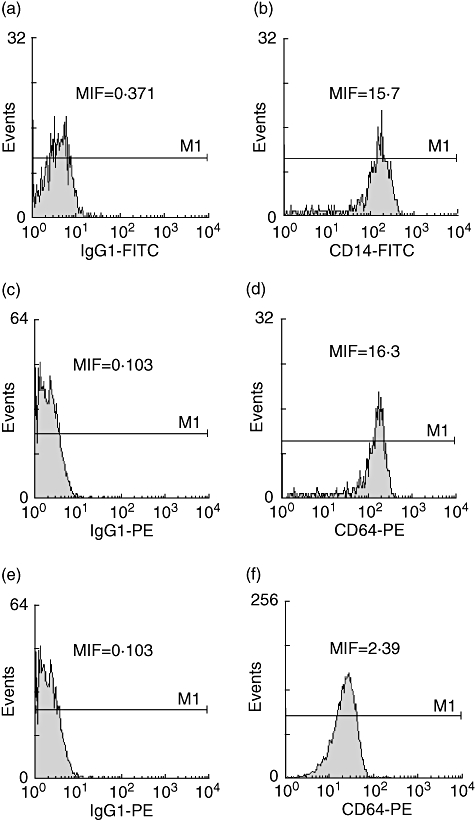
A representative fluorescence activated cell sorter experiment showing histograms comparing isotype controls with the expression of CD14 and CD64 antigens in monocytes (a–d) and the expression of CD64 in PMNs (e, f) in a survivor patient at the day of admission. MIF, mean intense fluorencence.
Statistical analysis
Values were expressed as mean ± standard deviation. Some of the patients' characteristics were expressed as prevalence rates.
Demographic data between groups were analysed using χ2 with Fischer exact two-tailed P value.
The data were checked with Kolmogorov-Smirnov's test and found to be normally distributed; therefore, parametric tests were used to compare values.
In the analysis of the study results, initial comparisons between the three groups were performed by one-way analysis-of-variance test. When significance arose, a post-hoc analysis of dyads with the Tukey's test for multiple comparisons was used for pairwise analyses.
Differences in quantitative variables in the same group were analysed by the paired t-test.
The area under a receiver operating characteristic curve (ROC) was calculated to predict the survival of the septic patients. The Youden Index (sensitivity and specificity-1) was used to identify the best cut-off point. The sensitivity, specificity, positive predictive value [PPV(%): true positive/(true positive + false positive)] and negative predictive value [NPV(%):true negative/(true negative + false negative)] are reported.
Correlations between variables on septic patients were evaluated by Pearson's correlation coefficient value. Stepwise multiple regression analysis was used to detect which variables could be predictive factors of the final outcome (dependent variable) in septic patients.
A P value of <0·05 was considered significant. All the statistical analyses were performed with spss, version 14·0 software.
Results
Characteristics of the study population
From the 41 patients enrolled in the study, 10 were excluded from further analysis due to prior antibiotic use, incomplete sampling or technical failures.
Of the remaining 31 patients, 20 patients survived while 11 patients died during their hospitalization. Twelve patients (38·7%) developed septic shock within 48 h after their admission and five of them (41·66%) died, whereas the remaining seven patients had no other complications during their hospital stay. Two patients were transferred to the intensive care unit. One of them survived.
Severe sepsis was the consequence of pneumonia (n = 9), cholangiitis (n = 5), osteomyelitis (n = 2), urinary tract infection-pyelonephritis (n = 9) and intra-abdominal infection (n = 6).
Microorganisms were isolated from a total of 10 patients (gram negative bacteria, n = 6, gram positive bacteria, n = 4). The most frequently isolated bacteria were E. coli (n = 3), Pseudomonas aeruginosa (n = 2), Staphylococcus aureus(n = 1) and Staphylococcus epidermidis (n = 3). Other gram(−) bacteria (Morganella morganii, Enterobacter cloacae, Klebsiella pneumoniae) were isolated from three patients.
Two patients developed polymicrobial bacteremia (more than one microorganism responsible for severe sepsis). More specifically, one patient had positive blood culture results for E. coli and Ps. aeruginosa while Kl. pneumoniae and Ps. aeruginosa were isolated from an another patient.
Clinical characteristics did not differ between survivors and non-survivors. The main patients' characteristics are shown in Table 1.
Table 1.
Characteristics of patients enrolled in the study.
| Characteristics | Survivors n = 20 | Non-survivors n = 11 | P-value* |
|---|---|---|---|
| Age (years) | 79(39–96)** | 80(72–89)** | n.s† |
| Sex(males/females) | 12/8 | 4/7 | |
| Simplified Acute Physiology Score II‡ | 45·6 ± 16·57 | 54·60 ± 9·87 | n.s† |
| No.(%) with gram(−) infection | 2(10%) | 4(36·36%) | n.s |
| No.(%) with gram(+) infection | 2(10%) | 2(18·18%) | n.s |
| No.(%) with hypoxemia§ | 5(25%) | 4(36·36%) | n.s |
| No.(%) with respiratory failure¶ | 0 | 3(27·27%) | n.s |
| No.(%) with renal impairment†† | 8(40%) | 5(45·45%) | n.s |
| No.(%) with disseminated intravascular coagulation‡‡ | 10(50%) | 7(63·6%) | n.s |
| Days of hospitalization | 11·58 ± 6 | 8·6 ± 6·2 | n.s† |
Fischer exact two-tailed P-value for the comparison of proportions.
Median (range).
Unpaired t-test was used for the comparison between the survivors and non-survivors. n.s., not significant.
Score on admission.
PaO2 ≤ 75 mmHg without evidence of primary lung disease.
Ratio of partial pressure of arterial oxygen to forced inspiratory oxygen fraction of inspired oxygen <300.
Creatinine concentration of >3·5 mg/dl or hemodialysis or oliguria of <250 ml/24 h.
Disseminated intravascular coagulation (disseminated intravascular coagulation) represents prothrombin time or partial thromboplastin time of ≥1·2 times the upper normal limit plus d-dimers ≥500 or platelets ≤100 000/µl.
Phagocytic activity
The phagocytic activity of PMNs on the day of admission was significantly reduced in patients with severe sepsis who did not survive compared with the healthy individuals (P = 0·0001). In contrast, the phagocytic activity of PMN of patients who survived did not differ from that of the healthy individuals (Fig. 2).
Fig. 2.
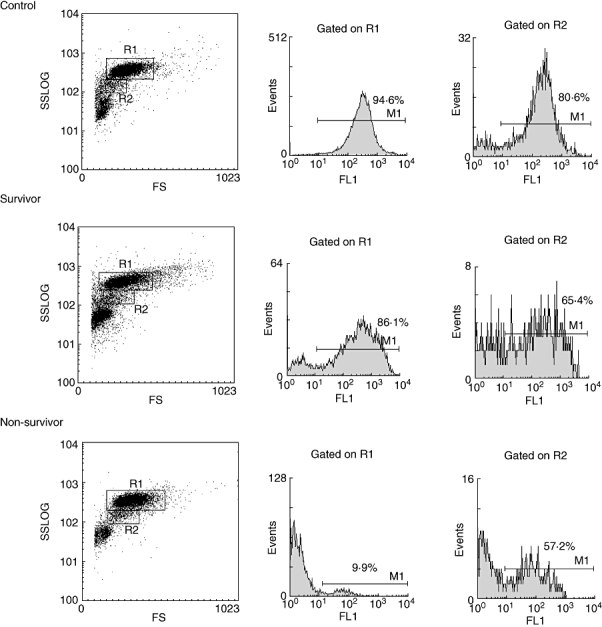
A representative fluorescence activated cell sorter experiment showing the phagocytic activity of PMNs (R1) and monocytes (R2) in a survivor patient (middle), a non-survivor patient (bottom) on the day of admission and a control (top), following incubation for 10 min at 37°C in the presence of flouroscein-labelled E. coli.
The phagocytic activity of monocytes on the day of admission was similar between the two groups of patients and the healthy controls. These data are shown in Table 2.
Table 2.
Receptor expression and phagocytic activity of PMN and monocytes on the day of admission for survivors, non-survivors and controls.
| Group | Mean ± SD | P-value* | |
|---|---|---|---|
| MIF CD14 on monocytes† | Controls | 15·56 ± 3·13 | n.s. |
| Survivors | 16·55 ± 8·44 | ||
| Non-survivors | 14·81 ± 5·14 | ||
| MIF CD64 on PMN‡ | Controls | 0·285 ± 0·119 | |
| Survivors | 3·75 ± 2·94 | 0·0001 | |
| Non-survivors | 1·08 ± 0·68 | n.s. | |
| MIF CD64 on monocytes§ | Controls | 6·70 ± 1·84 | |
| Survivors | 15·92 ± 7·39 | 0·0001 | |
| Non-survivors | 8·51 ± 2·85 | n.s. | |
| % Phago PMN¶ | Controls | 71·86 ± 14·17 | |
| Survivors | 61·48 ± 34·81 | n.s. | |
| Non-survivors | 11·82 ± 9·74 | 0·0001 | |
| % Phago monocytes†† | Controls | 52·45 ± 17·16 | n.s. |
| Survivors | 50·85 ± 20·79 | ||
| Non-survivors | 41·11 ± 18·90 |
The data are expressed as mean ± SD.
The post-hoc Tukey test was used to compare controls with survivors and controls with non-survivors.
Mean fluorescence intensity of monocyte receptor CD14 expression.
Mean fluorescence intensity of neutrophils receptor CD64 expression.
Mean fluorescence intensity of monocytes receptor CD64 expression.
Phagocytic activity of neotrophils (percentage).
Phagocytic activity of monocytes (percentage). n.s., not statistically significant.
In surviving patients the phagocytic activity of PMNs and monocytes remained similar on the day of admission and discharge. These data are shown in Table 3.
Table 3.
Receptor expression and phagocytic activity in survivors on the day of admission and the day of discharge.
| Survivors (n = 20) | |||
|---|---|---|---|
| Day of admission | Day of discharge | P-value* | |
| MIF CD14 on monocytes† | 16·55 ± 8·44 | 17·79 ± 5·18 | n.s. |
| MIF CD64 on PMN‡ | 3·75 ± 2·94 | 0·48 ± 0·31 | 0·002 |
| MIF CD64 on monocytes§ | 15·92 ± 7·39 | 9·35 ± 3·45 | 0·013 |
| % Phago PMN¶ | 61·48 ± 34·81 | 67·14 ± 33·6 | n.s. |
| % Phago monocytes†† | 50·85 ± 20·79 | 59·66 ± 19·00 | n.s. |
Data are expressed as mean ± SD.
P-values refer to the comparison between survivors on the day of admission and the day of discharge using paired t-test. n.s., not significant.
Mean fluorescence intensity of monocyte receptor CD14 expression.
Mean fluorescence intensity of neutrophils receptor CD64 expression.
Mean fluorescence intensity of monocytes receptor CD64 expression.
Phagocytic activity of neotrophils (percentage).
Phagocytic activity of monocytes (percentage).
Expression of CD64 antigen on PMNs and monocytes in patients with severe sepsis
The expression of the CD64 on PMNs and monocytes of patients with severe sepsis who survived was significantly increased in comparison with healthy controls (P = 0·0001). However, the expression of CD64 antigen on PMNs and monocytes did not differ between non-survivors and healthy controls (Table 2).
In surviving patients, a significant down-regulation of CD64 antigen expression was noted both on PMNs and monocytes on the day of discharge compared with the admission levels (P < 0·05).
Expression of CD14 antigen on monocytes in patients with severe sepsis
No statistical significance of CD14 expression on monocytes was detected between patients and the control group. The MIF of CD14 antigen on monocytes did not reveal any statistical significance between survivors and non-survivors.
Correlations
Univariate analysis showed a significant positive correlation between phagocytic activity of PMNs and expression of CD64 on monocytes and PMNs on the day of admission (r = 0·466, P = 0·025 and r = 0·518, P = 0·014 respectively). These data are depicted in Fig. 3.
Fig. 3.
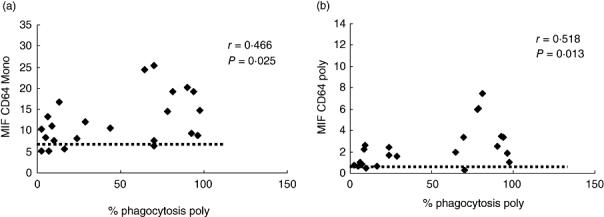
Correlation between phagocytic activity of PMNs and expression of CD64 on monocytes (a) and PMN (b) on the day of admission. Variables were evaluated by Pearson's correlation coefficient value. The horizontal dotted line represents the mean percentages of expression of CD64 on monocytes and PMN in the control group respectively.
The expression of CD64 on monocytes was strongly correlated with the expression of CD64 on PMNs (Fig. 4).
Fig. 4.
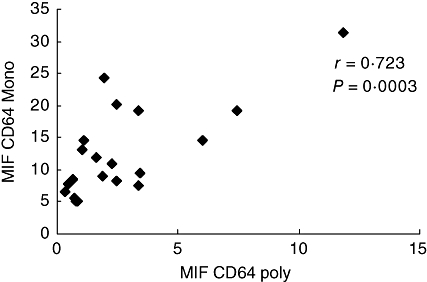
Correlation between expression of CD64 on monocytes and PMNs on the day of admission. Variables were evaluated by Pearson's correlation coefficient value.
In addition, the laboratory parameters were correlated to clinical outcome. Univariate analysis showed a significant positive correlation between the clinical outcome and the phagocytic activity of PMNs (r = 0·595, P = 0·001) as well as between the clinical outcome and the expression of CD64 on monocytes and PMNs upon admission (r = 0·521, P = 0·009 and r = 0·491, P = 0·015 respectively). No significant correlation was found between the clinical outcome and the phagocytic activity of monocytes (r = 0·314, P = 0·191). These findings indicate that increased phagocytic activity and CD64 expression on phagocytes have a favourable impact on survival.
Receiver operating characteristic curve analysis of CD64 on monocytes, CD64 on PMNs and phagocytosis of PMNs showed that phagocytosis of PMNs is the most sensitive predictor of survival in severe sepsis. These data are shown in Table 4.
Table 4.
ROC curve analysis data for CD64 expression on PMNs and monocytes and the phagocytosis of PMNs to predict survival.
| Variable | AUC | P-value | Cut-off point | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|
| MIF CD64 on PMNs | 0·892 | 0·002 | 2·45 | 60 | 100 | 100 | 53·8 |
| MIF CD64 on monocytes | 0·817 | 0·014 | 13·9 | 60 | 100 | 100 | 57 |
| % Phago PMNs | 0·889 | 0·001 | 36·1 | 72·2 | 100 | 100 | 67 |
AUC, area under the curve; PPV, positive predictive value; NPV, negative predictive value.
Simplified Acute Physiology Score II was evaluated in relation to all potentially related factors. No correlation was found between the severity of disease and the phagocytic activity of monocytes, PMNs and the expression of CD64 on PMNs upon admission. In contrast, the CD64 expression on monocytes was strongly correlated with the SAPS II score (r = −0·665, P = 0·001).
No correlation was found between traditional markers (CRP, total leucocyte count, absolute neutrophil count and monocyte count), the severity of disease and the final outcome. Furthermore, the evaluation of markers mentioned above with the phagocytic activity of phagocytes and the surface expression of CD14 and CD64 did not reveal any correlation.
The parameters found significant in univariate analysis were subsequently analysed by stepwise multiple regression analysis. The phagocytic activity of PMNs was proven to be the only independent predictor factor for survival in patients with severe sepsis, responsible for the 46% of variance in the clinical outcome (P = 0·001).
Discussion
The present study evaluated the impact of the phagocytic activity of PMN and monocytes on the outcome of patients with severe sepsis. A reduced phagocytic activity of neutrophils during the first 24 h after admission was a negative predictor for survival. In contrast, the phagocytic activity of patients' monocytes did not differ from that of normal controls and did not correlate to the final outcome.
The expression of CD64 antigen on monocytes and PMN was found increased in all patients during the first 24 h after admission compared with controls. The increased expression of CD64 on both populations correlated favourably to survival. The phagocytic activity of neutrophils was strongly correlated to the expression of CD64 on monocytes and PMN, proving the close biological significance between these parameters. ROC analysis showed that the phagocytic activity of neutrophils is a more sensitive predictor of patients' survival at 28 days compared with CD64 expression on monocytes and neutrophils. In multivariate analysis the phagocytic activity of PMN was the only independent prognostic factor for the clinical outcome of patients. These data collectively show that the increased capacity of PMN to kill bacteria and eliminate the aetiological factor of sepsis has a favourable impact on the survival of patients with severe sepsis.
The role of the effector function of neutrophils in sepsis has been poorly investigated. There are data showing that neutrophil adherence and transmigration are impaired in septic patients while in contrast production of reactive oxygen species is increased [42–44]. Hirsh et al. have previously shown that the phagocytic activity of neutrophils and monocytes is decreased in septic patients compared with controls. They have also demonstrated that in these patients the CD64+ monocytes have lower phagocytic ability than CD64− monocytes while the phagocytic ability of CD64+ PMN does not differ from that of CD64− PMN. In contrast, in the subgroup of patients with sepsis and ARDS the phagocytic activity was significantly reduced in CD64+ PMN compared with CD64− PMN.
Interestingly, our results show that patients with severe sepsis could be divided into two subgroups: those with decreased PMN phagocytic activity, lower levels of CD64 expression on PMNs and worse outcome and those with normal PMN phagocytic activity, higher levels of CD64 expression on PMNs and better outcome. This profile is difficult to interpret. Low PMN phagocytic function might represent a state of neutrophil deactivation similarly to the way that diminished HLA-DR expression on monocytes represents a state of monocytic deactivation in septic patients. Hirsh et al. have also suggested that the unresponsiveness of neutrophils noted in their study in patients with ARDS might be due to ‘exhaustion’ of cells by continuous stimulation by systemic cytokines or to a deregulation of intracellular signal pathways initiated by CD64 activation [36].
Ex vivo experiments have shown that down-regulation of HLA-DR expression on monocytes is mediated in septic patients by IL-10 and cortisol while clinical studies have correlated HLA-DR expression to IL-10 levels [6,7,13,45,46]. Many clinical studies have demonstrated that low HLA-DR expression on monocytes is predictive of mortality in these patients [7,44,47–49]. These data confirm the value of HLA-DR expression as a marker of CARS. Studies investigating the mechanisms underlying the deactivation of monocytes have shown that anti-inflammatory cytokines down-regulate the expression of HLA-DR antigen and decrease the production of pro-inflammatory cytokines in response to Toll-like receptor agonists (e.g. LPS) but not in response to whole bacteria [50,51]. Based on these findings investigators have suggested that during CARS in sepsis there is a reprogramming of leucocytes and not a true state of immunoparesis.
The experiments in the above studies have been performed after separation of mononuclear cells, therefore their results are not applied to neutrophils. The technique used in our study investigates phagocytosis of opsonized E. coli in whole blood and therefore can simultaneously evaluate both monocytes and neutrophils with minimal manipulations. Our results confirmed that monocytes of septic patients respond normally to whole bacteria. They also demonstrated that patients with severe sepsis can be subdivided into two groups based on the response of neutrophils to whole bacteria. Whether this distinction corresponds to different clinical phases of sepsis and whether impaired neutrophil phagocytosis is another manifestation of CARS are questions that can not be answered by our findings but deserve further investigations.
Alternatively, reduced PMN phagocytic activity of neutrophils might be due to genetic polymorphisms either of Fc receptors or C3 complement receptor. There is evidence that some polymorphisms of low affinity Fc receptors, FcRIIa, FcRIIIa and FcRIIIb, reduce their affinity and specifity to IgG [52]. It has been shown that PMNs of homozygous FcRIIa -R/R131 donors internalize IgG2 opsonized meningocci less than PMNs from FcRIIa -H/H131 donors and that individuals with the former polymorphism have increased susceptibility to meningococcal disease [53,54].
Many clinical studies have shown the sensitivity and specificity of CD64 expression on PMN for the diagnosis of sepsis compared with global markers of inflammation such as CRP and PCT and measurement of individual cytokines [55–57]. Even more, there are reports showing that the PMN CD64 expression distinguishes the clinical stages of sepsis (i.e sepsis, severe sepsis and septic shock) [37]. In contrast, the data on its predictive value are few. Livaditis et al. have recently shown that the increased CD64 neutrophil expression is adversely correlated to the outcome of patients with sepsis [37]. Muller et al., evaluating the prognostic value of a wide range of markers reflecting different stages of priming, adhesion and activation of monocytes and neutrophils, have shown that overall increase in the expression of markers of neutrophil and monocyte activation is related to favourable outcome in septic patients. The only exception was the expression of CD64, which was increased in the non-survivors and reduced in the survivors [47]. Recently, Song et al. have shown that increased neutrophil CD64 expression correlates to 28-day mortality of patients with disseminated intravascular coagulation, including patients with sepsis [56]. Our findings, in contrast to the above studies, show that increased PMNs expression is associated with a favourable outcome for patients with severe sepsis. This discrepancy might be due to differences in the study population, as the other studies included all subgroups of patients while we enrolled only patients with severe sepsis. However, it can also be due to the kinetics of CD64 antigen expression on PMNs that is induced by the pro-inflammatory cytokines, but its expression might persist during the compensatory phase of the anti-inflammatory response. In normal individuals CD64 antigen expression on PMNs increases rapidly, in 4 h, after administration of a single injection of G-CSF and returns to baseline levels in 5 days, while after IFN-γ it returns to baseline levels in 7 days [28,35]. Although CD64 expression has been proven to be induced on monocytes by IL-10, this has not been demonstrated for neutrophils, therefore it can not be argued that its expression is stimulated directly by anti-inflammatory cytokines [58].
The data on the kinetics of CD64 expression on PMNs indicate that its prognostic significance might be compromised by its relatively long persistence and that its combination with other markers such as HLA-DR might improve its performance.
The present study demonstrated that reduced PMNs phagocytosis upon admission is a negative predictor of survival of patients with severe sepsis. It is unclear whether this functional impairment of PMNs represents a state of functional deactivation and whether this is a manifestation of CARS. The use of a combination of markers at diagnosis of septic patients, including PMNs CD64 expression, HLA-DR expression on monocytes and phagocytic function of PMNs, would lead to better classification of patients and more accurate prediction, and would help in the use of targeted treatments.
References
- 1.Bone RC, Balk R, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest. 1992;101:1644–55. doi: 10.1378/chest.101.6.1644. [DOI] [PubMed] [Google Scholar]
- 2.Gullo A, Iscra F, Capua GDI, et al. Sepsis and organ dysfunction: an ongoing challenge. Minerva Anestesiol. 2005;71:671–99. [PubMed] [Google Scholar]
- 3.Davis BH. Improved diagnostic approaches to infection/sepsis detection. Expert Rev Mol Diagn. 2005;5:193–207. doi: 10.1586/14737159.5.2.193. [DOI] [PubMed] [Google Scholar]
- 4.Annane D, Bellisant E, Cavaillon JM. Septic shock. Lancet. 2005;365:63–78. doi: 10.1016/S0140-6736(04)17667-8. [DOI] [PubMed] [Google Scholar]
- 5.Reddy RC, Chen GH, Tekchandani PK, Standiford TJ. Sepsis-induced immunosuppression: from bad to worse. Immunol Res. 2001;24:273–87. doi: 10.1385/IR:24:3:273. [DOI] [PubMed] [Google Scholar]
- 6.Wolk K, Döcke W, von Baehr V, Volk H, Sabat R. Comparison of monocyte functions after LPS-or IL-10 induced reorientation: importance in clinical immunoparalysis. Pathobiology. 1999;67:253–6. doi: 10.1159/000028104. [DOI] [PubMed] [Google Scholar]
- 7.Monneret G, Finck M-E, Venet F, et al. The anti-inflammatory response dominates after septic shock: association of low monocyte HLA-DR expression and high interleukin-10 concentration. Immunol Lett. 2004;95:193–8. doi: 10.1016/j.imlet.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 8.Casey LC, Balk RA, Bone RC. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome. Ann Intern Med. 1993;119:771–8. doi: 10.7326/0003-4819-119-8-199310150-00001. [DOI] [PubMed] [Google Scholar]
- 9.Damas P, Canivet JL, de Groote D, et al. Sepsis and serum cytokine concentrations. Crit Care Med. 1997;25:405–12. doi: 10.1097/00003246-199703000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Lin RY, Astiz ME, Saxon JC, Saha DC, Rackow EC. Relationships between plasma cytokine concentrations and leukocyte functional antigen expression in patients with sepsis. Crit Care Med. 1994;22:1595–602. [PubMed] [Google Scholar]
- 11.Spittler A, Razenberger M, Kupper H, et al. Relationship between Interleukin 6 plasma concentration in patients with sepsis, monocyte phenotype, monocyte phagocytic properties, and cytokine production. Clin Infect Dis. 2000;31:1338–42. doi: 10.1086/317499. [DOI] [PubMed] [Google Scholar]
- 12.Gogos C, Drossou E, Bassiaris H, Skoutelis A. Pro- versus anti-inflammatory cytokine profile in patients with severe sepsis: a marker for prognosis and future therapeutic options. J Infect Dis. 2000;181:176–80. doi: 10.1086/315214. [DOI] [PubMed] [Google Scholar]
- 13.Lekkou A, Karakantza M, Mouzaki A, Kalfarentzos F, Gogos C. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired severe infections. Clin Diagn Lab Immunol. 2004;11:161–7. doi: 10.1128/CDLI.11.1.161-167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–60. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall JC. Much stuff as dreams are made on: mediator-directed therapy in sepsis. Nat Rev Drug Discov. 2003;2:391–405. doi: 10.1038/nrd1084. [DOI] [PubMed] [Google Scholar]
- 16.Lin MT, Albertson TE. Genomic polymomorphims in sepsis. Crit Care Med. 2004;32:569–79. doi: 10.1097/01.CCM.0000110878.49476.42. [DOI] [PubMed] [Google Scholar]
- 17.Mitaka C. Clinical laboratory differentiation of infectious versus non- infectious systemic inflammatory response syndrome. Clin Chim Acta. 2005;351:17–29. doi: 10.1016/j.cccn.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 18.Simon L, Gauvin F, Amre DK, Saint-Lewis P, Lacroix J. Serum procalcitonin and C-reactine protein levels as markers of bacterial infection: a systemic review and meta-analysis. Clin Infect Dis. 2004;39:206–17. doi: 10.1086/421997. [DOI] [PubMed] [Google Scholar]
- 19.Clec'h C, Ferriere F, Karoubi P, et al. Diagnostic and prognostic value of procalcitonin in patients with septic shock. Crit Care Med. 2004;32:1166–9. doi: 10.1097/01.ccm.0000126263.00551.06. [DOI] [PubMed] [Google Scholar]
- 20.Fadlon E, Vordermeier S, Pearson TC, et al. Blood polymorphonuclear leucocytes from the majority of sickle cell patients in the crisis phase of the disease show enhanced adhesion to vascular endothelium and increased expression of CD64. Blood. 1998;91:266–74. [PubMed] [Google Scholar]
- 21.van der Meer W, Pickkers P, Scott CS, van der Hoeven JG, Gunnewiek JK. Hematological indices, inflammatory markers and CD64 expression: comparative trends during experimental human endotoxemia. J Endotoxin Res. 2007;13:94–100. doi: 10.1177/0968051907079101. [DOI] [PubMed] [Google Scholar]
- 22.Haziot A, Chen S, Ferrero E, Low MG, Silber R, Goyert SM. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1998;141:547–52. [PubMed] [Google Scholar]
- 23.Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC. CD14, a receptor for complexes of lipopolysacharide (LPS) and LPS binding protein. Science. 1990;249:1431–3. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 24.Cleveland MG, Gorham JD, Murphy TL, Tuomanen E, Murphy KM. Lipoteichoic acid preparations of gram positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–12. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusonoki T, Hailman E, Juan TS, Lichenstein HS, Wright SD. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate responses. J Exp Med. 1995;182:1673–82. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pugin J, Heumann D, Tomasz A, et al. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–16. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 27.Akerley WL, 3rd, Guyre PM, Davis BH. Neutrophil activation through high-affinity Fc? receptor using a monomeric antibody with unique properties. Blood. 1991;77:607–15. [PubMed] [Google Scholar]
- 28.Schiff DE, Rae J, Martin TR, Davis B, Curnutte JT. Increased phagocyte CD64 expression and improved Fc-receptor mediated phagocytosis following in vivo recombinant human interferon-γ treatment of normal human subjects. Blood. 1997;90:3187–94. [PubMed] [Google Scholar]
- 29.Michon JM, Gey A, Moutel S, et al. In vivo induction of functional Fc-gamma RI CD64 on neutrophils and modulation of blood cytokine m RNA levels in cancer patients treated with G-CSF (rMetHuG-CSF) Br J Haematol. 1998;100:550–6. doi: 10.1046/j.1365-2141.1998.00597.x. [DOI] [PubMed] [Google Scholar]
- 30.Davis BH, Bigelow NC. Comparison of neutrophil CD64 expression, manual myeloid immaturity counts and automated haematology analyser flags as indicators of infection or sepsis. Lab Haematol. 2005;11:137–47. [PubMed] [Google Scholar]
- 31.Takano K, Kaganoi J, Yamamoto K, Takahashi A, Kido T, Sasada M. Rapid and prominent up-regulation of high-affinity receptor for immunoglobulin G (Fcgamma RI) by cross-linking of beta 2 integrins on polymorphonuclear leukocytes. Int J Hematol. 2000;72:48–54. [PubMed] [Google Scholar]
- 32.Guyre PM, Graziano RF, Vance BA, Morganelli PM, Fanger MW. Monoclonal antibodies that bind to distinct epitopes on FcγRI are able to trigger receptor function. J Immunol. 1989;143:1650–5. [PubMed] [Google Scholar]
- 33.Pfefferkorn LC, Fanger MW. Transient activation of the NADPH oxidase through FcγRI. J Immunol. 1989;143:2640–9. [PubMed] [Google Scholar]
- 34.Wallace PK, Keler T, Coleman K, et al. Humanized mab H22 binds the human high affinity Fc receptor for IgG(FcgammaR1), blocks phagocytosis and modulates receptor expression. J Leukoc Biol. 1997;62:469–79. doi: 10.1002/jlb.62.4.469. [DOI] [PubMed] [Google Scholar]
- 35.Kerst JM, de Haas M, van der Schoot CE, et al. Recombinant granulocyte colony stimulating factor administration to healthy volunteers: induction of immunophenotypically and functionally altered neutrophils via an effect on myeloid progenitor cells. Blood. 1993;82:3265–72. [PubMed] [Google Scholar]
- 36.Hirsh M, Mahamid E, Bashenko Y, Hirsh I, Krausz MM. Overexpression of the high affinity Fc-γ receptor (CD64) is associated with leucocyte dysfunction in sepsis. Shock. 2001;16:102–8. doi: 10.1097/00024382-200116020-00003. [DOI] [PubMed] [Google Scholar]
- 37.Livaditi O, Kotanidou A, Psarra A, et al. Neutrophil CD64 expression and serum IL-8: sensitive early markers of severity and outcome in sepsis. Cytokine. 2006;36:283–90. doi: 10.1016/j.cyto.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 38.American College of Chest Physicians/Society of Critical Medicine Consensus Conference. Definition for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 39.Hirt W, Nebe T, Birr C. Phagotest and Bursttest (Phagoburst), test kits for study of phagocyte functions. Wien Klin Wochenschr. 1994;106:250–2. [PubMed] [Google Scholar]
- 40.Cacciapuoti C, Terrazzano G, Barone L, et al. Glycosyl-phosphatidyl-inositol-defective granulocytes from paroxysmal nocturnal haemoglobinuria patients show increased bacterial ingestion but reduced respiratory burst induction. Am J Hematol. 2007;82:98–107. doi: 10.1002/ajh.20779. [DOI] [PubMed] [Google Scholar]
- 41.Shalekoff S, Tiemessen CT, Gray CM, Martin DJ. Depressed phagocytosis and oxidative burst in polymorphonuclear leukocytes from individuals with pulmonary tuberculosis with or without human immunodeficiency virus type 1 infection. Clin Diagn Lab Immunol. 1998;5:41–4. doi: 10.1128/cdli.5.1.41-44.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tellado JM, Christou NV. Critically ill anergic patients demonstrate polymorphonuclear neutrophil activation in the intravascular compartment with decreased cell delivery to inflammatory focci. J Leukoc Biol. 1991;50:547–53. doi: 10.1002/jlb.50.6.547. [DOI] [PubMed] [Google Scholar]
- 43.Terregrino CA, Lubkin CL, Thom SR. Impaired neutrophil adherence as an early marker of systemic inflammatory response syndrome and severe sepsis. Ann Emerg Med. 1997;29:400–3. doi: 10.1016/s0196-0644(97)70353-6. [DOI] [PubMed] [Google Scholar]
- 44.Alves-Filho JC, Tavares-Murta BM, Barja-Fidalgo C, et al. Neutrophil function in severe sepsis. Endocr Metab Immune Disord Drug Targets. 2006;6:151–8. doi: 10.2174/187153006777442404. [DOI] [PubMed] [Google Scholar]
- 45.Le Tulzo Y, Pangault C, Amiot L, et al. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med. 2004;169:1144–51. doi: 10.1164/rccm.200309-1329OC. [DOI] [PubMed] [Google Scholar]
- 46.Fumeaux T, Pugin J. Role of interleukin-10 in the intracellular sequestration of human leukocyte antigen-DR in monocytes during septic shock. Am J Respir Crit Care Med. 2002;166:1475–82. doi: 10.1164/rccm.200203-217OC. [DOI] [PubMed] [Google Scholar]
- 47.Muller Kobold AC, Tulleken JE, Zijlstra JG, et al. Leukocyte activation in sepsis; correlations with disease state and mortality. Int Care Med. 2000;26:883–92. doi: 10.1007/s001340051277. [DOI] [PubMed] [Google Scholar]
- 48.Xiao H, Siddiqui J, Remick DG. Mechanisms of mortality in early and late sepsis. Infect Immun. 2006;74:5227–35. doi: 10.1128/IAI.01220-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monneret G, Lepape A, Bohe J, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–83. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 50.Cavaillon JM, Adrie C, Fitting C, Adib-Conquy M. Reprogramming of circulatory cells in sepsis and SIRS. J Endotoxin Res. 2005;11:311–20. doi: 10.1179/096805105X58733. [DOI] [PubMed] [Google Scholar]
- 51.Adip-Conquy M, Moine P, Asehnoune K, et al. Toll-like receptor-mediated tumor necrosis factor and interleukin-10 production differ during systemic inflammation. Am J Respir Crit Care Med. 2003;168:158–64. doi: 10.1164/rccm.200209-1077OC. [DOI] [PubMed] [Google Scholar]
- 52.va der Pol W, van de Winkel JG. IgG receptor polymorphisms: risk factors for disease. Immunogenetics. 1998;48:222–32. doi: 10.1007/s002510050426. [DOI] [PubMed] [Google Scholar]
- 53.Fijen CA, Bredius RG, Kuijper EJ, et al. The role of Fcgamma receptor polymorphisms and C3 in the immune defence against Neisseria meningitidis in complement-deficient individuals. Clin Exp Immunol. 2000;120:338–45. doi: 10.1046/j.1365-2249.2000.01208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Platonov AE, Kuijper EJ, Vershinina IV, et al. Meningococcal disease and polymorphisms of Fc gammaRIIa (CD32) in late complement component-deficient individuals. Clin Exp Immunol. 1998;111:97–101. doi: 10.1046/j.1365-2249.1998.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis BH, Olsen SH, Ahmad E, Bigelow NC. Neutrophil CD64 is an improved indicator of infection or sepsis in emergency department patients. Arch Pathol Lab Med. 2006;130:654–61. doi: 10.5858/2006-130-654-NCIAII. [DOI] [PubMed] [Google Scholar]
- 56.Song SH, Kim HK, Park MH, Cho H-I. Neutrophil CD64 expression is associated with severity and prognosis of disseminated intravascular coagulation. Thromb Res. 2008;121:499–507. doi: 10.1016/j.thromres.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 57.Bhandari V, Wang C, Rinder C, Rinder H. Hematologic profile of sepsis in neonates: neutrophil CD64 as a diagnostic marker. Pediatrics. 2008;121:129–34. doi: 10.1542/peds.2007-1308. [DOI] [PubMed] [Google Scholar]
- 58.Bovolenta C, Gasperini S, Mc Donald PP, Cassatella MA. High affinity receptor for IgG (FcγR1/CD64) gene and STAT protein binding to the IFN-γ response region (GRR) are regulated differentially in human neutrophils and monocytes by IL-10. J Immunol. 1998;160:911–19. [PubMed] [Google Scholar]


