Abstract
In higher eukaryotic cells, the spindle forms along with chromosome condensation in mitotic prophase. In metaphase, chromosomes are aligned on the spindle with sister kinetochores facing toward the opposite poles. In anaphase A, sister chromatids separate from each other without spindle extension, whereas spindle elongation takes place during anaphase B. We have critically examined whether such mitotic stages also occur in a lower eukaryote, Schizosaccharomyces pombe. Using the green fluorescent protein tagging technique, early mitotic to late anaphase events were observed in living fission yeast cells. S. pombe has three phases in spindle dynamics, spindle formation (phase 1), constant spindle length (phase 2), and spindle extension (phase 3). Sister centromere separation (anaphase A) rapidly occurred at the end of phase 2. The centromere showed dynamic movements throughout phase 2 as it moved back and forth and was transiently split in two before its separation, suggesting that the centromere was positioned in a bioriented manner toward the poles at metaphase. Microtubule-associating Dis1 was required for the occurrence of constant spindle length and centromere movement in phase 2. Normal transition from phase 2 to 3 needed DNA topoisomerase II and Cut1 but not Cut14. The duration of each phase was highly dependent on temperature.
INTRODUCTION
The fission yeast Schizosaccharomyces pombe is an excellent model organism in which to study mitosis, because many genes required for mitosis have been identified, and their products have been characterized by cellular and molecular biological methods (e.g., Yanagida, 1995, 1998; Su and Yanagida, 1997). S. pombe cells in interphase have the nuclei positioned in the middle with well-developed cytoskeletal networks. Approximately two-thirds to three-fourths of the cell cycle is postreplicative G2 interphase, during which a rodlike cell becomes progressively longer. Cells cease growing, however, in mitosis, during which chromosomes condense and the spindle forms, followed by rapid sister chromatid separation and nuclear division.
In this study, mitotic events in living S. pombe cells were investigated by using green fluorescent protein (GFP)-tagged spindle pole body (SPB) protein and also centromeric DNA. The GFP tagging technique was successfully introduced in S. pombe to visualize the spindle using a GFP–Dis1 construct (Nabeshima et al., 1995), but it has not been possible to visualize the centromere DNA in living cells. We applied the method developed for observing the Saccharomyces cerevisiae centromere DNA by GFP-tagged Lac repressor (designated LacI hereafter), which was bound to the repeated LacO DNA sequences integrated onto the centromere proximal position (Robinett et al., 1996; Straight et al., 1996, 1997). The centromere DNA and the SPBs were thus visible simultaneously for the first time in living fission yeast cells.
The entry into and the exit from M phase of S. pombe are characterized, respectively, by the rise and the fall of Cdc2–Cdc13 (mitotic cyclin) kinase activity (e.g., Yamano et al., 1996; MacNeill and Nurse, 1997). During the early stages of mitosis, the SPBs separate from each other, the spindle forms between them, and cytoplasmic microtubules disappear (e.g., Masuda et al., 1992). The interphase centromeres are known to be clustered and located near the SPB (Takahashi et al., 1992; Funabiki et al., 1993). In situ hybridization with the centromere DNA indicated that this interphase centromere clustering was disrupted on entry into mitosis, and that the individual centromeres appeared to interact with the short spindle (Funabiki et al., 1993; Saitoh et al., 1997).
Mitosis in larger eukaryotic cells can be divided into four stages, prophase, prometaphase, metaphase, and anaphase. It is unknown, however, whether a stage identical to prophase exists in fission yeast cells, because nuclear envelope breakdown, a prophase event, does not take place in yeast cells. Therefore, the period of spindle formation was designated as a prophase-like stage. In the in situ hybridization study of fission yeast (Funabiki et al., 1993), metaphase was defined as the period in which cells had a short spindle, whose length was similar to the diameter of the interphase nucleus. Anaphase A was defined as the period of sister chromatid separation. The small size of fission yeast chromosomes did not allow us to observe precisely how individual chromosomes were positioned on the short spindle and when they established biorientation toward the spindle poles. The prometaphase stage in which chromosomes do not complete biorientation but move along the short spindle is thus difficult to assign and was included as a part of metaphase in the previous and present studies.
The kinase activity of Cdc2–Cdc13 complexes is thought to be high from prophase to metaphase. Critical mitotic proteins are degraded by anaphase-promoting complex (cyclosome)-mediated ubiquitination and subsequent proteasome-dependent proteolysis. The destruction of mitotic cyclin (Cdc13) leads to mitotic exit, and the destruction of Cut2 induces sister chromatid separation (Funabiki et al., 1996; Yamano et al., 1996). Previous experiments indicated that sister chromatids were separated without a significant increase in the distance between the SPBs (Funabiki et al., 1993; Saitoh et al., 1997) so that an anaphase A-like stage should occur in S. pombe. However, the precise timing of sister chromatid separation and the interval from prophase to metaphase were hard to determine. We used GFP to mark the SPBs and centromeres and performed microscopy on living cells to address the following questions: 1) how the centromere and SPB move in different stages of mitosis; 2) when sister centromeres are bioriented in the spindle; and 3) exactly when sister centromeres separate from each other.
MATERIALS AND METHODS
Preparation of Specimens
HM123 (h− leu1) was used as a wild-type strain of S. pombe. Wild-type cells carrying plasmid with the GFP-tagged sad1+ gene were exponentially grown to 3 × 106/ml in 20 ml minimal EMM2 medium. One or 2 ml of the culture were centrifuged at 10,000 rpm for 1 min and resuspended in 80–100 μl Edinburgh minimal medium 2 (EMM2). Twenty to 30 μl of resuspended culture were placed on a glass-bottom culture dish (P35G-0-10-C, MatTeck, Ashland, MA). The technical details were described by Nabeshima et al. (1997). The coverslip of the above culture dish was previously coated with concanavalin A (1 mg/ml). Cells were adsorbed to the coated coverslip by incubation for 30 min in the dish containing a wet Kimwipe paper (Kimberly-Clark, Dallas, TX) and sealed with parafilm. Under a microscope cells were incubated at 36, 33, 26, or 20°C with a temperature control unit (Chikashige et al., 1994; Ding et al., 1998). To observe mutant dis1 null cells cultured at 20°C, which was the restrictive temperature (Ohkura et al., 1988), the dis1 null strain was transformed with pSD8, which carried the Sad1–GFP gene. The resulting transformant cells were grown exponentially in 20 ml rich YPD (cell concentration, 1–2 × 106/ml) and transferred to 20°C for 1–2 h. Then an aliquot (1–2 ml) of the culture was taken, centrifuged, resuspended in 80–100 μl synthetic EMM2, and placed on the glass-bottom culture dish. Specimens were observed under a microscope in a room kept at 20°C. Temperature-sensitive strains top2, cut1, cut3, and cut14 carrying plasmid pSD8 were similarly treated and observed at the restrictive (36°C) and permissive (26°C) temperatures.
Construction of a Fission Yeast Strain for Visualization of Centromeric DNA
A haploid S. pombe strain h− lys1 his7 was simultaneously transformed with the two plasmids pMK24A and pMK2A. pMK24A carried the GFP–LacI–nuclear localization signal (NLS) (Straight et al., 1996) driven by the promoter region of dis1+ (Nabeshima et al., 1995) and the his7+ gene. The plasmid pMK2A contained the LacO repeat (7.8-kb SalI–XhoI fragment from pAFS59) and the lys1+ gene (2.2-kb HindIII, containing the N terminus), which is tightly linked to the cen1 locus (∼30 kb; Takahashi et al., 1992). Plasmids were linearized by the cleavage within the his7+ or the lys1+ gene, respectively (pMK24A with ClaI and pMK2A with HpaI) and used for transformation. Resulting Lys+ His+ stable transformants were isolated. The transformant MKY7A-4 contained the integrated GFP–LacI–NLS fusion gene at the his7 locus and the LacO array on the lys1 locus. Correct integration at the two chromosomal loci was verified by genomic Southern hybridization.
Plasmids and Mutant Strains
The fission yeast sad1+ gene codes for an SPB component (Hagan and Yanagida, 1995), and the plasmid pSD8 that was constructed in the present study contains a protein fusion between Sad1 and GFP. A 2.3-kb-long fragment containing the entire open reading frame and 0.5 kb of upstream sequences from the fission yeast sad1+ gene was inserted in front of the GFP gene so that the C terminus of Sad1 was joined to the N terminus of jellyfish GFP.
For visualizing the centromeric DNA–GFP and Sad1–GFP simultaneously, the S. pombe strain MKY7A-4 was transformed with pSD8. The temperature- and cold-sensitive mutant strains used in the present study were dis1 null (Nabeshima et al., 1995), cut1-206 (Uzawa et al., 1990), top2-191 (Uemura and Yanagida, 1984), and cut14-208 (Saka et al., 1994).
Microscopy
Fluorescence microscopy was as previously described (Chikashige et al., 1994; Nabeshima et al., 1997). Details of the temperature control and computer systems were described by Ding et al. (1998). Living cells were mounted in a glass-bottom culture dish. Time-lapse images were taken at 30- or 60-s intervals with each exposure of 0.2–0.5 s; data for each single cell were taken with a total exposure time of 12–50 s. A microscope focus was adjusted under a computer control and a single-focal plane was presented for each time point in most cases; full three-dimensional time-lapse images were obtained in some cases. Microscope image data were obtained using the Resolve3D program on a Silicon Graphics (Mountain View, CA) IRIS35/GT workstation (Hiraoka et al., 1991), and image processing, analysis, and display was carried out using the DeltaVision program (Applied Precision, Seattle, WA) on a Silicon Graphics Indigo2 workstation.
RESULTS
Visualization of SPB Movements in Mitosis
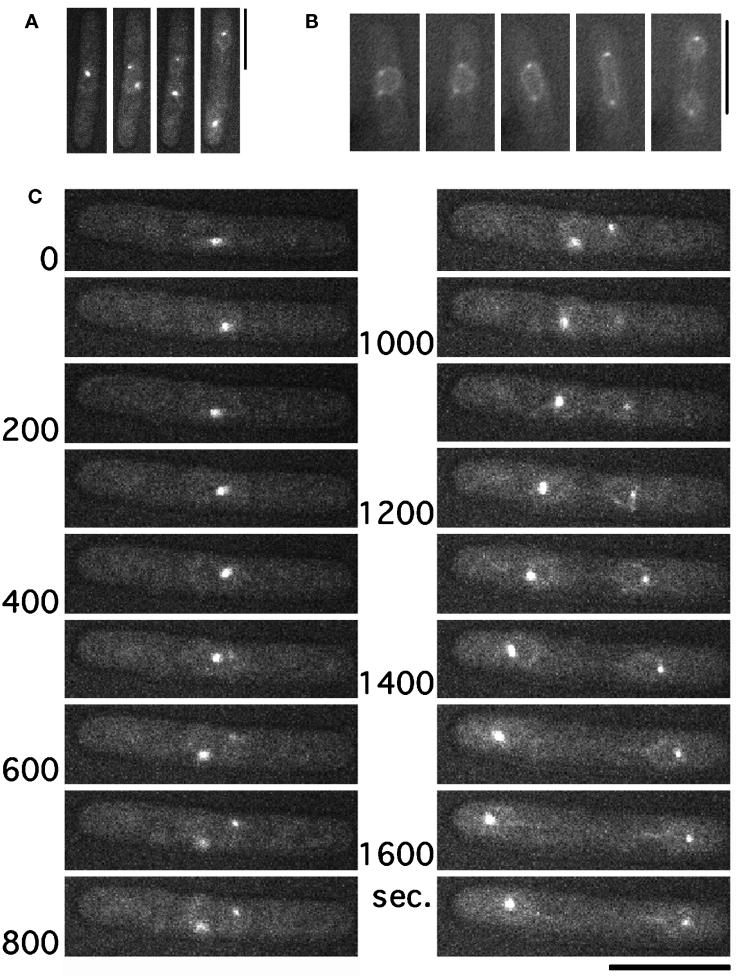
To observe the SPB in living cells, GFP-tagged Sad1 (designated hereafter Sad1–GFP) was expressed and found to be bound to the SPB throughout the cell cycle (Figure 1A), identical to immunolocalization data (Hagan and Yanagida, 1995). Fluorescence was also seen at the nuclear envelope (Figure 1B), most likely because of the increased dosage of Sad1 produced by a multicopy plasmid, although growth parameters were not affected (Hagan and Yanagida, 1995). This nuclear envelope fluorescence was convenient for monitoring nuclear division.
Figure 1.
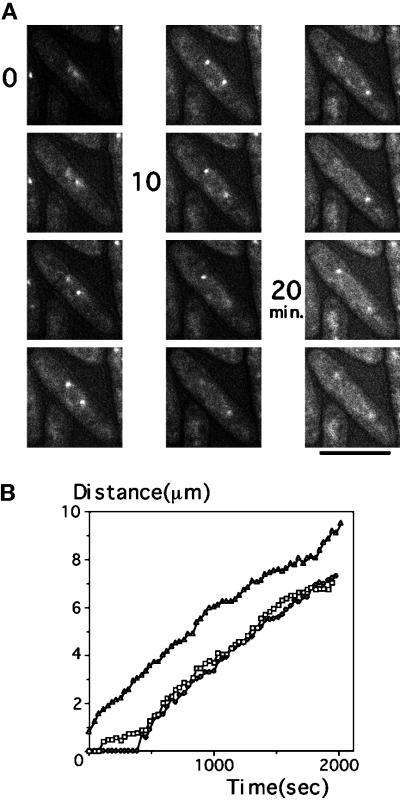
Visualization of GFP-tagged Sad1, an SPB protein, in living cells (A and B) Wild-type cells transformed with plasmid carrying the Sad1–GFP fusion gene were observed at 33°C. Green fluorescence was found at the SPB positions and also at the nuclear envelope, as seen in cells carrying an elevated gene dosage of sad1+ (Hagan and Yanagida, 1995). Bars, 10 μm. (C) Images of a single wild-type cell expressing Sad1–GFP (the number indicates time in seconds). One of multiple images obtained for each time point is shown. Through-focusing was particularly necessary during the short spindle stage to ensure the number and localization of the GFP signal; this was done because the nucleus at this stage often made rotatory movements. Bar, 10 μm.
Images of one living cell at 33°C (taken by a cooled charged-coupled device camera attached to a microscope in a temperature-controlled room) are shown in Figure 1C. The cell initially contained a single SPB, demonstrating that it was in G2 (at 0 s). Separation of the SPB (e.g., spindle formation), which marks entry into mitosis, occurred between 400 and 500 s. One of the duplicated GFP–SPB signals was weak, because it was not in the focal plane. The spindle length reached 2.5 μm and was roughly constant from 700 to 900 s. Spindle length began to increase again at 900 s and continued to 1500 s, reaching 15 μm, the maximal length. The spindle axis was initially oblique to the cell axis but rotated toward the direction parallel to the cell axis during elongation. Simultaneous focusing of the two SPBs was thus often difficult when the spindle length was short. The presence of two SPBs, however, could be seen by through-focusing at each time point. After full spindle elongation, the daughter nuclei made backward movement toward the center of daughter cells (1600 s), which is a microtubule-dependent process (Hagan and Yanagida, 1997), followed by cytokinesis and septation.
Three Distinct Phases of SPB Movements
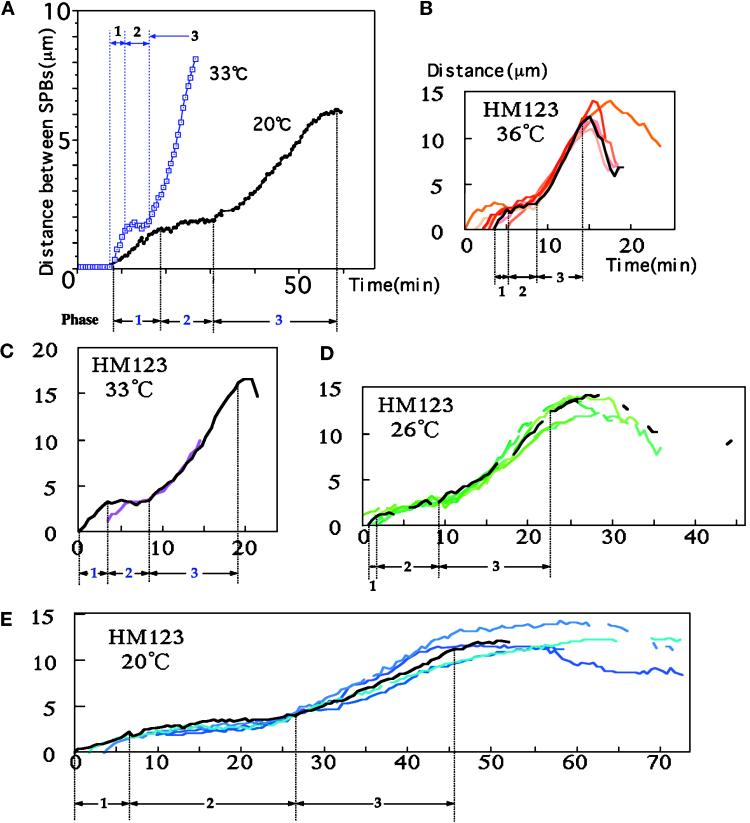
Distances between the separated SPBs were measured in living wild-type cells (strain HM123) cultured at different temperatures (20, 26, 33, and 36°C; Figure 2). Quantitative measurements revealed three phases in the movements of the SPBs (Figure 2A). Phase 1 corresponded to the period of spindle formation (e.g., a prophase-like stage), in which the spindle length increased from 0 to 2.5 μm; phase 2 represented the period of constant spindle length (e.g., metaphase–anaphase A); and phase 3 was the period of spindle extension from 2.5 to 12–15 μm (anaphase B). These three distinct phases were found in all the wild-type cells examined, although the duration of each phase was strongly dependent on the temperature used (Figure 2, B–E).
Figure 2.
Time course changes of the pole-to-pole distances during mitosis; 1, 2, and 3 represent the periods of phases 1, 2, and 3. (A) Time course changes for the distances between the mitotic SPBs (in micrometers) of two wild-type cells cultured at 33 or 20°C are shown for comparison. (B–E) Data of the distances between the SPBs obtained from cells cultured at 36°C (B), 33°C (C), 26°C (D), and 20°C (E) are plotted.
The average time duration of the three phases at 20, 26, and 36°C is shown in Table 1. The time from the initiation of spindle formation to the completion of late anaphase spindle extension was ∼12 min at 36°C and 3.75-fold longer (45 min) at 20°C. Note that the generation time at 20°C is 2.5-fold longer than that at 36°C (Table 1), so that the relative duration of mitosis became longer at a lower temperature. Phase 2 was particularly temperature dependent; it was only 4 min at 36°C but 19 min (4.8-fold increase) at 20°C. It may be noteworthy that a number of spindle-defective mutants were isolated as cold sensitive (e.g., Hiraoka et al., 1984).
Table 1.
Duration of three phases at different temperature
| Phases | Cell cycle stage | Spindle | Duration (min)
|
||
|---|---|---|---|---|---|
| 20°C | 26°C | 36°C | |||
| Phase 1 | Prophase | Formation | 4.34±1.56 | 2.50±1.08 | 1.36±0.52 |
| Phase 2 | Metaphase, Anaphase A | Constant length | 18.66±0.61 | 7.00±0.71 | 4.00±2.02 |
| Phase 3 | Anaphase B | Increasing length | 21.91±2.37 | 10.50±2.27 | 6.21±0.75 |
| Whole cell cycle | 330 | 200 | 130 | ||
Interestingly, the rates at which the SPBs separated from each other in phases 1 and 3 were similar (1.3 ± 0.3 and 1.4 ± 0.2 μm/min at 36°C, respectively) and temperature dependent (0.47 ± 0.1 and 0.38 ± 0.1 μm/min at 20°C, respectively). In phase 2, the spindle elongated very slowly; the rates of SPB separation were 0.08 and 0.2 μm/min at 20 and 36°C, respectively.
Visualization of the Centromeric DNA in Mitosis
Previous studies (Funabiki et al., 1993; Saitoh et al., 1997) strongly suggested that anaphase A existed in S. pombe. It was unknown, however, how and exactly when sister centromere DNAs actually moved during mitosis. Direct information on centromere DNA movements was needed to answer the question. The ability of GFP–LacI fusion to bind to long tandem arrays of the Lac operator allows chromosomes to be followed in living cells (Robinett et al., 1996; Straight et al., 1996, 1997). We integrated 256 copies of LacO at the lys1 gene, which is located 30 kb from the centromere of chromosome I (cen1) (Takahashi et al., 1992) in S. pombe (Figure 3A; see MATERIALS AND METHODS). A fusion gene encoding GFP–LacI tagged with an NLS was integrated at a second site in the genome. Correct integration was verified by genomic Southern hybridization (our unpublished result). The expressed GFP–LacI–NLS protein could thus enter the nucleus and specifically bind to the operator sequences linked to cen1, allowing visualization of the movements of cen1 in living cells.
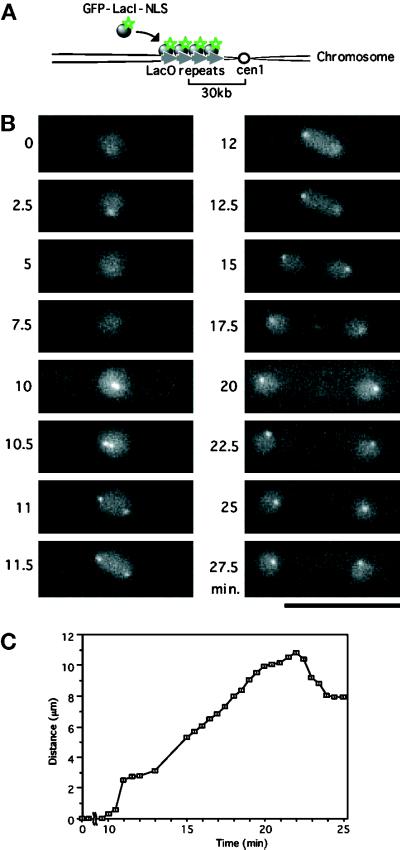
Figure 3.
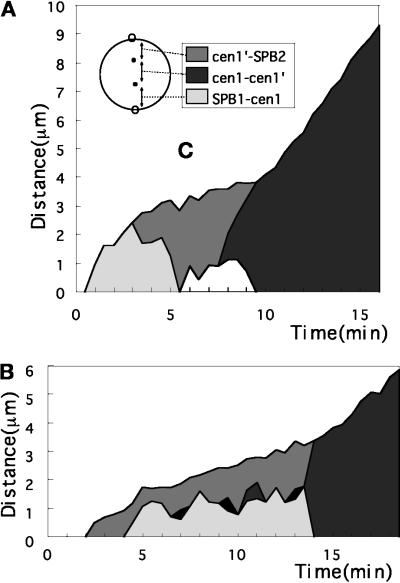
Visualization of the centromeric DNA in living cells. (A) Schematic representation for construction of an S. pombe strain expressing GFP–LacI–NLS, which can enter the nucleus and associates with the LacO array integrated near cen1. (B) Time-lapse GFP images of a single cell expressing the GFP–LacI–NLS located in the nucleus and associated with the cen1-linked DNA. At 10.5 min, two closely situated dots were observed, and these were further separated at 11 min; this represented sister centromere separation followed by nuclear elongation (12 min) and division (15 min). The distance between the separated signals increased continuously up to 20 min. Anaphase B occurred from 11 to 20 min. Bar, 10 μm. (C) The distances between the cen1 signals are plotted vs. time.
Fast Centromere Separation
Cells containing the cen1-linked LacO array and GFP–LacI–NLS contained one or two fluorescent dots in the nucleus (Figure 3B) against a background of fainter homogenous nuclear fluorescence. When GFP–LacI–NLS was expressed in control wild-type cells that did not contain the integrated LacO repeats, the fluorescent dot was absent, and only homogenous nuclear fluorescence was seen (our unpublished result). The GFP–LacI–NLS signal thus detected the integrated LacO repeats. Time-lapse microscopy of GFP–LacI–NLS in single cells taken at 33°C indicated that separation of a single dot into two occurred abruptly. Separation of the signal occurred within 1 min (starting at ∼10 min and completed at 11 min, as seen in Figure 3, B and C). The average time required for this separation was 0.5–1.0 min, and the rate of separation was thus 3–5 μm/min. The separated signals moved further apart and reached the ends of the cell at ∼20 min. The rate of this latter movement was slower than that at separation and identical to that of anaphase B spindle extension.
Occurrence of Centromere Separation at the End of Phase 2
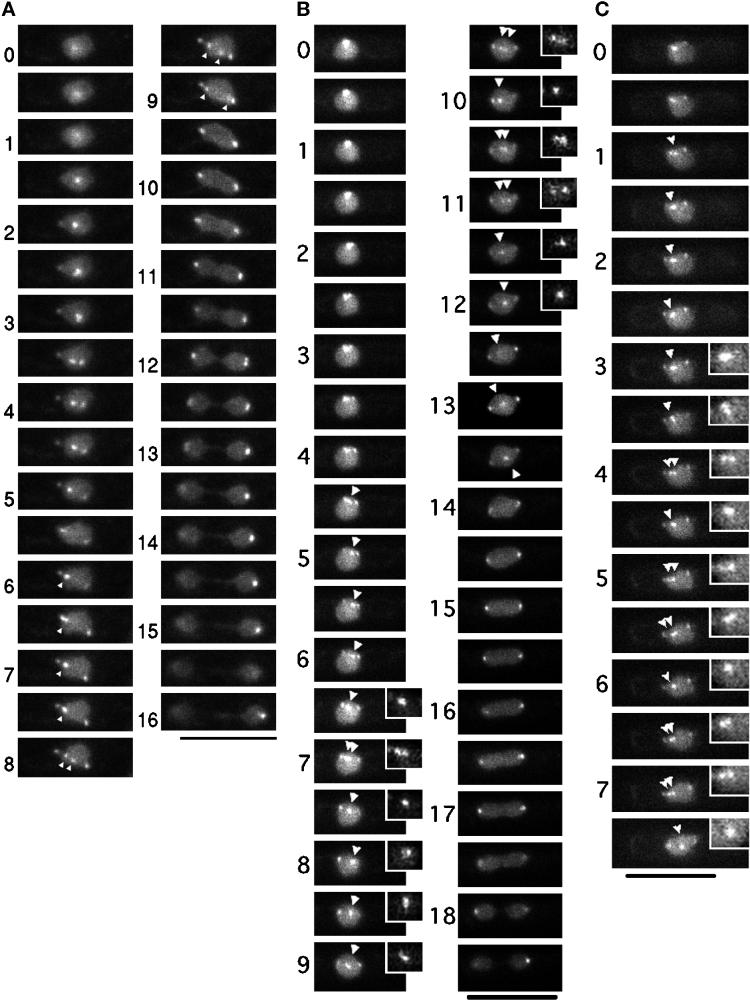
To precisely determine the timing of centromere separation with regard to spindle dynamics, we constructed the S. pombe strain expressing both Sad1–GFP and GFP–LacI–NLS with the integrated centromeric LacO repeats. The SPB and the cen1-adjacent DNA could thus be observed in the same cells. A series of images for three example cells (cultured at 26°C) are shown in Figure 4 (the numbers indicate minutes). In Figure 4A, the SPB signal was separated at 1.5–2.0 min (the left signal was weak because the SPB was out of focus). At 3.5 min, another signal, representing the cen1 DNA, dissociated from the SPB and was visible between the SPBs. This cen1 signal moved back and forth between the two SPBs in phase 2. The movement was fast and continued until 7.5 min. The centromere signal (indicated by the arrowhead) split into two at 8 min, and the separated signals moved swiftly toward the opposite poles. The duration of sister centromere separation (anaphase A) was thus a small part of the whole period of constant spindle length. This is clearly seen in the time course plot of the position of a pair of sister centromeres relative to the SPBs (Figure 5A). The distances between the cen1 signals (cen1–cen1′), and between the cen1 and either one of the two SPBs (SPB1–cen1, cen1′–SPB2) were measured. These results established that phase 2 contained not only metaphase but also a brief period of anaphase A at its end.
Figure 4.
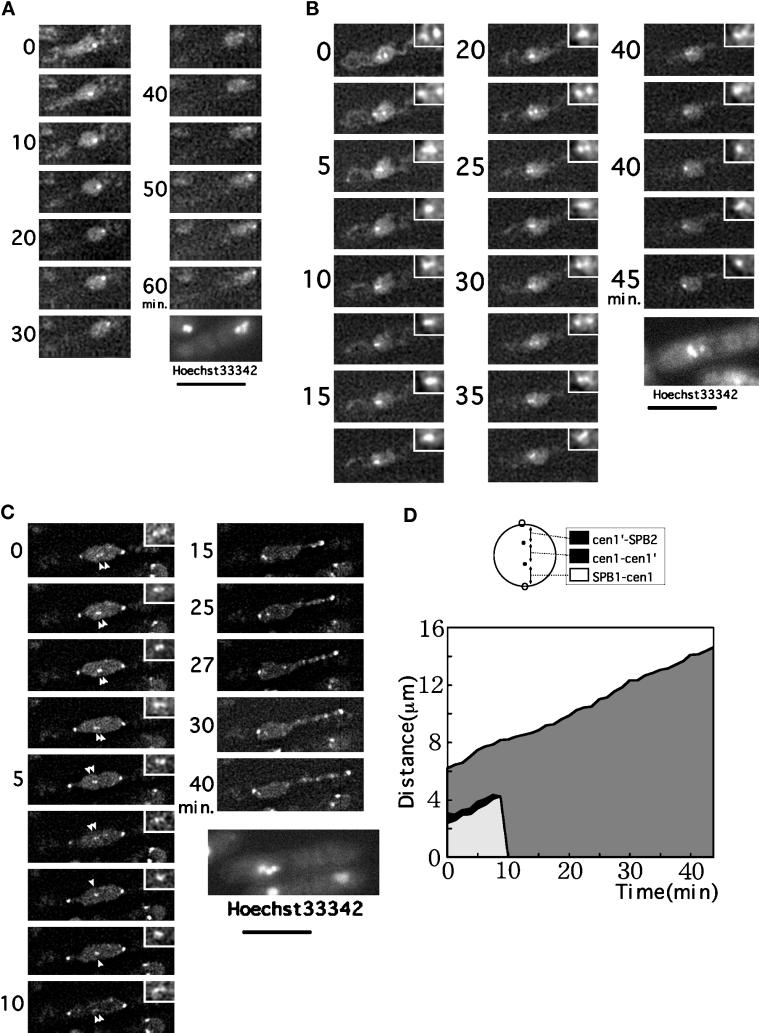
GFP images of the SPB and the cen1 DNA in the same cells. Three example cells are shown in A–C. The numbers indicate minutes. Bars, 10 μm. In A and B, the SPB and cen1 DNA signals initially reside at the same positions, whereas in C the SPB was already separated. In A, splitting of the signal into two takes place at 1.5–2.0 min, representing separation of the SPBs. The weak signal in one of the separated SPBs is due to defocusing. The third signal clearly seen at 3.5 min represents the cen1 DNA dissociated from the SPB. This cen1 DNA signal moves back and forth from 3.5 to 7.5 min and is separated into two at 8 min (indicated by arrowheads) and nearly completely separated at 9 min. Nuclear division takes place in the following 3 min. Anaphase B (spindle extension) continues until 16 min; the thin channel structure connecting the daughter nuclei contains the spindle (Tanaka and Kanbe, 1986). In B, SPB separation takes place at 2.5 min, and dissociation of the cen1 DNA from the SPB occurs at 4 min. The cen1 DNA (indicated by arrowheads) was transiently split three times and definitive separation occurred from 13.5 to 14 min. In C, the cen1 DNA is temporally split at 4, 5, and 7 min.
Figure 5.
Measurements of the distances between the SPBs and between the SPB and the sister centromeres. The distance between the SPBs is divided into three segments, the segments between the cen1 and the SPBs (cen1–SPB1, cen1′–SPB2) and between the sister cen1 DNA (cen1–cen1′). The distances of these three segments are plotted vs. time as indicated. Sister centromeres were transiently separated in (B). Sister centromere separation abruptly took place at the end of phase 2.
The second set of images (Figure 4B) showed that the cen1 DNA (indicated by the arrowhead) made fluctuating movements around the middle of the spindle but transiently split into two a short distance away before separation (also see enlarged [2×] inset for the centromere signals). This temporal splitting (two arrowheads) occurred multiple times before final centromere separation. The timing and extent of this transient centromere separation are shown in Figure 5B. In this cell, the period of constant spindle length was ∼10 min, somewhat longer than the average (7 min). In the third set of time-lapse images (Figure 4C), this splitting also occurred. The transient centromere separation suggested that sister centromeres might be pulled and briefly separated by the spindle force toward the opposite directions during the period of constant spindle length. Biorientation of sister centromeres toward the spindle ends was thus established in phase 2 (at least for the centromere visualized). The timing of this transient splitting indicated that biorientation could be established at an early stage of phase 2. It was unlikely that the split centromeres could rotate so that the signals might become single when the direction of the split centromeres was parallel to the light axis. The signals of the SPBs and the sister centromeres appeared to exist in the same focal plane. In addition, the direction of the split centromeres was always parallel to the spindle axis. Note, however, that splitting could not be detected if it occurred in the distances below light microscopic resolution.
Phase 2 Is Absent in dis1 Mutant
We then addressed the question of whether any mitotic mutations could alter the duration of phases 1, 2, and 3. Four mutants, dis1, top2, cut1, and cut14, were examined. These mutants show different types of defects in sister chromatid separation, although the spindle was made and at least partly elongated in all of them (Uemura and Yanagida, 1986; Uzawa et al., 1990; Saka et al., 1994; Nabeshima et al., 1995). Dis1 is associated with microtubules and the mitotic SPBs. In its absence, sister chromatid separation and cytokinesis are completely suppressed, although the spindle elongates to its full extent (Nabeshima et al., 1995). Neither mitotic cyclin (Cdc13) nor Cut2 was degraded in dis1 mutant cells.
Time-lapse images of Sad1–GFP (Figure 6A) showed that phase 2 was clearly lacking in dis1 mutant cells at the restrictive temperature (20°C). The spindle length measured in three dis1 mutant cells (Figure 6B) continuously increased and did not pause at the spindle length of 2.5 μm. The increase rate of the SPB distance (0.3 μm/min at 20°C) was similar to that of wild-type phases 1 and 3 at 20°C, suggesting that the machinery for spindle formation and elongation might be functioning. At the permissive temperature (33°C), phase 2 was clearly present in dis1, although the duration was longer (12 min) than in wild-type cells at 33°C (our unpublished result). Functional Dis1 was thus required for establishing phase 2 or completing phase 1, possibly by restraining spindle extension. Dis1 might be implicated in establishing the normal linkage between the kinetochores and the SPBs via the kinetochore microtubules. The linkage is necessary for generating the opposing force that would balance the spindle extension force. The constant spindle period may be maintained by balancing the two forces.
Figure 6.
Phase 2 is absent in dis1 mutant. (A) dis1 deletion mutant cell expressing Sad1–GFP at the restrictive temperature is shown. Separated SPBs continuously increased the distance between them. (B) Time-course increase of the distance between the Sad1–GFP signals is shown for three examples of dis1 mutant cells.
To also observe movement of the cen1 DNA, GFP–LacI–NLS was expressed in the dis1 mutant integrated at cen1 with the tandem LacO repeats. Cells observed at 20°C revealed two frequent types of cen1 behavior. In one type of cells, the cen1 signal was not separated but moved toward one end of the cell while the spindle extended (Figure 7A). In the other type of cells, the cen1 signal was situated in the middle of the cell but was split into two with a small separation for significant time length (∼15 min) and then reassociated (Figure 7B). The sister centromeres appeared to be separate from each other but not properly pulled during this period of transient splitting.
Figure 7.
Behavior of the cen1 DNA signal in dis1 mutant cells. (A) The cen1 DNA signal remains associated in dis1 deletion cell at the restrictive temperature, although the nucleus has been elongated by spindle extension. After 60 min the cell was stained by Hoechst 33342. (B) Another dis1 deletion cell revealing GFP–LacI–NLS. Two closely situated cen1 signals are seen for a considerable length of time without further separation. (C) Simultaneous observation of the cen1 DNA (indicated by arrowheads) and the GFP–Sad1 signals in dis1 deletion. Two closely situated cen1 signals are seen between two SPBs. Bars, 10 μm. (D) Kinetic data for the distances between the centromeres (cen1–cen1′) and between the sister centromeres and the SPBs (cen1–SPB1, cen1′–SPB2). Data collected from the cell in C are shown. The distance between the SPBs increased continuously.
We looked at the relationship between centromere splitting and spindle dynamics by constructing and observing a strain that could express both GFP–Sad1 and GFP–LacI–NLS. The split cen1 signals were seen while the spindle length was long and continuing to elongate (Figure 7C, arrowheads). Kinetic data obtained by measurements of the distances between the sister centromeres (cen1–cen1′) and between the SPBs and the sister centromeres (SPB1–cen1, cen1′–SPB2) are shown in Figure 7D. A striking feature in dis1 mutant cells was that the back-and-forth cen1 DNA movements seen in phase 2 of wild-type cells were entirely absent. After spindle elongation (the SPB distance, ∼8 μm), the cen1 signals were fused again and moved to one of the SPBs. Such prolonged centromere splitting while the spindle was elongating was never seen in wild-type or any of the other mutant cells examined so far.
Transition to Phase 3 Was Abnormal in top2 and cut1 Mutants
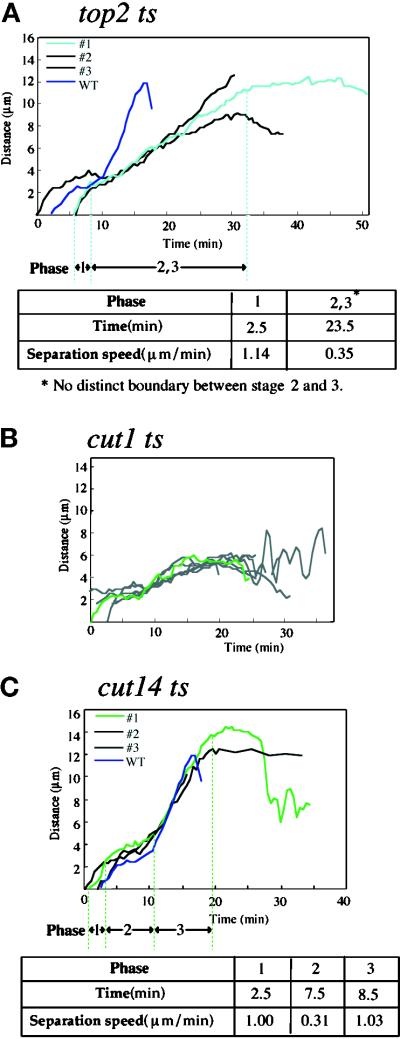
Type II topoisomerase activity is required to allow complete separation of sister chromatids in fission yeast and several other eukaryotes. Three sets of time-lapse series for Sad1–GFP were taken from top2–191 mutant cells, which lack type II topoisomerase activity at the restrictive temperature (36°C). The average rate of SPB separation in phase 3 was much slower (0.35 μm/min) than that of wild-type cells (Figure 8A, WT). There was no clear transition from phase 2 to phase 3 in top2 (Figure 8A). Spindle elongation in phase 3 appeared to be strongly inhibited, probably by the inability to fully separate the entangled sister chromatid DNAs formed in top2 mutant cells. At the permissive temperature (26°C), the three phases were clearly present in top2 mutant (our unpublished result).
Figure 8.
Spindle dynamics in top2, cut1, and cut14 mutants. The distances between the SPBs were measured for top2–191 (A), cut1–206 (B), and cut14–208 (C) mutant cells that expressed Sad1–GFP. In A, the wild-type control is shown by a dark blue line, and three mutant cells that exhibited aberrant initiation of phase 3 are also shown. In B, phase 3 only partially took place.
Cut1 is a protein that is required for sister chromatid separation and is activated when its regulator, Cut2, is destroyed by anaphase-promoting complex (cyclosome)- and ubiquitin-mediated proteolysis. Seven sets of time-lapse images for Sad1–GFP were taken from cut1–206 cultured at 36°C (Figure 8B). Phase 2 occurred, but spindle elongation in phase 3 was only partial, and spindle length increase was arrested at ∼6–7 μm. The terminal archery bow phenotype of the cut1 mutant (Hirano et al., 1986; Uzawa et al., 1990) occurred with this size of partial spindle extension. Although the sister centromeres and a significant part of sister chromatids were separated in this mutant (Funabiki et al., 1993), the defect in phase 3 (anaphase B) suggests that the anaphase B spindle elongation is restrained by physical connection between the remaining chromatids. Alternatively, Cut1 may be needed to activate the anaphase spindle so that the anaphase spindle force generated in this mutant is weak (Kumada et al., 1998). At the permissive temperature (26°C), the three phases were clearly present in the cut1 mutant (our unpublished result).
Cut14 is a condensin subunit that plays a role in mitotic chromosome condensation (Hirano et al., 1997). In cut14–208 mutant cells, the three phases of mitosis were clearly observed (Figure 8C). Defects in chromosome condensation thus did not appear to affect the occurrence of these phases in spindle dynamics. This is surprising because only a tiny portion of the sister chromatids containing the centromeres were separated in this mutant at the restrictive temperature (Saka et al., 1994). The tension generated by paired centromeres opposing the force exerted on the kinetochore microtubules appeared to exist in this mutant, although most of the chromatids remained uncondensed. Cohesion in the sister centromeres might then be released on the onset of anaphase, but other parts of the chromatids remained associated, perhaps because of the lack of condensation.
DISCUSSION
We examined the movements of the SPBs and the centromere DNA in living fission yeast cells. Images of wild-type cells and mitotic mutants were analyzed to determine how spindle and chromosome dynamics were spatially and temporally regulated during mitosis. We showed that the normal spindle dynamics consisted of three distinct periods, phase 1 (spindle formation), phase 2 (constant spindle length), and phase 3 (spindle elongation). Phase 1, corresponding to a prophase-like stage, probably occurred after Cdc2 kinase was activated (Hagan and Yanagida, 1992; Masuda et al., 1992), but this remains to be experimentally verified. The duplicated SPBs enter the nuclear envelope and gain access to the nucleoplasm for mitosis (Ding et al., 1997). In phase 2, spindle elongation was inhibited, but centromere DNA moved rapidly back and forth along the spindle. The greater part of phase 2 was prometaphase and metaphase, whereas sister chromatid separation (anaphase A) occurred at the end of this phase. Phase 3 began immediately after or simultaneously with the onset of anaphase A. Because the rate of anaphase A is fast, it is difficult to distinguish the onset of anaphases A and B. These three phases had a strong resemblance to principal events in higher eukaryotic mitosis (e.g., Mitchison, 1989; Rieder and Salmon, 1994) and also in budding yeast (Straight et al., 1997). Distinguishing mitotic stages with regard to chromosome condensation was, however, difficult in this study, because the degree of condensation has not been visualized in living cells. Timing for the onset of interaction between condensed chromosome and spindle microtubules also remains to be determined.
A striking feature revealed in this study was that the centromeric DNA moved along the spindle throughout phase 2. Such movements were observed neither in interphase nor in other mitotic stages, indicating that the movements might correspond to prometaphase oscillation in higher eukaryotic mitosis. S. pombe thus appears to have a stage equivalent to prometaphase in which the sister centromeres moved together in the same directions, whereas in anaphase A, they moved in the opposite directions, leading to sister chromatid separation. The maximal rates of movements in metaphase and anaphase A were 2 and 4 μm/min (at 33°C), respectively, much faster than that of anaphase B spindle extension. The direct cause of these fast centromere movements in a prometaphase-like stage was unclear. Certain mitotic motors or factors affecting the properties of mitotic microtubules might be implicated. The rates of these centromere movements in phase 2 are faster than the rate of poleward microtubule flux in vertebrate tissue culture cells (Mitchison, 1989; Sawin, 1991), but roughly comparable with those seen in extracts of frog eggs (Desai et al., 1998). The movements were abolished in dis1 mutant cells at the nonpermissive temperature. This was an important observation, because phase 2 was absent in dis1 mutant cells, suggesting that the centromere movements were an intrinsic character of this phase.
Another feature in phase 2 was that the centromere signal was often transiently split into two. The separation distance was <0.6 μm, and the direction of separation was always parallel to the spindle axis. We interpreted this transient sister centromere separation to be due to the tension force exerted on sister centromeres in opposite directions by the kinetochore microtubules, possibly generated by microtubule depolymerization or an associated motor protein (Mitchison et al., 1986; Rieder et al., 1986; Li and Nicklas, 1995; Skibbens et al., 1995; Waters et al., 1996; Inoue, 1997; Waterman-Storer and Salmon, 1997). The chromosomes in phase 2 were thus positioned on the spindle in a bioriented manner, implying that both sister kinetochores were already caught by kinetochore microtubules directed toward different poles. This bioriented interaction between chromosomes and the spindle occurs in higher eukaryotic prometaphase and metaphase. In this regard, fission yeast centromere behavior is similar to that of higher eukaryotes. It remains to be determined whether this transient separation is confined to the centromeres.
The spindle in phase 2 was not quiescent, because sister centromeres moved quite rapidly. How, then, was the duration of phase 2 determined? The time span of this phase was three times longer than that of phase 1 and also greatly dependent on temperature (Table 1). Mitotic cells might have to spend a significant period of time in the execution of certain unidentified functions to ensure correct sister chromatid separation. Polyubiquitination of certain essential proteins such as cyclin and Cut2 for their destruction (e.g., Funabiki et al., 1996; Yamano et al., 1996) is one possibility. Alternatively, proteins essential for the spindle checkpoint may have to monitor the states of metaphase spindle (e.g., Straight et al., 1997). An essential component implicated in anaphase proteolysis has been recently shown to be tightly connected to Mad2, which is required for spindle checkpoint control (Hwang et al., 1998; Kim et al., 1998). There must be a mechanism to restrain spindle extension before the onset of anaphase, and it might be relatively time consuming. Phase 2 could thus be a central issue in understanding the regulatory mechanisms in fission yeast mitosis.
A curious phenotype in a fraction (∼30%) of dis1 mutant cells was that sister centromeres were slightly separated for a significant time period while the spindle was continually elongating. These sister centromeres did not move, remained in the middle of the spindle, and eventually reassociated each other. In the remaining mutant cells, the sister centromeres were associated, did not make oscillatory movements, and moved to one of the poles while the spindle was continuously elongating. Dis1 protein contains motifs that bind to microtubules and the mitotic SPBs (Nabeshima et al., 1995, Nakaseko et al., 1996). This microtubule-associated protein is thus required for the oscillatory movements of the centromere DNA and the constant spindle length observed in phase 2, suggesting a mechanistic connection between the phenomena. Dis1 might be required for completing the formation of the bipolar spindle structure in metaphase and inhibiting spindle extension in anaphase. The dis1 mutant phenotype might be interpreted as the failure in ending the phase 1. Either or both sister kinetochores might fail to make the normal connection to the spindle apparatus in dis1 mutant cells. Dis1 protein locates at the mitotic SPBs and the metaphase spindle microtubules. Overproduction of the C-terminal Dis1 fragment, which locates only at the SPBs, can suppress dis1 mutation. The SPBs in dis1 mutant cells might thus fail in interacting with the kinetochore-bound microtubules.
One explanation of the dis1 phenotype is that the microtubule-mediated linkage between the sister kinetochores and the spindle poles of the mutant cells may be deficient. In normal cells, the linkage plays an important role in restraining the length of the metaphase spindle, which is determined by the balance between forces that pull the poles together and those that push them apart. Because the sister kinetochores are linked to each other, forces that pull the kinetochores toward the poles also pull the poles toward each other, thus balancing other microtubule-dependent forces that would push the poles apart. If the kinetochores cannot be connected to the SPBs via microtubules, the pulling forces will be absent and the spindle will elongate continuously. Experiments in budding yeast show that microtubule-dependent forces are not required for sister chromatid separation (Guacci et al., 1997; Michaelis et al., 1997) but are needed to segregate the sister chromatids once they have separated, showing that the long periods of partial separation seen in dis1 cells are consistent with a loss of the kinetochore–microtubule–SPB interactions. There are obviously many other possible explanations of the role of Dis1 in mitosis, but our results suggest that the dynamic behavior of the centromere DNA in phase 2 is a prerequisite for normal anaphase, and that the SPB or the minus ends of microtubules might play an important role in such spindle dynamics.
In higher eukaryotic cells, condensed mitotic chromosomes are congregated and aligned at the middle of the spindle in metaphase. In fission yeast, the presence of metaphase was previously anticipated from the images of fluorescence in situ hybridization analyses, which showed that the centromere signals were congregated on the spindle (Funabiki et al., 1993; Saitoh et al., 1997). The present study suggested, however, that the duration of metaphase might be very brief in fission yeast; prometaphase thus might be predominant. Resolving this question will require simultaneous observation of at least two of the three centromeres in living cells. In budding yeast, the centromere and the spindle apparatus were simultaneously observed (Straight et al., 1997), and it was concluded that metaphase was absent but anaphase A was present. In many of the fission yeast cells we observed, the centromere signals were found midway between the spindle poles just before sister chromatid separation. In some mitotic mutants of S. pombe deficient in ubiquitin-mediated anaphase-promoting proteolysis, condensed chromosomes were arranged in the middle of the short spindle (Hirano et al., 1988; Samejima and Yanagida, 1994). Further work is needed to determine whether all the centromeres are congregated on the spindle before anaphase even for a very short period.
Two mechanisms that might induce anaphase are as follows: first, sister chromatid separation destroys the opposition between preexisting forces on the kinetochores and spindle poles, thus allowing the kinetochores to move to the spindle poles (anaphase A) and the poles to separate from each other (anaphase B); and, second, changes in the cell cycle machinery alter the magnitude of the microtubule-dependent forces acting on spindle components. Mutating the budding yeast homologue of Cut1 blocks microtubule-independent sister chromatid separation, suggesting that the primary function of Cut1 is in sister chromatid separation. Our observation that cut1 mutants lack anaphases A and B supports the idea that the main trigger for anaphase movements is dissolving the linkage between sister chromatids, although we cannot rule out the possibility that Cut1 also has direct roles in force generation (Kumada et al., 1998). The lack of topoisomerase II activity in temperature-sensitive top2 mutant cells did not induce precocious spindle elongation, but the transition from phase 2 to phase 3 was unclear, possibly because of the physical constraints of topoisomerase II-deficient mitotic chromosomes. It is of interest to determine whether cohesion molecules remain in the mutant chromosomes after spindle elongation in phase 3. The same question may be applied to mitotic chromosomes in cut14 mutant cells. Three phases are clearly present in the condensin-deficient mutant. The behavior of the cen1 DNA in these mutant cells is of considerable interest but has not been studied at 36°C, because fluorescence of the GFP–LacI–NLS construct used was greatly diminished at 36°C. We are currently making other GFP constructs that can be used for visualization at 36°C.
ACKNOWLEDGMENTS
This study was supported by the CREST project of the Japan Science Technology Corporation and research grants of the Human Frontier Science Program Organization (to M.Y.) and the National Institutes of Health (to A.W.M.).
REFERENCES
- Chikashige Y, Ding D-Q, Funabiki H, Haraguchi T, Mashiko S, Yanagida M, Hiraoka Y. Telomere-led premeiotic chromosome movement in fission yeast. Science. 1994;264:270–273. doi: 10.1126/science.8146661. [DOI] [PubMed] [Google Scholar]
- Desai A, Maddox PS, Mitchison TJ, Salmon ED. Anaphase chromosome movement and poleward spindle microtubule flux occur at similar rates in Xenopus extract spindles. J Cell Biol. 1998;141:703–713. doi: 10.1083/jcb.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding DQ, Chikashige Y, Haraguchi T, Hiraoka Y. Oscillatory nuclear movement in fission yeast meiotic prophase is driven by astral microtubules, as revealed by continuous observation of chromosomes and microtubules in living cells. J Cell Sci. 1998;111:701–712. doi: 10.1242/jcs.111.6.701. [DOI] [PubMed] [Google Scholar]
- Ding R, West RR, Morphew M, Oakley BR, McIntosh JR. The spindle pole body of Schizosaccharomyces pombe enters and leaves the nuclear envelope as the cell cycle proceeds. Mol Biol Cell. 1997;8:1461–1479. doi: 10.1091/mbc.8.8.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Hagan I, Uzawa S, Yanagida M. Cell cycle-dependent specific positioning and clustering of centromeres and telomeres in fission yeast. J Cell Biol. 1993;121:961–976. doi: 10.1083/jcb.121.5.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabiki H, Yamano H, Kumada K, Nagao K, Hunt T, Yanagida M. Cut2 proteolysis required for sister-chromatid separation in fission yeast. Nature. 1996;381:438–441. doi: 10.1038/381438a0. [DOI] [PubMed] [Google Scholar]
- Guacci V, Koshland D, Strunnikov AV. A direct link between sister chromatid cohesion and chromosome condensation revealed through the analysis of MCD1 in S. cerevisiae. Cell. 1997;91:47–57. doi: 10.1016/s0092-8674(01)80008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan IM, Yanagida M. Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature. 1992;356:74–76. doi: 10.1038/356074a0. [DOI] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. The product of the spindle formation gene sad1+ associates with the fission yeast spindle pole body and is essential for viability. J Cell Biol. 1995;129:1033–1047. doi: 10.1083/jcb.129.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan I, Yanagida M. Evidence for cell cycle-specific, spindle pole body-mediated, nuclear positioning in the fission yeast Schizosaccharomyces pombe. J Cell Sci. 1997;110:1851–1866. doi: 10.1242/jcs.110.16.1851. [DOI] [PubMed] [Google Scholar]
- Hirano T, Funahashi S, Uemura T, Yanagida M. Isolation and characterization of Schizosaccharomyces pombe cut mutants that block nuclear division but not cytokinesis. EMBO J. 1986;5:2973–2979. doi: 10.1002/j.1460-2075.1986.tb04594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Hiraoka Y, Yanagida M. A temperature-sensitive mutation of Schizosaccharomyces pombe gene nuc2+ that encodes a nuclear scaffold-like protein blocks spindle elongation in mitotic anaphase. J Cell Biol. 1988;106:1171–1183. doi: 10.1083/jcb.106.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Kobayashi R, Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–521. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- Hiraoka Y, Swedlow JR, Paddy MR, Agard DA, Sedat JR. Three-dimensional multiple-wavelength fluorescence microscopy for the structural analysis of biological phenomena. Semin Cell Biol. 1991;2:153–165. [PubMed] [Google Scholar]
- Hiraoka Y, Toda T, Yanagida M. The NDA3 gene of fission yeast encodes β-tubulin: a cold sensitive nda3 mutation reversibly blocks spindle formation and chromosome movement in mitosis. Cell. 1984;39:349–358. doi: 10.1016/0092-8674(84)90013-8. [DOI] [PubMed] [Google Scholar]
- Hwang LH, Lau LF, Smith DL, Mistrot CA, Hardwick KG, Hwang ES, Amon A, Murray AW. Budding yeast Cdc20: a target of the spindle checkpoint. Science. 1998;279:1041–1044. doi: 10.1126/science.279.5353.1041. [DOI] [PubMed] [Google Scholar]
- Inoue S. The role of microtubule assembly dynamics in mitotic force generation and functional organization of living cells. J Struct Biol. 1997;118:87–93. doi: 10.1006/jsbi.1996.3839. [DOI] [PubMed] [Google Scholar]
- Kim SH, Lin DP, Matsumoto S, Kitazono A, Matsumoto T. Fission yeast Slp1: an effector of the Mad2-dependent spindle checkpoint. Science. 1998;279:1045–1047. doi: 10.1126/science.279.5353.1045. [DOI] [PubMed] [Google Scholar]
- Kumada K, Nakamura T, Nagao K, Funabiki H, Nakagawa T, Yanagida M. Cut1 is loaded onto the spindle by binding to Cut2 and promotes anaphase spindle movement upon Cut2 proteolysis. Curr Biol. 1998;8:633–641. doi: 10.1016/s0960-9822(98)70250-7. [DOI] [PubMed] [Google Scholar]
- Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- MacNeill SA, Nurse P. Cell cycle control in fission yeast. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1997. pp. 697–763. [Google Scholar]
- Masuda H, Sevik M, Cande Z. In vitro microtubule-nucleating activity of spindle pole bodies in fission yeast Schizosaccharomyces pombe: cell cycle dependent activation in Xenopus cell-free extracts. J Cell Biol. 1992;117:1055–1066. doi: 10.1083/jcb.117.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis C, Ciosk R, Nasmyth K. Cohesins: chromosome proteins that prevent premature separation of sister chromatids. Cell. 1997;91:35–45. doi: 10.1016/s0092-8674(01)80007-6. [DOI] [PubMed] [Google Scholar]
- Mitchison T, Evans L, Schulze E, Kirschner M. Sites of microtubule assembly and disassembly in the mitotic spindle. Cell. 1986;45:515–527. doi: 10.1016/0092-8674(86)90283-7. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ. Poleward microtubule flux in the mitotic spindle: evidence from photoactivation of fluorescence. J Cell Biol. 1989;109:637–652. doi: 10.1083/jcb.109.2.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima K, Kurooka H, Takeuchi M, Kinoshita K, Nakaseko Y, Yanagida M. p93dis1 required for sister chromatid separation is a novel microtubule and spindle pole body associating protein phosphorylated at the Cdc2 target sites. Genes Dev. 1995;9:1572–1585. doi: 10.1101/gad.9.13.1572. [DOI] [PubMed] [Google Scholar]
- Nabeshima K, Saitoh S, Yanagida M. Use of green fluorescent protein for intracellular localization in living fission yeast cells. Methods Enzymol. 1997;283:459–471. doi: 10.1016/s0076-6879(97)83037-6. [DOI] [PubMed] [Google Scholar]
- Nakaseko Y, Nabeshima K, Kinoshita K, Yanagida M. Dissection of fission yeast microtubule associating protein p93Dis1: regions implicated in regulated localization and microtubule interaction. Genes Cells. 1996;1:634–644. doi: 10.1046/j.1365-2443.1996.00253.x. [DOI] [PubMed] [Google Scholar]
- Ohkura H, Adachi Y, Kinoshita N, Niwa O, Toda T, Yanagida M. Cold-sensitive and caffeine supersensitive mutants of the Schizosaccharomyces pombe dis genes implicated in sister chromatid separation during mitosis. EMBO J. 1988;7:1465–1473. doi: 10.1002/j.1460-2075.1988.tb02964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Davison EA, Jensen LC, Salmon ED. Oscillatory movements of monooriented chromosomes and their position relative to the spindle pole result from the ejection properties of the aster half-spindle. J Cell Biol. 1986;103:581–591. doi: 10.1083/jcb.103.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder CL, Salmon ED. Motile kinetochores and polar ejection forces dictate chromosome position on the vertebrate mitotic spindle. J Cell Biol. 1994;124:223–233. doi: 10.1083/jcb.124.3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinett CC, Straight A, Li G, Willhelm C, Sudlow G, Murray A, Belmont AS. In vivo localization of DNA sequences and visualization of large-scale chromatin organization using lac operator/repressor recognition. J Cell Biol. 1996;135:1685–1700. doi: 10.1083/jcb.135.6.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh S, Takahashi K, Yanagida M. Mis6, a fission yeast inner centromere protein, acts during G1/S and forms specialized chromatin required for equal segregation. Cell. 1997;90:131–143. doi: 10.1016/s0092-8674(00)80320-7. [DOI] [PubMed] [Google Scholar]
- Saka Y, Sutani T, Yamashita Y, Saitoh S, Takeuchi M, Nakaseko Y, Yanagida M. Fission yeast cut3 and cut14, members of the ubiquitous protein family, are required for chromosome condensation and segregation in mitosis. EMBO J. 1994;13:4938–4952. doi: 10.1002/j.1460-2075.1994.tb06821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima I, Yanagida M. Bypassing anaphase by fission yeast cut9 mutation: requirement cut9+ gene to initiate anaphase. J Cell Biol. 1994;127:1665–1670. doi: 10.1083/jcb.127.6.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE. Poleward microtubule flux mitotic spindles assembled in vitro. J Cell Biol. 1991;112:941–954. doi: 10.1083/jcb.112.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skibbens RV, Rieder CL, Salmon ED. Kinetochore motility after severing between sister centromeres using laser microsurgery: evidence that kinetochore directional instability and position is regulated by tension. J Cell Sci. 1995;108:2537–2548. doi: 10.1242/jcs.108.7.2537. [DOI] [PubMed] [Google Scholar]
- Straight AF, Belmont AS, Robinett CC, Murray AW. GFP tagging of budding yeast chromosomes reveals that protein-protein interactions can mediate sister chromatid cohesion. Curr Biol. 1996;6:1599–1608. doi: 10.1016/s0960-9822(02)70783-5. [DOI] [PubMed] [Google Scholar]
- Straight AF, Marshall WF, Sedat JW, Murray AW. Mitosis in living budding yeast: anaphase A but no metaphase plate. Science. 1997;277:574–578. doi: 10.1126/science.277.5325.574. [DOI] [PubMed] [Google Scholar]
- Su SSY, Yanagida M. Mitosis and cytokinesis in the fission yeast, Schizosaccharomyces pombe. In: Pringle JR, Broach JR, Jones EW, editors. The Molecular and Cellular Biology of the Yeast Saccharomyces. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1997. pp. 765–825. [Google Scholar]
- Takahashi K, Murakami S, Chikashige Y, Niwa O, Funabiki H, Yanagida M. A low copy number central sequence with strict symmetry and unusual chromatin structure in the fission yeast centromere. Mol Biol Cell. 1992;3:819–835. doi: 10.1091/mbc.3.7.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Kanbe T. Mitosis in the fission yeast Schizosaccharomyces pombe as revealed by freeze-substitution electron microscopy. J Cell Sci. 1986;80:253–268. doi: 10.1242/jcs.80.1.253. [DOI] [PubMed] [Google Scholar]
- Uemura T, Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984;3:1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Yanagida M. Mitotic spindle pulls but fails to separate chromosomes in type II DNA topoisomerase mutants: uncoordinated mitosis. EMBO J. 1986;5:1003–1010. doi: 10.1002/j.1460-2075.1986.tb04315.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzawa S, Samejima I, Hirano T, Yanagida M. The fission yeast cut1+ gene regulates spindle pole body duplication and has homology to the budding yeast ESP1 gene. Cell. 1990;62:913–925. doi: 10.1016/0092-8674(90)90266-h. [DOI] [PubMed] [Google Scholar]
- Waterman-Storer CM, Salmon ED. Microtubule dynamics: treadmilling comes around again. Curr Biol. 1997;7:R369–R372. doi: 10.1016/s0960-9822(06)00177-1. [DOI] [PubMed] [Google Scholar]
- Waters JC, Mitchison TJ, Rieder CL, Salmon ED. The kinetochore microtubule minus-end disassembly associated with poleward flux produces a force that can do work. Mol Biol Cell. 1996;7:1547–1558. doi: 10.1091/mbc.7.10.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamano H, Gannon J, Hunt T. The role of proteolysis in cell cycle progression in Schizosaccharomyces pombe. EMBO J. 1996;15:5268–5279. [PMC free article] [PubMed] [Google Scholar]
- Yanagida M. Frontier questions about sister chromatin separation in anaphase. Bioessays. 1995;17:519–526. doi: 10.1002/bies.950170608. [DOI] [PubMed] [Google Scholar]
- Yanagida M. Fission yeast cut mutations revisited: control of anaphase. Trends Cell Biol. 1998;8:144–149. doi: 10.1016/s0962-8924(98)01236-7. [DOI] [PubMed] [Google Scholar]