Abstract
AIMS
Korean red ginseng (unskinned Panax ginseng before it is steamed or otherwise heated and subsequently dried) is one of the most widely used herbal remedies. This systematic review evaluates the current evidence for the effectiveness of red ginseng for treating erectile dysfunction.
METHODS
Systematic searches were conducted on 20 electronic databases without language restrictions. Hand-searches included conference proceedings and our files. All randomized clinical studies (RCT) of red ginseng as a treatment of erectile dysfunction were considered for inclusion. Methodological quality was assessed using the Jadad score.
RESULTS
Seven RCTs met all the inclusion criteria. Their methodological quality was low on average. Six of the included RCTs compared the therapeutic efficacy of red ginseng with placebo. The meta-analysis of these data showed a significant effect (n = 349, risk ratio, 2.40; 95% CI of 1.65, 3.51, p < 0.00001, heterogeneity: tau2 = 0.05, χ2 = 6.42, p = 0.27, I2 = 22%). Subgroup analyses also showed beneficial effects of red ginseng in psychogenic erectile dysfunction (n = 135, risk ratio, 2.05; 95% CI of 1.33, 3.16, p = 0.001, heterogeneity: χ2 = 0.08, p = 0.96, I2 = 0%).
CONCLUSIONS
Collectively these RCTs provide suggestive evidence for the effectiveness of red ginseng in the treatment of erectile dysfunction. However, the total number of RCTs included in the analysis, the total sample size and the methodological quality of the primary studies were too low to draw definitive conclusions. Thus more rigorous studies are necessary.
Keywords: erectile dysfunction, meta-analysis, red ginseng, systematic review
Introduction
Erectile dysfunction (ED) affects 30–50% of men aged 40–70 years. Age, smoking and obesity are the main risk factors. In about 20% of cases psychological problems are the cause [1]. Current medical interventions for the management of ED include oral drugs, intrapenile therapies (intra-urethral suppositories and intracavernous injections) and penile prosthesis implantation. Although considerable advances have been made, the ideal treatment of ED has not been identified [2]. Herbal therapies for ED include yohimbine which is burdened with serious adverse effects [2, 3] and red ginseng (Panax ginseng) [2].
Ginseng cultivated in Korea is classified into three types, depending on how it is processed: fresh ginseng (less than 4 years old), white ginseng (4–6 years old and dried after peeling), and red ginseng (harvested when 6 years old, steamed and dried) [4]. Red ginseng is not skinned before it is steamed or otherwise heated and subsequently dried. In the course of the steaming process, ginseng starch is gelatinized, causing an increase in saponin content. Traditionally red ginseng has been used to restore and enhance normal well-being, and is often referred to as an adaptogenic [5]. One of the therapeutic claims for red ginseng is that it enhances sexual function. Recent guidelines on the treatment of ED [1, 2] for evidence of effectiveness of red ginseng were based only on one or two randomized clinical trials and ignored studies not published in English [6, 7]. The aim of this systematic review is to update, complete and critically evaluate the evidence from RCTs for or against the effectiveness of red ginseng for patients with ED.
Methods
Data sources
The following electronic databases were searched from their inceptions up to January 2008: Medline, AMED, British Nursing Index, CINAHL, EMBASE, PsycInfo, The Cochrane Library 2008 (Issue 1), six Korean Medical Databases (Korean Studies Information, DBPIA, Korea Institute of Science and Technology Information, and Research Information Center for Health Database, Korea Medline, National Assembly Library), and four Chinese Medical Databases (China Academic Journal, Century Journal Project, China Doctor/Master Dissertation Full Text DB, and China Proceedings Conference Full Text DB) and three Japanese electronic databases (Japan Science and Technology Information Aggregator Electronic, Journal@rchive and Science Link Japan). The search phrase used was (red ginseng AND [ED or impotence or sexual dysfunction]). We also manually searched our departmental files and relevant journals (FACT [Focus on Alternative and Complementary Therapies], up to December 2007). In addition, the references in all located articles were manually searched for further relevant articles.
Study selection
All articles were included that reported an RCT in which human patients with any type of ED were treated with any type of red ginseng. Studies comparing two different forms of ginseng and those in which no clinical data were reported were excluded. No language restrictions were imposed.
Data extraction and quality assessment
Hard copies of all articles were obtained and read in full. All articles were read by three independent reviewers (DJJ, MSL, BCS) and data from the articles were validated and extracted according to pre-defined criteria (Table 1). Allocation concealment was assessed using the Cochrane classification [8]. To assess methodological quality the Jadad scale [9] was used. Discrepancies were resolved through discussion between two reviewers (DJJ, MSL) and if needed, by seeking the opinion of a third reviewer (EE).
Table 1.
Summary of clinical studies of Korean red ginseng for erectile dysfunction compared with placebo control
| Reference | Study design, allocation concealment | Number of patient in study and ED aetiology | Age range (years) | Severity of ED Duration of ED (years) | Dose (mg × 3/days) | Treatment duration (weeks) | Main outcome measures | Results (sample size) | Adverse effect | Jadad score* |
|---|---|---|---|---|---|---|---|---|---|---|
| Choi et al. [6] | Parallel, PB, n.r. | 90† Psychogenic ED | 25–70 | Mild or mild to moderate (1–30) | 600 | 12 | Report of improvement of erection and sexual satisfaction by patients and partner (structured interview) | Positive response RG (60) vs. placebo (9), p < 0.05 | (+) None | 2 (1 + 1 + 0 + 0 + 0) |
| Choi & Choi [10] | Parallel, PB, n.r. | 50 Psychogenic ED | 27–68 | Erectile failure: mean IIEF Q3: 2.43 mean IIEF Q4: 1.82 (1–29) | 600 | 8 | 1) Response to global efficacy question 2) IIEF | 1) Positive response RG(14) vs. placebo (6) 2) Intergroup difference of score, P < 0.05 | Gastric upset (RG: 1; P: 1) (+) | 1 (1 + 0 + 0 + 0 + 0) |
| Choi et al. [11] | Parallel, PB, n.r. | 28 Psychogenic ED | 24–68 | Erectile failure: mixed (1–29) | 600 | 4 | Total IIEF score and global efficacy question | Improvement RG (12) vs. placebo (3) | Headache, insomnia (RG:3) (+) | 1 (1 + 0 + 0 + 0 + 0) |
| Kim & Paick [12] | Parallel, PB, n.r. | 26 Mild vasculogenic impotence | 29–61 | Mild ED PSV (20 to 35 cm s–1) (n.r.) | 900 | 12 | Watts sexual function questionnaire | Response sample size was not reported Intergroup difference of score, NS Within group (RG: P = 0.014) | n.r. (−) | 3 (1 + 0 + 1 + 1 + 0) |
| Choi et al. [13] | Parallel, DB, n.r. | 64 Any kind of ED | 39–50 | Rigidity <70% (mean duration, 1.7 to 4.5) | 600 | 12 | Self reported questionnaire related with ED | Improvement RG (18) vs. placebo (6) | Constipation: (RG, 2) Gastric upset (RG, 2; P, 3) (+) | 1 (1 + 0 + 0 + 0 + 0) |
| Hong et al. [7] | Cross-over, DB, n.r. Assessor blind | 45 Any kind of ED | 54 (mean) | Inability to archive and maintain erection sufficient for normal sexual satisfaction (n.r.) | 900 | 8 | 1) Response to global efficacy question (erection) 2) Total IIEF score | 1) Improvement RG (27) vs. placebo (9) 2) Intergroup difference of score, P < 0.01 | Gastric upset (RG: 1) (+) | 5 (1 + 1 + 1 + 1 + 1) |
| de Andrade et al. [14] | Parallel, DB, n.r. | 60 Any kind of ED | 26–70 | IIEF-5 score: 13–21 (mild or mild to moderate) (n.r.) | 1000 | 12 | 1) Response to global efficacy question (erection) 2) Total IIEF5 | 1) Improvement RG (20) vs. placebo (0) 2) Intergroup difference of score, P = 0.00003 | Headache, insomnia (RG:3) (+) | 2 (1 + 0 + 0 + 1 + 0) |
Jadad scores were expressed as total score (randomization + appropriate randomization methods + describing withdrawals and dropouts + double-blinding + appropriate double-blinding methods).
This study is a three-arm parallel design with RG (n = 30), placebo (n = 30), and trazodone group (n = 30). To avoid contamination of analysis, we included only RG and placebo groups. DB, double-blind; ED, erectile dysfunction; IIEF, International Index of Erectile Function; n.r., not reported; NS, not significant; RG, red ginseng; PB, patient blind; (+) = mentioned in text; (−) = not mentioned in text.
Data synthesis
The response rates in the red ginseng and placebo arms were used as a basis for calculating the risk ratio, or relative risk (RR) (weighted according to sample size). RR and 95% confidence interval (CI) were calculated using the response rates for red ginseng (successful improvement of sexual function) as a basis. Standard mean differences (SMD) and 95% CI were also calculated for total scores of sexual function (international using the Cochrane Collaboration's software (Review Manager (RevMan) Version 5.0 for Windows. Copenhagen: The Nordic Cochrane Centre). The variance of the change was imputed using a correlation factor of 0.4 suggested by the Cochrane Collaboration. If appropriate, we then pooled data across studies using random effects models if excessive statistical heterogeneity did not exist The tau2, chi-square test and the Higgins I2 test were used to assess heterogeneity [8]. Homogeneous datasets were statistically pooled using a random effects model because of clinical heterogeneity between each study such as age, dose of red ginseng, treatment duration and etiology of ED.
Results
The searches identified 28 potentially relevant studies, of which seven met our inclusion criteria (Figure 1). The key data from all included RCTs are summarized in Table 1[6, 7, 10–14]. One RCT [15] which employed different types of ginseng was not included. Two duplicated RCTs were excluded [6, 7]. Choi et al. [6] published their trial twice in Korean and English and the English version is included. The other duplicated RCT was published in a master's thesis [7]. Six RCTs originated from Korea [6, 7, 10–13] and one from Brazil [14]. Five of the included trials adopted a two-armed parallel group design [10–14], one three-armed parallel group design [6] and one cross-over design [7]. The seven trials evaluated 363 men aged from 24 to 70 years old. The range of duration of ED was from 1 to 30 years. The duration of treatment ranged from 4 to 12 weeks. The adopted doses of red ginseng were 600 mg, three times daily in four trials [6, 10, 11, 13], 900 mg in two studies [7, 12] and 1000 mg in one trial [14]. Three trials were conducted in psychogenic ED patients [6, 10, 11], one in vasculogenic impotence patients [12] and three in mixed kinds of ED [7, 13, 14]. The subjective outcome measures in these trials were the International Index of Erectile Function (IIEF) [7, 10, 11, 14], Watts sexual function questionnaire [12], global efficacy question [7, 10, 11, 14] (mainly for erection sufficient for normal satisfaction) and author made structural interview questionnaires related to erectile function [6, 13] (without testing validity and reliability). Baseline comparisons of symptoms of ED were reported in three trials [7, 12, 14]. Others reported only the mean average of outcome measures, statistical p values and standard deviations [10, 13] or no reporting of baseline comparisons [6, 11].
Figure 1.
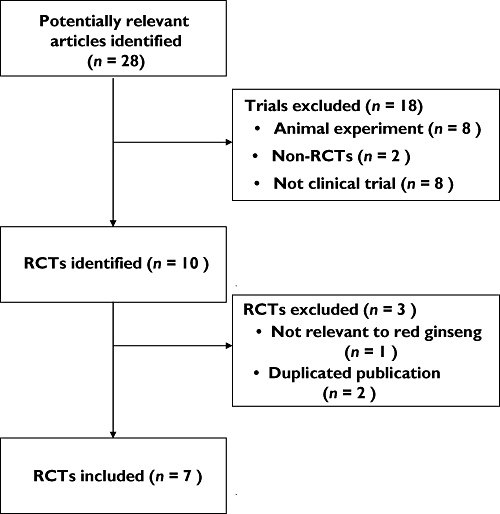
Flowchart of trial selection process. RCT: randomized clinical trial
Methodological quality
The methodological quality of the RCTs was variable (from 1 to 5). Only one described the method of randomization [7], and only one RCT described the method of double-blinding [7]. Details of drop-outs and withdrawals were described in three trials [6, 7, 12]. Dropout rates ranged from 0% [6, 7] to 19%. None reported details on allocation concealment. One RCT adopted assessor blinding [7]. Two trials [12, 14] mentioned that they had adopted double-blind methods but did not report details.
Outcomes
Response rate
Six RCTs reported the therapeutic efficacy (improvement of erectile function) of red ginseng compared with placebo control and all favoured red ginseng [6, 7, 10, 11, 13, 14]. The meta-analysis of the RCTs suggests red ginseng to be superior to placebo (n = 349, RR, 2.40; 95% CI of 1.65, 3.51, p < 0.0001, heterogeneity: tau2 = 0.05, χ2 = 6.42, p = 0.27, I2 = 22%, Figure 2A). Sensitivity analysis demonstrated that the value did not vary much despite changes in the statistical model [random effect model, 2.40 (1.65, 3.51) and fixed effects model, 2.87 (2.07, 3.96)]. Subgroup analyses also showed beneficial effects of red ginseng in psychogenic ED (n = 135, RR, 2.05; 95% CI of 1.33, 3.16, p = 0.001, heterogeneity: χ2 = 0.08, p = 0.96, I2 = 0%, Figure 2B) [6, 10, 11]. There was no difference between the random effect model and the fixed effects model.
Figure 2.
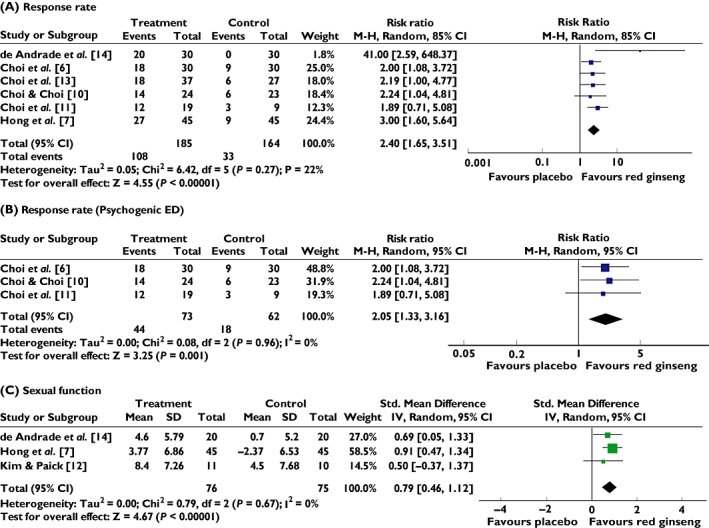
Forest plot of red ginseng for ED on response effectiveness in all kinds of ED (A), psychogenic ED (B) and on sexual function on questionnaires (C). ED: erectile dysfunction; IIEF: International Index of Erectile function
Sexual functions
Four RCTs tested the effects of red ginseng for sexual function on questionnaires compared with placebo and all trials reported positive effects of red ginseng [7, 10, 12, 14]. Three trials used the International Index of Erectile Function (IIEF) [7, 10, 14], while one RCT employed Watts sexual function questionnaire [12]. The meta-analysis of these three studies with available data [7, 12, 14] shows an effect in favour of red ginseng on sexual function compared with placebo (n = 151, SMD, 0.79, 95% CI = 0.46, 1.12, p < 0.00001; heterogeneity: tau2 = 0.00, χ2 = 0.79, p = 0.67, I2 = 0%; Figure 2C).
Discussion
To the best of our knowledge this is the first systematic review and meta-analysis of RCTs on the effectiveness of red ginseng for ED. Its results suggest that red ginseng is more effective than placebo in treating ED. However, the number of trials, the total sample size and the methodological quality of the primary studies are low.
A standard scoring system was used to quantify the likelihood of bias inherent in the studies based on the description of randomization, blinding and withdrawals.[9] Of the seven RCTs, only one RCT [7] reported details of double-blinding procedures. Two trials [12, 14] mentioned that they had adopted double-blind methods but did not report details. Only one RCT was of good methodological quality with maximum Jadad scores of 5 [7]. The remainder only reached a score of 1 [10, 11, 13, 14], 2 [6, 7, 10–14], or 3 [6, 12]. None of the RCTs reported the concealment of treatment allocation. Trials with inadequate blinding and inadequate allocation concealment may lead to selection bias and are likely to show exaggerated treatment effects. Only one RCT adopted assessor blinding [7]. Others failed to do so and contained detection bias. Details of drop-outs and withdrawals were described in three trials [6, 7, 12] and the others did not report this information. This may lead to exclusion or attrition bias. Thus the reliability of the evidence presented here is clearly limited.
Although all included RCTs adopted placebo control, none reported success of blinding. Three RCTs [7, 12, 14] used starch with ginseng flavour for placebo but others [6, 10, 11, 13] did not report details about the placebo. Unblinding is, therefore, a further possibility for potential overestimation of the treatment effects which is known as performance bias [16]. None of the studies reported a power calculation, and sample sizes were very small in some RCTs, with two having less than 30 participants. In addition, all included trials seem to have failed to report details about ethical approval. Baseline comparisons of symptoms of ED were reported in three trials [7, 12, 14]. The baseline imbalances may lead to erroneous conclusions from the statistical analyses. Some studies poorly described the outcome in their articles [6, 11]. In some trials only the mean average of all participants was reported or results were presented only with statistical language. In some trials P values or standard deviations were not provided [10, 13]. Further studies should follow CONSORT procedures [17].
Self-reported subjective questionnaires completed by patients and their partners are the most convenient method of collecting data on ED. Two [6, 13] of the included RCTs adopted questionnaires which assessed the symptoms of ED without testing validity and reliability, while others employed validating inventories for ED. However, it seems important that only validated questionnaires are used. Unless the outcome measures used have established reliability and validity, data derived from them are subject to bias, and comparisons between the results of different studies are difficult.
The extent to which red ginseng's therapeutic effects depend on the availability and amount of the various constituents in the preparation is unclear. The optimum dose of red ginseng is unknown. The single dose studies used quantities ranging from 1800 mg to 3000 mg of its extracts. Four of trials employed 600 mg, three times daily as treatment, while two trials used 900 mg and one study 1000 mg. However, a clinical trial comparing dose dependency has not yet been performed.
Possible mechanisms of action of red ginseng include hormonal effects similar to those of testosterone. However, direct measurements of testosterone concentrations seem to refute this hypothesis [6, 7, 13, 14]. Others have postulated that red ginseng might induce relaxation of the smooth muscles of the corpus carvernosum via the nitric oxide (NO) pathway [14, 18, 19]. Ginsenosides, which are thought to be the principle active constituents of red ginseng, have been shown to cause a dose-dependent relaxation of the corpus caverndosal smooth muscle in rabbits by increasing release of NO [18, 20–22]. More basic research is needed to understand fully the mechanisms of action of red ginseng.
Reports of adverse events with red ginseng were scarce and those that were reported were mild. Adverse effects of red ginseng were reported in five of the reviewed RCTs [7, 10, 11, 13, 14]. Six cases of headache or insomnia, four cases of gastric upset and two cases of constipation were reported, while three cases of gastric upset occurred with placebo. Currently, no post-marketing surveillance studies for red ginseng exist. A review of the safety profile of red ginseng reported no evidence of adverse drug reactions in humans with normal doses of red ginseng but pointed out the lack of data on long-term use.
Our review has a number of important limitations. Although strong efforts were made to retrieve all RCTs on the subject, we cannot be absolutely certain that our searches located all relevant RCTs. Moreover, selective publishing and reporting are other major causes for bias, which have to be considered. It is conceivable that several negative RCTs have remained unpublished and thus have distorted the overall picture [23–26]. It is noteworthy that a number of studies were supported by manufacturers of ginseng products, which may have introduced a degree of bias. Most trials sponsored by the industry revealed a positive outcome. In this review, three [6, 11, 12] of them were supported by a company associated with red ginseng (KT&G Corp) and two [7, 13] of them provided the red ginseng for trials from KT&G [13] and Korea Ginseng and Tobacco Research Institute [7]. This is one concern about possible bias of this systematic review. Another possible bias is that six [6, 7, 10–13] of the included trials were carried out in Korea. This is one of the regions which has been shown to produce largely positive results [27]. Further limitations include the paucity and the often suboptimal methodological quality of the primary data. Some of the RCTs included in the present review were not successful in minimizing bias. These facts limit the conclusiveness of this systematic review.
In conclusion, the results of our systematic review and meta-analysis provide suggestive evidence for the effectiveness of red ginseng in treating ED. However, the total number of RCTs that could be included in this analysis, the total sample size and the average methodological quality of the primary studies was too low to draw firm conclusions. More high quality studies are necessary to establish whether or not red ginseng has a place in the treatment of ED.
The authors especially thank Kate Boddy, Peninsula Medical School, Universities of Exeter & Plymouth, Exeter, UK for editing this manuscript and Jae-Cheol Kong, Wonkwang University, Iksan, South Korea for searching extensive databases. D.J.J. and Y.C.L. were supported by the Korean Intellectual Property Office and Korea Institute of Science and Technology Information.
REFERENCES
- 1.Tharyan P, Gopalakrishanan G. Erectile dysfunction. Clin Evid. 2006;15:1227–51. [PubMed] [Google Scholar]
- 2.American Urological Association. Management of erectile dysfunction: an update. [5 May 2008]. Available at http://www.auanet.org/guidelines/edmgmt.cfm.
- 3.Ernst E, Pittler MH. Yohimbine for erectile dysfunction: a systematic review and meta-analysis of randomized clinical trials. J Urol. 1998;159:433–6. doi: 10.1016/s0022-5347(01)63942-9. [DOI] [PubMed] [Google Scholar]
- 4.Yun TK. Panax ginseng – a non-organ-specific cancer preventive? Lancet Oncol. 2001;2:49–55. doi: 10.1016/S1470-2045(00)00196-0. [DOI] [PubMed] [Google Scholar]
- 5.Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf. 2002;25:323–44. doi: 10.2165/00002018-200225050-00003. [DOI] [PubMed] [Google Scholar]
- 6.Choi HK, Seong DH, Rha KH. Clinical efficacy of Korean red ginseng for erectile dysfunction. Int J Impot Res. 1995;7:181–6. [PubMed] [Google Scholar]
- 7.Hong B, Ji YH, Hong JH, Nam KY, Ahn TY. A double-blind crossover study evaluating the efficacy of korean red ginseng in patients with erectile dysfunction: a preliminary report. J Urol. 2002;168:2070–3. doi: 10.1016/S0022-5347(05)64298-X. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT, Greens S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, Ltd; 2006. [Google Scholar]
- 9.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 10.Choi HK, Choi YJ. Evaluation of clinical efficacy of Korea red ginseng for erectile dysfunction by international index of erectile function. J Ginseng Res. 2001;25:112–7. in Korean. [Google Scholar]
- 11.Choi HK, Choi YJ, Kim JH. Penile blood change after oral medication of Korean red ginseng in erectile dysfunction patients. J Ginseng Res. 2003;27:165–70. in Korean. [Google Scholar]
- 12.Kim SW, Paick JS. Clinical efficacy of Korean red ginseng on vasculogenic impotent patients. Korean J Androl. 1999;17:23–8. in Korean. [Google Scholar]
- 13.Choi HK, Choi YD, Adaikan PG, Jiang Y. Effectiveness of Korean red ginseng in erectile dysfunction: multi-national approach. J Ginseng Res. 1999;23:247–56. in Korean. [Google Scholar]
- 14.de Andrade E, de Mesquita AA, Claro Jde A, de Andrade PM, Ortiz V, Paranhos M, et al. Study of the efficacy of Korean Red Ginseng in the treatment of erectile dysfunction. Asian J Androl. 2007;9:241–4. doi: 10.1111/j.1745-7262.2007.00210.x. [DOI] [PubMed] [Google Scholar]
- 15.Kim HS, Woo SH, Jo S, Hahn EJ, Youn NY, Lee HL. Double-blind, placebo-controlled, multi-center study for therapeutic effects of mountain Panax Ginseng C.A. Meyer extract in men with erectile dysfunction: a preliminary report. Korean J Androl. 2006;24:84–99. [Google Scholar]
- 16.Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA. 1995;273:408–12. doi: 10.1001/jama.273.5.408. [DOI] [PubMed] [Google Scholar]
- 17.Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, Pitkin R, Rennie D, Schulz KF, Simel D, Stroup DF. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637–9. doi: 10.1001/jama.276.8.637. [DOI] [PubMed] [Google Scholar]
- 18.Choi YD, Xin ZC, Choi HK. Effect of Korean red ginseng on the rabbit corpus cavernosal smooth muscle. Int J Impot Res. 1998;10:37–43. doi: 10.1038/sj.ijir.3900300. [DOI] [PubMed] [Google Scholar]
- 19.O'Hara M, Kiefer D, Farrell K, Kemper K. A review of 12 commonly used medicinal herbs. Arch Fam Med. 1998;7:523–36. doi: 10.1001/archfami.7.6.523. [DOI] [PubMed] [Google Scholar]
- 20.Choi YD, Rha KH, Choi HK. In vitro and in vivo experimental effect of Korean red ginseng on erection. J Urol. 1999;162:1508–11. [PubMed] [Google Scholar]
- 21.Kim HJ, Woo DS, Lee G, Kim JJ. The relaxation effects of ginseng saponin in rabbit corporal smooth muscle: is it a nitro oxide donor? Br J Urol. 1998;82:744–8. doi: 10.1046/j.1464-410x.1998.00811.x. [DOI] [PubMed] [Google Scholar]
- 22.MacKay D. Nutrients and botanicals for erectile dysfunction: examining the evidence. Altern Med Rev. 2004;9:4–16. [PubMed] [Google Scholar]
- 23.Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA. 1990;263:1385–9. [PubMed] [Google Scholar]
- 24.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–6. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ernst E, Pittler MH. Alternative therapy bias. Nature. 1997;385:480. doi: 10.1038/385480c0. [DOI] [PubMed] [Google Scholar]
- 26.Pittler MH, Abbot NC, Harkness EF, Ernst E. Location bias in controlled clinical trials of complementary/alternative therapies. J Clin Epidemiol. 2000;53:485–9. doi: 10.1016/s0895-4356(99)00220-6. [DOI] [PubMed] [Google Scholar]
- 27.Vickers A, Goyal N, Harland R, Rees R. Do certain countries produce only positive results? A systematic review of controlled trials. Control Clin Trials. 1998;19:159–66. doi: 10.1016/s0197-2456(97)00150-5. [DOI] [PubMed] [Google Scholar]


