Abstract
AIMS
Data on efavirenz in HIV/viral hepatitis co-infected patients is non-consensual, probably due to liver function heterogeneity in the patients included.
METHODS
A case control study was performed on 27 HIV-infected patients, with controlled and homogenous markers of hepatic function, either mono-infected or co-infected with HBV/HCV, to ascertain the influence of viral hepatitis on efavirenz concentrations over a 2-year follow-up period.
RESULTS
No differences were found in efavirenz concentrations between groups both during and at the end of the follow-up period: control (2.43 ± 1.91 mg l–1) vs. co-infected individuals (2.37 ± 0.37 mg l–1).
CONCLUSION
It was concluded that HBV/HCV infections in themselves do not predispose to an overexposure to efavirenz.
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
HIV-1 co-infection with HBV/HCV is the most important factor determining efavirenz-induced liver toxicity. Higher efavirenz plasma concentrations have been reported in these patients facilitating concentration drug-related adverse effects.
It is not known whether changes in efavirenz disposition are due to the hepatitis infection/inflammation or to liver failure. As a consequence, the guidelines for the application of therapeutic drug monitoring of efavirenz in HBV/HCV co-infected patients have not been established.
WHAT THIS STUDY ADDS
The present study has shown that HBV/HCV infection in itself does not predispose to higher efavirenz plasma concentrations. In the absence of hepatic failure, the risk of efavirenz concentration-dependent toxicity is not increased.
Thus, therapeutic drug monitoring indications in co-infected patients with hepatic function within the normal range should be the same as in HIV-1 mono-infected patients.
Keywords: efavirenz concentrations, liver function, viral hepatitis co-infection
Introduction
Chronic viral hepatitis by-hepatitis B virus (HBV) and hepatitis C virus (HCV) is common among people with human immunodeficiency virus (HIV-1) infection because of shared modes of transmission [1–3] and has been largely responsible for end-stage liver disease [4]. Hepatic disease can reduce the clearance of drugs that are metabolized by the liver, increasing the risk of dose-related toxicities.
Efavirenz is a non-nucleoside reverse transcriptase inhibitor extensively metabolized to inactive hydroxylated metabolites by the cytochrome P450 enzymatic system, with subsequent urinary and biliary excretion of these metabolites after glucuronidation [5]. Exposure to high efavirenz plasma concentrations has been associated with an increased risk of experiencing neuropsychiatric adverse events [6–8]. Liver toxicity (severe increase in liver enzymes) related to efavirenz occurs in 1 to 8% of patients [9] and has been attributed to hypersensitivity reactions to efavirenz [10, 11] or to an accumulation or dose-dependent mechanisms [12].
HBV/HCV co-infection is the most important factor associated with efavirenz-induced liver toxicity [13–15] but it is not known what the contribution of infection/inflammation by hepatitis virus is to efavirenz disposition.
Data on efavirenz plasma concentrations in HBV/HCV co-infected patients is scant and contradictory and most of the data corresponds exclusively to one sample measurement per patient. Some reports, without data on hepatic liver function [8, 16] or with ‘normal’ liver function tests [17], show indirect indications that HBV/HCV infection does not necessarily predispose to higher efavirenz plasma concentrations in co-infected patients. However, association with high efavirenz plasma concentrations and cirrhosis or elevated hepatic enzymes was found in HBV/HCV co-infected patients [18–21].
In the present work a case control study was performed by monitoring efavirenz concentrations in HBV/HCV co-infected patients with controlled hepatic function, during 24 months in order to know whether the presence of HBV/HCV co-infection per se does influence plasma concentrations of efavirenz in the absence of severe hepatic damage.
Methods
All eligible patients were adults on efavirenz-containing regimens either as or not as initial therapy. The protocol received prior approval from the Hospital Ethics Committee, patients gave their written informed consent and adherence was controlled through a questionnaire.
Patients on concomitant medication other than antiretroviral drugs were also included. Only one patient was treated for HCV with pegylated-interferon combined with ribavirin in the second half of the study. No adjustments in efavirenz dose were made throughout the study.
Efavirenz determinations and blood collecting time were performed as previously described [22].
The assessment of the liver function was based on the analysis of key biochemical markers in hepatic systems and function [23]: markers of hepatocellular integrity (alanine aminotransferase (ALT) and aspartate aminotransferase (AST)); cholestasis (alkaline phosphatase (AP); γ-glutamyltranspeptidase (GGT); total bilirubin and direct bilirubin) and liver function (prothrombine international normalized ratio (PT) and albumin). The AST : ALT ratio was also recorded as a non-invasive method to differentiate the various degrees of hepatic disease severity: an AST : ALT ratio <1 has been associated with chronic hepatitis fibrosis score 0–3; an AST : ALT ratio ≥1 and <1.5 is common in patients with chronic hepatitis fibrosis score 4; and an AST : ALT ratio ≥1.5 means cirrhosis Child-Pugh class B and C [24].
Results were shown as mean ± SEM. Differences between groups were compared with Student's t-test. GraphPad Prism version 4 was used to perform analysis.
Results
This study was conducted in 27 HIV-infected patients on efavirenz (600 mg once daily) for more than 1 month: 18 were negative for HBV and HCV (control group) and nine were positive for HBV (n = 2) or HCV (n = 7) positive (study group).
The characteristics of the patients included in both groups were similar. The control group included 14 males and four females, aged 43 ± 3 years and with a body mass index (BMI) of 23 ± 3 kg m–2. These patients were on efavirenz for 14 ± 3 months, 57% were on efavirenz plus zidovudine and lamivudine and 10 patients were on their first antiretroviral regimen. The patients (seven males and two females) included in the study group were aged 39 ± 3 years and had a mean BMI of 23 ± 3 kg m–2. These patients were on efavirenz for 20 ± 3 months, 67% were on efavirenz plus zidovudine plus lamivudine and three patients were on their first antiretroviral regimen.
The CD4+ cell count at baseline was not different between the mono-infected (504 ± 66 cell mm−3) and the co-infected group (401 ± 32 cell mm−3). In both groups more than 70% of the patients had viral load <50 copies · mL–1.
At the baseline, no differences between groups in hepatocyte integrity and cholestasis were found (Table 1). Liver mass markers were similar between groups: baseline albumin mean value was 4.0 ± 0.1 g dl–1 in both groups (reference range 3.4–4.8 g dl–1) and the baseline PT was 1.04 ± 0.06 in the mono-infected group and 1.02 ± 0.04 in the co-infected group.
Table 1.
Comparison of hepatocellular integrity and cholestasis markers between, study (HIV and HBV or HCV co-infected patients) and control (HIV mono-infected patients) at the baseline and after 24 months of follow-up
| Marker (Reference range) | Study point | HIV mono-infected (n = 18) | HBV or HCV co-infected (n = 9) | P value |
|---|---|---|---|---|
| AST (<31 U l–1) | Baseline | 27 ± 3 | 35 ± 4 | NS |
| Month 24 | 24 ± 2 | 36 ± 7 | NS | |
| P value | NS | NS | ||
| ALT (<31 U l–1) | Baseline | 49 ± 5 | 59 ± 9 | NS |
| Month 24 | 34 ± 3 | 58 ± 9 | 0.0297 | |
| P value | 0.0222 | NS | ||
| AST : ALT (<1) | Baseline | 0.56 ± 0.04 | 0.71 ± 0.16 | NS |
| Month 24 | 0.85 ± 0.12 | 0.68 ± 0.04 | NS | |
| P value | 0.0267 | NS | ||
| AP (35–104 U l–1) | Baseline | 89 ± 5 | 94 ± 9 | NS |
| Month 24 | 80 ± 5 | 84 ± 9 | NS | |
| P value | NS | NS | ||
| GGT (<36 U l–1) | Baseline | 67 ± 13 | 114 ± 31 | NS |
| Month 24 | 55 ± 10 | 140 ± 44 | NS | |
| P value | NS | NS | ||
| Total bilirubin (<1.00 mg l–1) | Baseline | 0.31 ± 0.04 | 0.37 ± 0.06 | NS |
| Month 24 | 0.52 ± 0.07 | 0.57 ± 0.13 | NS | |
| P value | 0.0118 | NS | ||
| Direct bilirubin (<0.30 mg l–1) | Baseline | 0.11 ± 0.01 | 0.11 ± 0.03 | NS |
| Month 24 | 0.24 ± 0.03 | 0.21 ± 0.02 | NS | |
| P value | 0.0002 | 0.0352 |
Data are expressed in mean ± SEM. ns, non significant (p > 0.05). ALT, alanine aminotransferase; AP, alkaline phosphatase; AST, aspartate aminotransferase. GGT: γ-glutamyltranspeptidase.
Inter-individual variability in efavirenz plasma concentrations, calculated as coefficient of variation was 45% for the control group and 53% for the co-infected group.
At the end of the follow-up, no differences were found in the markers of hepatic function between mono-infected and co-infected patients. Although at month 24, increases in bilirubin (direct and total), in both mono- and co-infected patients (Table 1), were observed.
The efavirenz concentrations (Figure 1), at the baseline were higher (p = 0.036) in mono-infected individuals (1.86 ± 0.16 mg l–1) than in co-infected individuals (1.56 ± 0.93 mg l–1). However, efavirenz concentrations remained within the therapeutic range (1–4 mg l–1) [6], similar in both case and control groups during the follow-up and no differences were observed in intra-individual variability between the control (27 ± 18%) and co-infected group (37 ± 14%).
Figure 1.
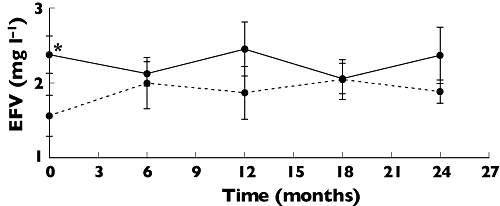
Time course of plasma concentrations of efavirenz. The solid line represents plasma concentrations of HIV mono-infected patients (n = 18) and the dotted line refers to plasma concentrations in HIV patients co-infected with HBV or HCV (n = 9). Month 0 corresponds to the inclusion of the patients in the study. Each point represents mean ± SEM. *p < 0.05 Student's t-test
Discussion
Changes in the hepatic drug metabolism of HIV patients co-infected with HBV/HCV can be affected by both the specific infection/inflammation caused by these viruses and by the severity of liver disease.
In our knowledge, this is the first work to analyze efavirenz concentrations in patients, with long-term controlled and homogeneous characteristics of hepatic function, without advanced stages of liver disease. It is concluded that: the infection by itself did not modify efavirenz concentrations; that the risk of efavirenz concentration-dependent toxicity is not increased in co-infected patients with normal hepatic function and that therapeutic drug monitoring (TDM) indications for these co-infected patients and HIV mono-infected patients are the same.
The 2 years of follow-up contributes to minimizing the limitation of the small number of patients included. This pilot study contributed to surpass the lack of information in the literature, not only about the true difference in efavirenz plasma concentrations between the mono and the co-infected patients but also about the variability of those measurements. It also generated data on within- and between-subject variations that will allow sample size calculation in further studies.
Recently the influence of liver fibrosis on efavirenz plasma concentrations was reported [21]. In that study, no functional data were analyzed and hepatic impairment was assessed exclusively by quantifying fibrosis using elastometry. This allowed authors to conclude that efavirenz concentrations are significantly higher in cirrhotic patients. This data fits with other previously described [18, 20] and, as it occurs in general with drugs that undergo hepatic elimination, cirrhosis increases efavirenz concentrations.
In the present work, we have looked into the hepatic function of patients with no clinical indications to do liver biopsies, particularly in those considered ‘well compensated’, where the degree of fibrosis could not be precisely discriminated by non-invasive methods.
The present work does not allow us to ascertain if high GGT concentrations were due to virus infection or to efavirenz, because at the baseline patients were on efavirenz for at least 14 months. Increases in GGT have been associated with HIV-infection [25] and efavirenz concentrations higher than 2.18 mg l–1, but only during the firsts 6 weeks of treatment [12].
At month 24, the ALT was significantly lower in mono-infected patients, although no changes in the AST/ALT or other analytical markers were observed. It is known that HCV-infection is characterized by a pattern of aminotransferase concentrations fluctuating around the upper reference value and elevated ALT values [26, 27].
It is known that hepatic drug clearance is depressed by most viruses, including HIV [28]. The CYP3A4 and CYP2D6 activities were significantly lower in patients with chronic hepatitis than those observed in healthy volunteers [28]. Besides efavirenz being a substrate for CYP3A4 and CYP2B6, we did not find differences in efavirenz concentrations.
The absence of toxic concentrations (>4 mg l–1, [6]) found in co-infected patients is consistent with the results obtained by Gutierrez et al., who did not find that co-infected patients are at a higher risk to neuropsychiatric adverse events due to overexposure to efavirenz [8]. In addition to normal efavirenz concentrations, hepatitis co-infection by itself did not apparently modify either intra- or inter-patient variability. The coefficients of variation herein observed in mono-infected patients were lower than those previously described in more heterogeneous groups of patients, like those included in TDM services. [6, 22, 29, 30].
No explanation for the lower efavirenz plasma concentrations observed in co-infected patients at baseline can be proposed because, at that point, efavirenz steady state concentrations have already been achieved and the diagnosis of hepatitis was performed simultaneously with HIV infection.
The concentrations of direct and total bilirubin at the end of the 24 months were higher than at baseline in both the mono-infected and the co-infected patients. This was an unexpected finding observed with drug concentrations within the therapeutic range that could be attributed to efavirenz and related to its glucuronidation metabolism and this needs further investigation.
In conclusion, the inflammation caused by hepatitis viruses B/C infections in the absence of liver function impairment did not modify efavirenz exposition and no special indications for TDM are apparently needed in these patients.
Conflict of interest: The authors do not have a commercial or any other association that might pose a conflict of interest.
Financial support was provided by POCTI FCB/42664/2001 and the Comissão Nacional de Luta contra a Sida. Samples of pure Efavirenz were kindly provided by Merck Sharp and Dohme. The authors are grateful to Ms Eunice Silva for technical support and to Mr Michael Bright for reviewing the English.
REFERENCES
- 1.Lok AS, McMahon BJ. Practice Guidelines Committee, American Association for the Study of Liver Diseases (AASLD). Chronic hepatitis B: update of recommendations. Hepatology. 2004;39:857–61. doi: 10.1002/hep.20110. [DOI] [PubMed] [Google Scholar]
- 2.Dodig M, Tavill AS. Hepatitis C and human immunodeficiency virus coinfections. J Clin Gastroenterol. 2001;33:367–74. doi: 10.1097/00004836-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Staples CT, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29:150–4. doi: 10.1086/520144. [DOI] [PubMed] [Google Scholar]
- 4.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32:492–7. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 5.Mutlib AE, Chen H, Nemeth G, Gan LS, Christ DD. Liquid chromatography/mass spectrometry and high-field nuclear magnetic resonance characterization of novel mixed diconjugates of the non-nucleoside human immunodeficiency virus-1 reverse transcriptase inhibitor, efavirenz. Drug Metab Dispos. 1999;27:1045–56. [PubMed] [Google Scholar]
- 6.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS. 2001;15:71–5. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 7.Nunez M, Gonzalez de Requena D, Gallego L, Jiminez-Nacher I, Gonzalez-Lahoz J, Soriano V. Higher efavirenz plasma levels correlate with development of insomnia. J Acquir Immune Defic Syndr. 2001;28:399–400. doi: 10.1097/00126334-200112010-00015. [DOI] [PubMed] [Google Scholar]
- 8.Gutierrez F, Navarro A, Padilla S, Anton R, Masiá M, Borrás J, Martín-Hidalgo A. Prediction of neuropsychiatric adverse events associated with long-term efavirenz therapy, using plasma drug level monitoring. Clin Infect Dis. 2005;41:1648–53. doi: 10.1086/497835. [DOI] [PubMed] [Google Scholar]
- 9.Rivero A, Mira JA, Pineda JA. Liver toxicity induced by non-nucleoside reverse transcriptase inhibitors. J Antimicrob Chemother. 2007;59:342–6. doi: 10.1093/jac/dkl524. [DOI] [PubMed] [Google Scholar]
- 10.Bossi P, Colin D, Bricaire F, Caumes E. Hypersensitivity syndrome associated with efavirenz therapy. Clin Infect Dis. 2000;30:227–8. doi: 10.1086/313629. [DOI] [PubMed] [Google Scholar]
- 11.Angel-Moreno-Maroto A, Suarez-Castellano L, Hernandez-Cabrera M, Perez-Arellano JL. Severe efavirenz-induced hypersensitivity syndrome (not-DRESS) with acute renal failure. J Infect. 2006;52:39–40. doi: 10.1016/j.jinf.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Kappelhoff BS, van Leth F, Robinson PA, MacGregor TR, Baraldi E, Montella F, Uip DE, Thompson MA, Russell DB, Lange JM, Beijnen JH, Huitema AD, 2NN Study Group Are adverse events of nevirapine and efavirenz related to plasma concentrations? Antivir Ther. 2005;10:489–98. [PubMed] [Google Scholar]
- 13.Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD. Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: role of hepatitis C and B infections. Hepatology. 2002;35:182–9. doi: 10.1053/jhep.2002.30319. [DOI] [PubMed] [Google Scholar]
- 14.Martin-Carbonero L, Nunez M, Gonzalez-Lahoz J, Soriano V. Incidence of liver injury after beginning antiretroviral therapy with efavirenz or nevirapine. HIV Clin Trials. 2003;4:115–20. doi: 10.1310/N4VT-3E9U-4BKN-CRPW. [DOI] [PubMed] [Google Scholar]
- 15.Ena J, Amador C, Benito C, Fenoll V, Pasquau F. Risk and determinants of developing severe liver toxicity during therapy with nevirapine-and efavirenz-containing regimens in HIV-infected patients. Int J STD AIDS. 2003;14:776–81. doi: 10.1258/09564620360719840. [DOI] [PubMed] [Google Scholar]
- 16.Rodriguez-Novoa S, Barreiro P, Rendon A, Jimenez-Nacher I, Gonzalez-Lahoz J, Soriano V. Influence of 516G>T polymorphisms at the gene encoding the CYP450-2B6 isoenzyme on efavirenz plasma concentrations in HIV-infected subjects. Clin Infect Dis. 2005;40:1358–61. doi: 10.1086/429327. [DOI] [PubMed] [Google Scholar]
- 17.Gibbons S, Taylor C, Waldron S, Back DJ, Weber J, Khoo SH. Therapeutic drug monitoring of NNRTIs in patients with hepatic dysfunction. 6th International Congress on Drug Therapy in HIV Infection, Glasgow. Abstract P174.
- 18.Maserati R, Villani P, Seminari E, Pan A, Lo Caputo S, Regazzi MB. High plasma levels of nelfinavir and efavirenz in two HIV-positive patients with hepatic disease. AIDS. 1999;13:870–1. doi: 10.1097/00002030-199905070-00025. [DOI] [PubMed] [Google Scholar]
- 19.Bickel M, Stephan C, Rottmann C, Carlebach A, Haberl A, Kurowski M, Staszewski S. Severe CNS side-effect and persistent high efavirenz plasma levels in a patient with HIV/HCV coinfection and liver cirrhosis. Scand J Infect Dis. 2005;37:520–2. doi: 10.1080/00365540410020901. [DOI] [PubMed] [Google Scholar]
- 20.Robertson SM, Scarsi KK, Postelnick MJ, Lynch P. Elevated plasma concentrations of protease inhibitors and nonnucleoside reverse transcriptase inhibitors in patients coinfected with human immunodeficiency virus and hepatitis B or C: case series and literature review. Pharmacotherapy. 2005;25:1068–72. doi: 10.1592/phco.2005.25.8.1068. [DOI] [PubMed] [Google Scholar]
- 21.Barreiro P, Rodriguez-Novoa S, Labarga P, Ruiz A, Jiménez-Nácher I, Martín-Carbonero L, Gonzalez-Lahoz J, Soriano V. Influence of liver fibrosis stage on plasma levels of antiretroviral drugs in HIV-infected patients with chronic hepatitis C. J Infect Dis. 2007;195:973–9. doi: 10.1086/512086. [DOI] [PubMed] [Google Scholar]
- 22.Pereira SA, Branco T, Caixas U, Côrte-Real RM, Germano I, Lampreia F, Monteiro EC. Intra-individual variability in efavirenz plasma concentrations supports therapeutic drug monitoring based on quarterly sampling in the first year of Therapy. Ther Drug Monit. 2008;30:60–6. doi: 10.1097/FTD.0b013e318160ce76. [DOI] [PubMed] [Google Scholar]
- 23.Giannini EG, Testa R, Savarino V. Liver enzyme alteration: a guide for clinicians. CMAJ. 2005;172:367–79. doi: 10.1503/cmaj.1040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giannini E, Risso D, Botta F, Chiarbonello B, Fasoli A, Malfatti F, Romagnoli P, Testa E, Ceppa P, Testa R. Validity and clinical utility of the aspartate aminotransferase-alanine aminotransferase ratio in assessing disease severity and prognosis in patients with hepatitis C virus-related chronic liver disease. Arch Intern Med. 2003;163:218–24. doi: 10.1001/archinte.163.2.218. [DOI] [PubMed] [Google Scholar]
- 25.Geffriaud C, Poynard T, Delfraissy JF, Bedossa P, Naveau S, Bourée P, Dubreuil P, Chaput JC. Hepatic involvement in HIV 1 virus infection. Gastroenterol Clin Biol. 1988;12:465–72. [PubMed] [Google Scholar]
- 26.Fonquernie L, Serfaty L, Charrois A, Wendum D, Lefebvre B, Girard PM, Meynard JL. Significance of hepatitis C virus coinfection with persistently normal alanine aminotransferase levels in HIV-1-infected patients. HIV Med. 2004;5:385–90. doi: 10.1111/j.1468-1293.2004.00239.x. [DOI] [PubMed] [Google Scholar]
- 27.Butt AA, Tsevat J, Ahmad J, Shakil AO, Mrus JM. Biochemical and virologic parameters in patients co-infected with hepatitis C and HIV versus patients with hepatitis C mono-infection. Am J Med Sci. 2007;333:271–5. doi: 10.1097/MAJ.0b013e31805341f0. [DOI] [PubMed] [Google Scholar]
- 28.Renton KW. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metab. 2004;5:235–43. doi: 10.2174/1389200043335559. [DOI] [PubMed] [Google Scholar]
- 29.Csajka C, Marzolini C, Fattinger K, Decosterd LA, Fellay J, Telenti A, Biollaz J, Buclin T. Population pharmacokinetics and effects of efavirenz in patients with human immunodeficiency virus infection. Clin Pharmacol Ther. 2003;73:20–30. doi: 10.1067/mcp.2003.22. [DOI] [PubMed] [Google Scholar]
- 30.Stahle L, Moberg L, Svensson JO, Sonnerborg A. Efavirenz plasma concentrations in HIV-infected patients: inter- and intraindividual variability and clinical effects. Ther Drug Monit. 2004;26:267–70. doi: 10.1097/00007691-200406000-00008. [DOI] [PubMed] [Google Scholar]


