Abstract
AIMS
To measure and compare the concentration–time profiles of oxazepam and oxazepam glucuronide in blood, serum and oral fluid within the scope of roadside testing.
METHODS
Biological samples were collected from eight male subjects after ingestion of 15 or 30 mg oxazepam on separate dosing occasions with an interval of 7 days. The concentration–time profiles of oxazepam and oxazepam glucuronide were fitted by using a one-compartment model.
RESULTS
For oxazepam and oxazepam glucuronide, the mean oral fluid/blood ratios were 0.05 (range 0.04–0.07) and 0.004 (range 0.002–0.006), respectively. The concentration–time profiles in oral fluid paralleled those in blood.
CONCLUSION
After oral administration of therapeutic doses of oxazepam, concentrations in oral fluid are very much lower than those in blood, and those of oxazepam glucuronide are much lower than those of the parent compound. Nevertheless, assay of oral fluid for oxazepam can be used to detect recent ingestion of the drug in drivers suspected of impaired driving performance.
WHAT IS ALREADY KNOWN ABOUT THE SUBJECT
Concentration–time profiles of drugs in oral fluid generally run parallel to those in blood.
In general, oral fluid contains more parent drug than metabolites.
For some benzodiazepines it has been shown that concentrations are lower in oral fluid than in blood.
WHAT THIS STUDY ADDS
The concentration–time profile of oxazepam in oral fluid after a single dose of oxazepam runs parallel to blood and is dose dependent.
Concentration ratios (oral fluid/blood and oral fluid/serum) of oxazepam and oxazepam glucuronide after controlled intake of a single dose of oxazepam are presented.
Keywords: oral fluid, oxazepam, oxazepam glucuronide, pharmacokinetics
Introduction
Benzodiazepines, especially oxazepam, are the most commonly detected medicinal drugs in blood from drivers [1]. Benzodiazepines may increase the risk of a road accident [1, 2]. The preferred specimen for roadside testing is oral fluid, because venepuncture is needed to obtain blood, and violation of privacy may be necessary for urine. However, the relationship between concentrations of oxazepam and its glucuronide metabolite in oral fluid and blood is unknown. To our knowledge, pharmacokinetic studies of oxazepam and oxazepam glucuronide in both oral fluid and blood have not been published until now. The aim of this study was to compare the concentration–time profiles of oxazepam and oxazepam glucuronide in blood, serum and oral fluid within the scope of roadside testing.
Methods
Subjects and study design
Eight healthy volunteers (healthy men, age 18–27 years) were recruited. The study design was double-blind crossover, in which the subjects received, in random order, 15 mg and 30 mg oxazepam orally, in identically appearing formulations, on 2 days separated by 1 week in order to exclude carry-over effects. The study was conducted according to the code of ethics on human experimentation established by the declaration of Helsinki (1964) and amended in Edinburgh (2000). The study protocol was approved by the medical ethics committee Medisch-Ethische Toetsing Onderzoek Patiënten en Proefpersonen (Tilburg, the Netherlands). All subjects gave written informed consent.
Bioanalysis
Oral fluid samples (n = 17) and blood samples (n = 13) were collected over specified intervals up to 8.5 h after oral administration. Oral fluid samples (approximately 1 ml) were obtained by spitting into a plastic container. Venous blood samples (approximately 5 ml) were collected by a physician and equally divided over two glass tubes. One tube was used for whole blood measurements, the other for serum measurements after centrifugation.
The concentrations of oxazepam and oxazepam glucuronide in oral fluid, serum and whole blood were determined by using liquid chromatography–mass spectrometry/mass spectrometry. Internal standards (d5-oxazepam and lorazepam-glucuronide) were added to a 200-µl sample before protein precipitation by using 600 µl methanol. Samples were centrifuged and the supernatant was evaporated and reconstituted in 75 µl methanol/water 40% v/v. The chromatographic column was Acquity UPLC™ BEH C 18 (100 × 2.1 mm, 1.7 µm; Waters, Etten-Leur, the Netherlands). Injection volume was 20 µl. The gradient was methanol/formic acid (0.010 M, pH 3.0), 45% v/v to 100% v/v methanol. For extracts of whole blood, a modified gradient was used in order to minimize ion suppression.
The linear range for the assay was 0.5–100 (oral fluid); 1–1000 (serum) ng ml−1 for oxazepam as well as for oxazepam glucuronide. The limit of detection was 0.25 (oral fluid), respectively 0.5 (serum) ng ml−1 for oxazepam and 0.25 (oral fluid), respectively 0.5 (serum) ng ml−1 for oxazepam glucuronide. The limit of quantification was 0.5 (oral fluid), respectively 1 (serum) ng ml−1 for oxazepam and 0.25 (oral fluid), respectively 1 (serum) ng ml−1 for oxazepam glucuronide. Interday precision was <20%, except for oxazepam at 0.5 ng ml−1 (23% in oral fluid and 20% in serum), and for oxazepam glucuronide at 0.5 ng ml−1 in serum (21%). The accuracy was 101% for oxazepam in serum and 100% for oxazepam glucuronide (a stock solution was used with a certified concentration). An external control sample of oxazepam in oral fluid was not available.
In whole blood, the linear range was 5–1000 ng ml−1 for oxazepam as well as for oxazepam glucuronide. The limit of detection was 2 ng ml−1 for oxazepam and 3 ng ml−1 for oxazepam glucuronide. The limit of quantification was between 1 and 5 ng ml−1 for oxazepam and between 5 and 10 ng ml−1 for oxazepam glucuronide. Intraday precision and interday precision were ≤20% for oxazepam and oxazepam glucuronide, except for the intraday precision for oxazepam glucuronide 5 ng ml−1 (22%). The accuracy of the measurement of oxazepam in blood was 106%.
Pharmacokinetic analysis
The concentration–time profiles in serum, whole blood and oral fluid were fitted by using a one-compartment model (KinFit, MWPharm® 3.50; Mediware, Groningen, the Netherlands) [3]. For the calculation of clearance, the bioavailability of oxazepam in serum and whole blood was assumed to be 1 [4]. The parameters of oxazepam and oxazepam glucuronide were calculated as the means of the individual parameters after intake of 15 and 30 mg oxazepam (except for Cmax and AUC), assuming linear pharmacokinetics. The blood/serum (B/S) ratio, oral fluid/blood (OF/B) ratio and oral fluid/serum (OF/S) ratio were calculated from the area under the curve (AUC0–8.5) values, calculated by the trapezoidal rule.
Results
Figure 1 shows the mean concentration–time profiles of oxazepam and oxazepam glucuronide in serum (Figure 1a) and oral fluid (Figure 1b) for the individual subjects (1–8) after intake of 15 and 30 mg oxazepam, respectively. The mean concentration–time profiles in whole blood were very similar to those of serum (not shown in Figure 1 but see Table 1).
Figure 1.
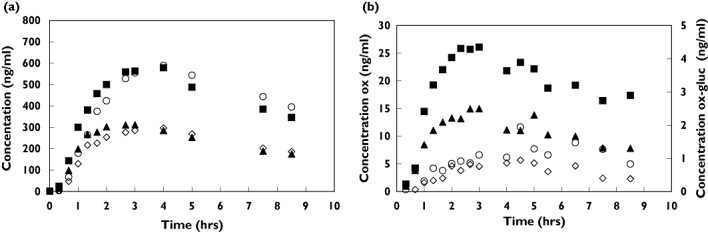
(a) mean concentration-time profiles of oxazepam and oxazepam glucuronide in serum (b) the mean concentration-time profiles in oral fluid. oxazepam (ox) concentrations after 15 mg dose (▴) and 30 mg dose (▪); oxazepam-glucuronide (ox-gluc) concentrations after 15 mg dose (◊) and 30 mg dose (○)
Table 1.
Pharmacokinetic data of oxazepam and oxazepam glucuronide in whole blood, serum and oral fluid
| Oxazepam | Whole blood (n = 8) | Serum (n = 8) | Oral fluid (n = 7)* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | rsd (%) | Min | Max | Median | Mean | rsd (%) | Min | Max | median | mean | rsd (%) | min | max | |
| CL (l h−1) | 5.75 | 5.83 | 33 | 2.11 | 8.36 | 5.07 | 5.07 | 41 | 1.23 | 8.01 | † | † | † | † | † |
| Vd (l kg−1) | 0.52 | 0.52 | 17 | 0.35 | 0.66 | 0.45 | 0.43 | 15 | 0.34 | 0.50 | † | † | † | † | † |
| ke (h−1) | 0.15 | 0.15 | 38 | 0.06 | 0.26 | 0.16 | 0.16 | 47 | 0.05 | 0.27 | † | † | † | † | † |
| tlag (h) | 0.38 | 0.44 | 59 | 0.20 | 0.95 | 0.32 | 0.40 | 57 | 0.23 | 0.93 | 0.27 | 0.30 | 29 | 0.19 | 0.43 |
| tmax (h) | 2.70 | 2.85 | 21 | 2.24 | 3.99 | 2.76 | 3.23 | 38 | 2.34 | 5.97 | 2.70 | 3.11 | 39 | 2.23 | 5.60 |
| after intake of 15 mg oxazepam | |||||||||||||||
| Cmax (ng ml−1) 15 mg | 263 | 279 | 20 | 217 | 391 | 317 | 317 | 20 | 229 | 441 | 11 | 13 | 44 | 8 | 24 |
| AUC0–8.5h trapezoidal (ng h ml−1) | 1673 | 1723 | 22 | 1303 | 2522 | 2006 | 1939 | 24 | 1307 | 2841 | 153 | 193 | 65 | 115 | 474 |
| AUC0–8.5h model (ng h ml−1) | 1772 | 1865 | 26 | 1356 | 2850 | 2125 | 2132 | 27 | 1376 | 3256 | 147 | 192 | 64 | 126 | 469 |
| after intake of 30 mg oxazepam | |||||||||||||||
| Cmax (ng ml−1) 30 mg | 510 | 515 | 15 | 423 | 662 | 555 | 599 | 25 | 497 | 967 | 19 | 24 | 44 | 15 | 45 |
| AUC0–8.5h trapezoidal (ng h ml−1) | 3177 | 3208 | 21 | 2485 | 4524 | 3413 | 3532 | 13 | 2955 | 4272 | 283 | 374 | 73 | 146 | 971 |
| AUC0–8.5h model (ng h ml−1) | 3723 | 3825 | 18 | 2907 | 4973 | 4373 | 4842 | 32 | 3539 | 8396 | 265 | 384 | 86 | 152 | 1117 |
| Oxazepam glucuronide | Whole blood (n = 8) | Serum (n = 8) | Oral fluid (n = 7)* | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean | rsd (%) | Min | Max | Median | Mean | rsd (%) | Min | Max | median | mean | rsd (%) | min | max | |
| ke (h−1) | 0.16 | 0.15 | 46 | 0.05 | 0.25 | 0.20 | 0.18 | 52 | 0.05 | 0.33 | † | † | † | † | † |
| tlag (h) | 0.51 | 0.60 | 43 | 0.38 | 1.11 | 0.52 | 0.58 | 35 | 0.42 | 1.02 | 0.77 | 0.85 | 29 | 0.57 | 1.24 |
| tmax (h) | 3.85 | 3.79 | 14 | 3.13 | 4.87 | 3.64 | 3.75 | 19 | 2.66 | 5.05 | 4.17 | 3.74 | 38 | 1.53 | 5.63 |
| after intake of 15 mg oxazepam | |||||||||||||||
| Cmax (ng ml−1) 15 mg | 206 | 184 | 26 | 101 | 236 | 299 | 293 | 24 | 193 | 420 | 1 | 1 | 41 | 0 | 1 |
| AUC0–8.5h trapezoidal (ng h ml−1) | 1294 | 1205 | 25 | 688 | 1550 | 1906 | 1880 | 24 | 1197 | 2737 | 12 | 11 | 35 | 5 | 15 |
| AUC0–8.5h model (ng h ml−1) | 1434 | 1383 | 28 | 768 | 1877 | 2199 | 2176 | 22 | 1427 | 3042 | 12 | 11 | 35 | 5 | 15 |
| after intake of 30 mg oxazepam | |||||||||||||||
| Cmax (ng ml−1) 30 mg | 376 | 358 | 24 | 203 | 457 | 595 | 567 | 25 | 387 | 787 | 1 | 1 | 39 | 1 | 2 |
| AUC0–8.5h trapezoidal (ng h ml−1) | 2363 | 2245 | 22 | 1423 | 2831 | 3828 | 3650 | 26 | 2433 | 5068 | 18 | 20 | 63 | 6 | 40 |
| AUC0–8.5h model (ng h ml−1) | 3064 | 2930 | 30 | 1573 | 4060 | 4909 | 4802 | 29 | 3168 | 7369 | 20 | 21 | 64 | 7 | 41 |
The test results in oral fluid of one subject were excluded because the results were not satisfactory.
Parameter not estimated.
AUC, area under the curve; CL, clearance; Cmax, maximal drug concentration; ke, elimination rate constant; tlag, lagtime; tmax, time to reach Cmax; Vd, volume of distribution.
The corrected Akaike information criterion (AICc) can be used as a measure of the goodness of fit of the pharmacokinetic model [5]. Based on the AICc, a one-compartment model was preferred over a two-compartment model for seven out of eight subjects. In oral fluid, oxazepam as well as oxazepam glucuronide was detected. Concentrations in oral fluid were relatively low.
Table 1 shows the pharmacokinetic data of oxazepam and oxazepam glucuronide in whole blood, serum and oral fluid.
Concentrations of oxazepam in blood and serum were comparable (mean B/S = 0.90; range 0.83–0.97). As a consequence, the OF/B ratio (mean 0.05; range 0.04–0.07) and the OF/S ratio (mean 0.04; range 0.03–0.07) were almost identical. Concentrations of oxazepam glucuronide were higher in serum than in blood (mean B/S = 0.64; range 0.46–0.81), which explains the difference in OF/B ratio (mean 0.004; range 0.002–0.006) and OF/S ratio (mean 0.002; range 0.001–0.004) for oxazepam glucuronide.
Discussion
After a single oral dose of 15 or 30 mg oxazepam, oxazepam and oxazepam glucuronide were detectable in oral fluid for at least 8.5 h. This period is long enough to demonstrate recent use of oxazepam in oral fluid from drivers.
The data in blood and serum were best described by using a one-compartment model in seven out of eight subjects. Other authors have suggested that a two-compartment model may be more appropriate [4]. However, we did not observe a distinct distribution phase in the concentration–time curves. In some subjects, the measured concentrations deviated from the fitted curves, especially in the first hours. This may be explained by variation in absorption (fluid intake at t = 1 h 5 min and t = 2 h 10 min).
The pharmacokinetic parameters calculated for oxazepam in blood are in agreement with other studies [6–8]. The B/S ratio for oxazepam (mean 0.90, range 0.83–0.97) is in agreement with the plasma/blood ratio (1.04 ± 0.05) reported by Shull et al. [9]. The ratios OF/B and OF/S for oxazepam are in correspondence with the expected free fraction in plasma. Oxazepam has pKa values of 1.7 and 11.6 [10] and is un-ionized in saliva (pH 6–8) and plasma (pH 7.4). About 95% of oxazepam is bound to plasma proteins [10]. Because un-ionized and unbound molecules are able to pass membranes, concentrations of free (unbound) oxazepam are expected to be equal in blood (or serum) and oral fluid.
The concentration–time profile of oxazepam in oral fluid was dose related and mimicked the concentration–time curves in blood and serum. Unfortunately, oral fluid concentrations fitted the pharmacokinetic model poorly, and the variation in, for example, Cmax and tmax was high. As a result, the correlation between concentrations in blood and oral fluid was variable. This variation may be partly due to variation from the analytical measurements at low concentrations. Better analytical methods may improve the correlation, but biological influencing factors such as changes in salivary composition or protein binding may also cause a significant variation in the results. To our knowledge, literature about the transfer of oxazepam glucuronide from blood to oral fluid is lacking. The variation in oral fluid concentrations will be higher in samples obtained from roadside testing than in this controlled study.
In summary, oral fluid can be used for the detection of the recent use of a single dose of oxazepam in (suspected) impaired drivers. A relation between the concentrations of oxazepam in blood (or serum) and oral fluid has been established in this study. The predictive value of oral fluid concentrations for values in blood is as yet unclear. As a consequence, a relationship between oral fluid concentrations and effects related to driving performance cannot be established at the moment. Oral fluid as matrix for roadside screening may be promising, but only in view of a zero-tolerance legislation.
The authors thank D. Botter, H. N. J. M. van Venrooij and U. J. L. Reijnders, forensic physicians, and the staff of Forum Educatief in Utrecht for their assistance in carrying out this study.
REFERENCES
- 1.Kelly E, Darke S, Ross J. A review of drug use and driving: epidemiology, impairment, risk factors and risk perceptions. Drug Alcohol Rev. 2004;23:319–44. doi: 10.1080/09595230412331289482. [DOI] [PubMed] [Google Scholar]
- 2.Movig KL, Mathijssen MP, Nagel PH, van Egmond T, de Gier JJ, Leufkens HGM, Egberts ACG. Psychoactive substance use and the risk of motor vehicle accidents. Accid Anal Prev. 2004;36:631–6. doi: 10.1016/S0001-4575(03)00084-8. [DOI] [PubMed] [Google Scholar]
- 3.Proost JH, Meijer DK. MW/Pharm, an integrated software package for drug dosage regimen calculation and therapeutic drug monitoring. Comput Biol Med. 1992;22:155–63. doi: 10.1016/0010-4825(92)90011-b. [DOI] [PubMed] [Google Scholar]
- 4.Sonne J, Loft S, Dossing M, Vollmer-Larsen A, Olesen KL, Victor M, Andreasen F, Andreasen PB. Bioavailability and pharmacokinetics of oxazepam. Eur J Clin Pharmacol. 1988;35:385–9. doi: 10.1007/BF00561369. [DOI] [PubMed] [Google Scholar]
- 5.Glatting G, Kletting P, Reske SN. Choosing the optimal fit function: comparison of the Akaike information criterion and the F-test. Med Phys. 2007;34:4285–92. doi: 10.1118/1.2794176. [DOI] [PubMed] [Google Scholar]
- 6.Greenblatt DJ, Divoll M, Harmatz JS, Shader RI. Oxazepam kinetics: effects of age and sex. J Pharmacol Exp Ther. 1980;215:86–91. [PubMed] [Google Scholar]
- 7.Ochs HR, Greenblatt DJ, Otten H. Disposition of oxazepam in relation to age, sex, and cigarette smoking. Klin Wochenschr. 1981;59:899–903. doi: 10.1007/BF01721923. [DOI] [PubMed] [Google Scholar]
- 8.Sonne J. Factors and conditions affecting the glucuronidation of oxazepam. Pharmacol Toxicol. 1993;73(Suppl)(1):1–23. doi: 10.1111/j.1600-0773.1993.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 9.Shull HJ, Wilkinson GR, Johnson R, Schenker S. Normal disposition of oxazepam in acute viral hepatitis and cirrhosis. Ann Intern.Med. 1976;84:420–5. doi: 10.7326/0003-4819-84-4-420. [DOI] [PubMed] [Google Scholar]
- 10.Moffat AC, Osselton MD, Widdop B, editors. Clarke's Analysis of Drugs and Poisons. London, Chicago: Pharmaceutical Press; 2004. Oxazepam; pp. 1375–6. [Google Scholar]


