Abstract
Ex-vivo-activated B cells are an alternative source of antigen-presenting cells (APCs) and a potential replacement for dendritic cells (DCs) in immunotherapy. However, the ability of ex-vivo-activated B cells to function as potent APCs has been a concern, especially when compared to DCs. Our study investigated whether modification of activated B cells with immune stimulatory molecules could enhance the ability of activated B cells to stimulate T cells. We show that murine splenic B cells, activated with a combination of Toll-like receptor agonist and agonistic anti-CD40, stimulated antigen-specific CD8+ T cells more efficiently than cells activated with Toll-like receptor agonist or anti-CD40 alone, probably by down-regulation of the immune regulatory cytokine interleukin-10 (IL-10). However, the activated B cells were still poor T-cell stimulators compared to mature DCs. Therefore, we modified the activated B cells by simultaneous electroporation of multiple messenger RNAs encoding costimulatory molecules (OX40L and 4-1BBL), cytokines (IL-12p35 and IL-12p40) and antigen. We found that de novo expression or overexpression of OX40L, 4-1BBL and IL-12p70 on activated B cells synergistically enhanced proliferation as well as IL-2 and interferon-γ production by CD8+ T cells. Furthermore, the RNA-modified activated B cells induced antigen-specific cytotoxic T lymphocyte responses as efficiently as mature DCs in vitro. Unexpectedly, modified activated B cells were inferior to mature DCs at in vivo induction of CD8+ T-cell responses. In summary, activated B cells modified to express immune stimulatory molecules are a potent alternative to DCs in immunotherapy.
Keywords: B cell, costimulatory molecule, cytokine, dendritic cell, RNA modification
Introduction
Ex-vivo-activated B cells have been shown to be a potential substitute for dendritic cells (DCs) in immunotherapy.1–9 In spite of their practical advantages over DCs, including their abundance in peripheral blood, ease of expansion, simple preparation and homogeneity, activated B cells have been shown to be less efficient stimulators of T cells than DCs. Croft et al.10 demonstrated that DCs were far superior to activated B cells in inducing proliferation and interleukin-2 (IL-2) production by naïve CD4+ T cells. Furthermore, Cassell et al.11 reported that activated B cells induced significantly lower IL-2 production by CD4+ T cells than DCs, which was a result of their suboptimal costimulation. Moreover, Subklewe et al.12 showed that B cells transformed by Epstein–Barr virus (EBV) were able to activate EBV-specific CD8+ T cells but their efficiency as antigen-presenting cells (APCs) was about 10-fold lower than that of DCs because of their lower expression of costimulatory and adhesion molecules.
Previously, DCs genetically modified to either express de novo or overexpress immune stimulatory molecules, such as costimulatory molecules, cytokines, chemokines and adhesion molecules, have enhanced cellular immunity.13 However, there are no such studies with on B cells activated ex vivo to enhance their APC functions. Consequently, we hypothesized that ex-vivo-activated B cells modified to express immune stimulatory molecules would have enhanced ability to stimulate T cells.
OX40L and 4-1BBL are costimulatory molecules expressed on APCs. In general, OX40L and 4-1BBL play a pivotal role in the augmentation of survival, proliferation and memory responses of CD4+ and CD8+ T cells, respectively.14,15 Dannull et al.16 previously demonstrated that overexpression of OX40L on human and mouse DCs by cotransfection of OX40L messenger RNA (mRNA) and tumour mRNA enhanced both CD4+ T-cell and CD8+ T-cell responses against tumour cells expressing the corresponding antigens and prolonged tumour-free survival of tumour-bearing mice. It has also been reported that combinatorial signals of OX40 and 4-1BB using agonistic antibodies had synergistic effects on CD8+ T-cell responses.17,18
IL-12p70 is a heterodimeric molecule comprised of IL-12p35 and IL-12p40. It is an immune stimulatory cytokine that is important for polarization of T helper type 1 (Th1) cells, interferon-γ (IFN-γ) production by T cells, macrophages and natural killer (NK) cells as well as enhancement of cellular immune responses.19 Recently, Tatsumi et al.20 reported that transduction of murine bone marrow-derived DCs with IL-12 cDNAs enhanced the inhibition of tumour growth and that this effect was dependent on CD4+ and CD8+ T cells as well as NK cells. Moreover, Bontkes et al.21 demonstrated that human monocyte-derived DCs transfected with IL-12 mRNAs enhanced IFN-γ production and cytotoxic T-lymphocyte (CTL) responses by CD8+ T cells.
Transfection of mRNAs has been broadly used for the delivery of antigen or immune stimulatory molecules into DCs.13 Compared to viral transduction and DNA transfection, RNA transfection has several advantages including safety, cost and simplicity of preparation. However, the efficiency of cotransfection of multiple mRNAs has not been extensively studied. In this study, we first investigated the feasibility of cotransfection of multiple mRNAs to coexpress a diverse array of molecules (at least five different molecules) in activated B cells. Second, we asked whether coexpression of IL-12p70, OX40L and 4-1BBL on activated B cells would synergize to enhance the induction of antigen-specific CD8+ T-cell responses.
Materials and methods
Mice
Five- to six-week-old C57BL/6 mice (H-2b) were obtained from Charles River Laboratories (Wilmington, MA). OT-1 transgenic mice (H-2Kb) were generously provided by Dr Duane Mitchell (Duke University, Durham, NC). In conducting the research described in this paper, the investigators adhered to the Guide for the Care and Use of Laboratory Animals as proposed by the committee on care of the Laboratory Animal Resources Commission on Life Sciences, National Research Council. The facilities at the Duke vivarium are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Preparation of APCs
CD19+ B cells were isolated from spleens of C57BL/6 mice by negative selection using the EasySep® mouse B-cell enrichment kit (StemCell Technologies, Seattle, WA). The purity of B cells was determined by flow cytometry after staining with phycoerythrin--conjugated (PE–) anti-mouse CD19 (MB19-1) (eBioscience, San Diego, CA). Over 98% of the isolated cells were CD19+ while CD11b and CD11c were negligibly detected (data not shown). The B cells were cultured for 2 days in a complete medium including RPMI-1640 with 10% heat-inactivated fetal bovine serum (FBS), penicillin, streptomycin, sodium pyruvate (1 mm), minimal essential medium non-essential amino acids (1 mm), HEPES (10 mm), l-glutamine (2 mm), minimal essential medium amino acids (1 mm) and β-mercaptoethanol (5 × 10−2 mm) (all from Invitrogen, Carlsbad, CA) at 37° in a humidified atmosphere with 5% CO2. To activate B cells ex vivo, the medium was supplemented with phosphorothioate B-type CpG ODN 1668 (5′-TCCATGACGTTCCTGATGCT-3′) (CpG) (1 μm) (IDT, Coralville, IA), lipopolysaccharide (LPS; 100 ng/ml) (Sigma-Aldrich, St Louis, MO), anti-mouse CD40 (HM40-3) (10 μg/ml) (eBioscience) or a combination on day 0. On day 2, the activated B cells were harvested and transfected with in-vitro-transcribed RNAs.
Murine bone marrow-derived DCs were obtained as previously described.22 After 6 days of culture, non-adherent cells were harvested and used as immature DCs.
Flow cytometry
Immunophenotypes of B cells and DCs were determined by flow cytometry after staining with PE–anti-mouse CD86 (GL1), PE–anti-mouse 4-1BBL (TKS-1), PE–anti-mouse OX40L (RM134L), fluorescein isothiocyanate-conjugated (FITC–) anti-mouse CD11b (M1/70), FITC–anti-mouse CD54 (YN1/1.7.4), FITC–anti-mouse major histocompatibility complex (MHC) class II (M5/114.15.2) (all from eBioscience), PE–anti-mouse CD11c (HL3) and PE–anti-mouse CD80 (16-10A1) (both from BD Biosciences, Franklin Lakes, NJ). PE–rat immunoglobulin G2b (IgG2b), κ, PE–rat IgG2a, κ, FITC–rat IgG2b, κ (all from eBioscience) and PE–hamster IgG1, λ (BD Biosciences) were used as isotype controls. Cells were washed, fixed and analysed on a fluorescence-activated cell sorter (FACS) Caliber (BD Biosciences).
Cytokine secretion from APCs
Isolated primary CD19+ B cells were cultured for 24 hr in the complete medium with various stimuli. As a control, immature DCs were cultured for 24 hr in the complete medium supplemented with LPS (100 ng/ml). Culture supernatants were collected and stored at −80° for later analyses. The production of IL-6, IL-10 and IL-12p70 was analysed with BD OptEIA™ enzyme-linked immunosorbent assay (ELISA) sets (BD Biosciences) by following the manufacturer's instructions. The lowest detection limit of the ELISA was defined as two standard deviations (SD) above the mean optical density of 10 replicates of zero standards, according to the manufacturer's instructions.
Preparation of RNAs
Cloning of pSP73-Sph/mIL-12p35/A64, pSP73-Sph/mIL-12p40/A64, pSP73-Sph/m4-1BBL/A64, pGEM4Z-mOX40L/A64 and pGEM4Z-OVA/A64
Murine IL-12p35 and IL-12p40 have been cloned by Wei Huang (Duke University) and 4-1BBL was cloned by Dr Kent New (Duke University). Cloning of murine OX40L16 and chicken ovalbumin (OVA)23 has been described previously.
Amplification of T7/mCD80/A64
The entire coding region of murine CD80 was amplified from a cDNA library of mature murine DCs using 5′-TATCGAAATTAATACGACTCACTATAGGGAGACCGGCCTCGAGCAGCTGAAGCTTGCCACCATGGCTTGCAATTGTCAGTTGATGCAGGAT-3′ and 5′- TTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTTAATTAAGAATTCCTAAAGGAAGACGGTCTGTTC-3′ primers. The polymerase chain reaction (PCR) fragment includes the entire murine CD80 coding region flanked with T7 promoter and poly (A) tail at 5′ and 3′end, respectively. The size of the PCR product was 1058 base pairs. This PCR product has been directly used for in vitro transcription reactions to generate mRNAs of murine CD80.
In vitrotranscription and transfection of mRNA
In vitro transcription and transfection were performed as described previously.22 Briefly, plasmids were rendered linear with SpeI followed by in vitro transcription using the mMESSAGE mMACHINE T7 RNA transcription kit (Ambion, Austin, TX). Activated B cells (2 × 106) or immature DCs (2 × 106) suspended in 200 μl Opti-MEM (Invitrogen) were mixed with 25 μg/ml in-vitro-transcribed RNAs in 2-mm cuvettes and were electroporated at 340 V for 500 μs using an Electro Square Porator ECM 830 (BTX, San Diego, CA). Transfected B cells were harvested after 2 hr of incubation in the complete medium. Transfected immature DCs were cultured for 20 hr in the granulocyte–macrophage colony-stimulating factor/IL-4-containing complete medium. For maturation of DCs, LPS was added during the last 4 hr of culture.
In vitroproliferation and cytokine production of antigen-specific CD8+ T cells
CD8+ T cells were isolated from spleens of OT-1 mice using the EasySep® mouse CD8+ T-cell enrichment kit (StemCell Technologies). CD8+ T cells (1 × 107) were incubated in 5 ml phosphate-buffered saline with 5% FBS and 3 μm carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) for 5 min at room temperature. After washing thrice with phosphate-buffered saline with 5% FBS, the cell pellet was resuspended in the complete medium. Then, 1 × 105 CFSE-labelled CD8+ T cells were added to either 2·5 × 104, 6·25 × 103 or 1·5625 × 103 RNA-transfected APCs in a final volume of 200 μl of the complete medium in a 96-well, V-bottom tissues culture plate and cocultured for 48 hr at 37° in a humidified atmosphere with 5% CO2. After 48 hr incubation, culture supernatants were collected for analysis of IL-2 and IFN-γ production and cell pellets were stained with PE–anti-mouse CD8α (Ly-2) (eBioscience) and analysed to determine the proliferation of the CD8+ T cells by flow cytometry.
CTL induction
In vitro CTL induction was performed as previously described.23 Briefly, RNA-modified and unmodified activated B cells and mature DCs transfected with OVA or actin mRNAs were used as stimulators. T cells (2 × 106 cells/ml) isolated from the spleen of a C57BL/6 mouse (H-2b) were cocultured with stimulator cells (2 × 105 cells/ml) in RPMI with 10% FBS, penicillin, streptomycin, sodium pyruvate and β-mercaptoethanol in a 96-well, U-bottom culture plate at 37° and 5% CO2. After a 5-day incubation, cells were used as effector cells in a standard 4-hr europium release assay.
Cytotoxicity assay
The europium release assays were performed as previously described.24 Briefly, 5 × 106 to 10 × 106 EL4, murine thymoma (H-2b), or EG7-OVA, chicken OVA-transduced EL4 cells (H-2b), were labelled with europium diethylenetriamine pentaacetate (europium) for 20 min at 4°. The europium-labelled cells were used as target cells; 1 × 104 target cells and serial dilutions of effector cells at varying effector : target (E : T) ratios were incubated in 200 μl complete medium in 96-well, V-bottom plates. The plates were centrifuged at 500 g for 3 min and incubated at 37° for 4 hr. Supernatant (50 μl) was harvested and europium release was measured by time-resolved fluorescence (Delta fluorometer; Wallac Inc, Gaithersburg, MD). Specific cytotoxic activity was determined using the following formula: % specific release = [(experimental release − spontaneous release)/(total release − spontaneous release)] × 100. Spontaneous release of the target cells was < 25% of total release by detergent in all assays. Standard errors of the means of triplicate cultures were < 5%.
Statistical analysis
The paired two-tailed Student's t-test was applied for determination of statistical significance. A probability of < 0·05 was considered statistically significant.
Results
B cells activated with a combination of Toll-like receptor (TLR) agonist and anti-CD40 enhanced stimulation of CD8+ T cells, but less effectively than mature DCs
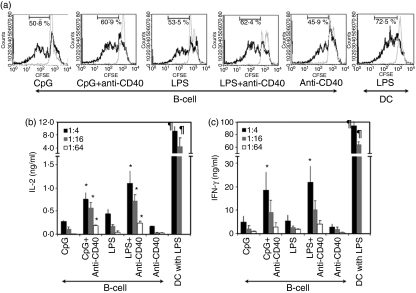
Several studies have compared the APC function of activated B cells to that of DCs and have demonstrated that activated B cells are less effective T-cell stimulators than DCs.2,10–12,25 Consistent with these studies, we showed that B cells activated with TLR agonists (CpG and LPS) or agonistic anti-CD40 and transfected with OVA mRNAs induced significantly lower proliferation of CD8+ T cells isolated from OT1 mice and 15-fold to 40-fold less IL-2 and IFN-γ production by the CD8+ T cells compared to mature DCs (P < 0·01) (Fig. 1). Interestingly, B cells activated with a combination of TLR agonist and anti-CD40 enhanced the proliferation of antigen-specific CD8+ T cells and induced threefold to fivefold more IL-2 and IFN-γ production by the CD8+ T cells compared to B cells activated with TLR agonist or anti-CD40 alone (P < 0·05).
Figure 1.
B cells activated with a combination of Toll-like receptor (TLR) agonist and anti-CD40 induce stronger antigen-specific CD8+ T-cell responses than B cells activated with TLR agonist or anti-CD40 alone. B cells were activated for 2 days with CpG, lipopolysaccharide (LPS), anti-CD40 or combinations of these. The activated B cells were transfected with either ovalbumin (OVA) mRNA (black solid line) or actin mRNA (grey solid line). These mRNA-transfected B cells were used as stimulators. CD8+ T cells were isolated from the spleen of an OT1 mouse, labelled with carboxyfluorescein succinimidyl ester (CFSE) and used as responders; 1 × 105 responders were cocultured with stimulators at 1 : 4 (black bar), 1 : 16 (grey bar) or 1 : 64 (white bar) (stimulator : responder). After 48 hr of coculture, cell pellets and culture supernatants were harvested. These cell pellets and culture supernatants were analysed for CFSE expression and interleukin-2 (IL-2) and interferon-γ (IFN-γ) production, respectively. (a) Cells were stained with phycoerythrin-conjugated anti-CD8α. After gating CD8+ T cells, CFSE-expressing cells were analysed by flow cytometry. The data represent one of three individual experiments and 1 : 16 ratio of stimulator : responder. (b,c) IL-2 and IFN-γ production was determined by enzyme-linked immunosorbent assay as described in Materials and methods. The data are means of three individual experiments and the error bars are SD. *P < 0·05, TLR ligand alone versus TLR ligand + anti-CD40; ¶P < 0·01, B cells versus dendritic cells.
Insufficient costimulatory molecule expression and unfavourable cytokine production by activated B cells caused inefficient CD8+ T-cell stimulation compared to mature DCs
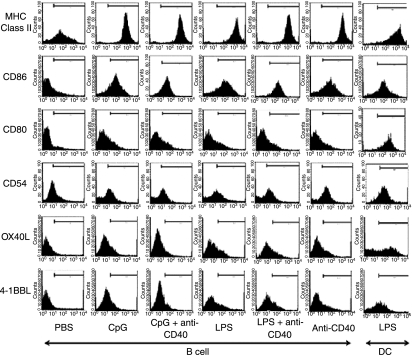
Insufficient expression of costimulatory molecules in activated B cells has been a potential explanation of the inefficient APC functions of activated B cells compared to mature DCs.11,12 As shown in Fig. 2, activated B cells up-regulated CD54, CD86 and MHC class II and the amount of these molecules on activated B cells was comparable with that on mature DCs. However, activated B cells expressed significantly less CD80, OX40L and 4-1BBL than mature DCs. These data explained, at least in part, why activated B cells stimulated CD8+ T cells less effectively than mature DCs. However, because the expression of costimulatory molecules in activated B cells was not significantly different between stimulatory conditions, it could not explain why B cells activated with a combination of TLR agonist and anti-CD40 enhanced CD8+ T-cell stimulation compared to B cells activated with only one or the other.
Figure 2.
Immunophenotype of murine splenic B cells activated with CpG, lipopolysaccharide (LPS), anti-CD40, or combinations of these compared to mature dendritic cells (DCs). Freshly isolated CD19+ B cells were cultured with CpG, LPS, anti-CD40 or combinations. After a 2-day culture, B cells were harvested and analysed for expression of major histocompatibility complex (MHC) class II molecules, CD86, CD80, CD54, OX40L, and 4-1BBL by flow cytometry. As a control, LPS-stimulated, bone-marrow-derived DCs were used. Bars in the histograms indicate the areas beyond background. Representative data from three individual experiments are shown.
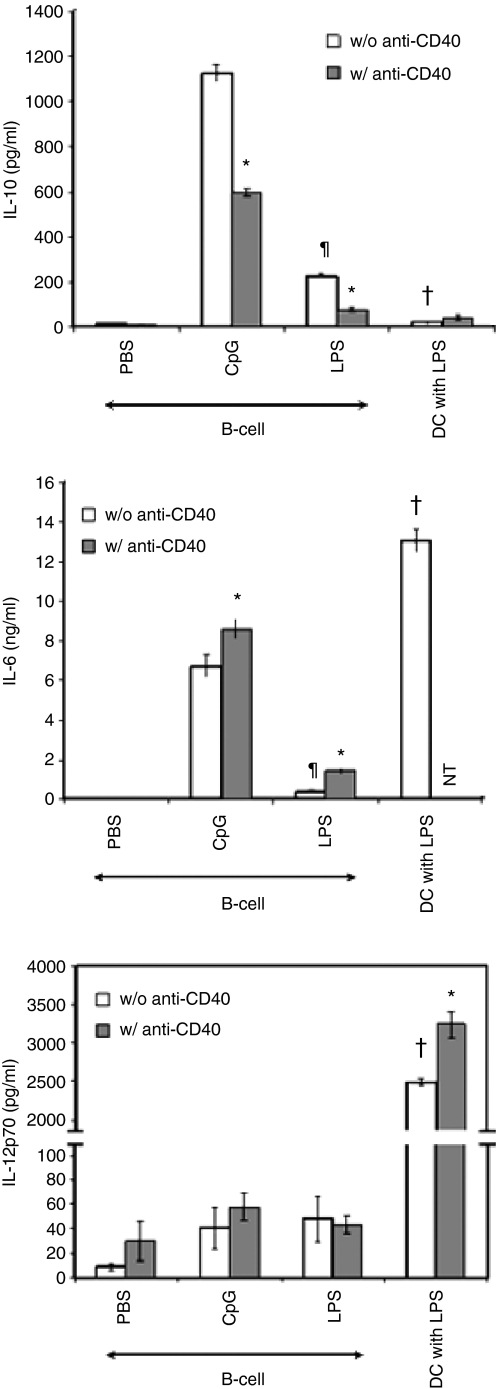
After activation or maturation, APCs produced diverse cytokines that play a critical role in the induction of cellular immunity.26 For example, mature DCs increase the production of immune stimulatory cytokine (IL-12p70) and proinflammatory cytokines (tumour necrosis factor-α and IL-6) that enhance Th1 responses and CTL induction whereas immature or semimature DCs produce the immune inhibitory cytokine (IL-10) but no or low levels of immune stimulatory cytokine/proinflammatory cytokines that regulate cellular immunity.26 It has been shown that activated B cells produce IL-10 as well as IL-12p70, even under specific activation conditions.3,7,19,27,28 In Fig. 3, B cells treated with CpG or LPS but not anti-CD40 produced IL-10 and IL-6 while LPS-matured DCs produced no IL-10 but did produce significantly greater amounts of IL-6 than activated B cells (P < 0·05). Mature DCs produced a large amount of IL-12p70 after LPS maturation, but B cells activated with CpG, LPS, or anti-CD40 produced a negligible amount of IL-12p70. Interestingly, B cells treated with a combination of anti-CD40 and either CpG or LPS produced significantly less IL-10 but more IL-6 than cells treated with either CpG or LPS alone (P < 0·005) (Fig. 3). Furthermore, the amount of IL-10 produced by B cells activated with TLR agonist, anti-CD40, or a combination was inversely correlated with CD8+ T-cell stimulation by activated B cells (Figs 1 and 3). Our data suggest that the inefficient APC function of activated B cells compared to mature DCs probably results from inadequacy of costimulatory molecules and unfavourable cytokine production by activated B cells. Therefore, we next investigated whether expression de novo or overexpression of costimulatory molecules and immune stimulatory cytokines would overcome inefficient T-cell stimulation by activated B cells.
Figure 3.
Downregulation of interleukin-10 (IL-10) but upregulation of IL-6 in B cells activated with a combination of Toll-loke receptor (TLR) agonist and anti-CD40 as compared to cells activated with TLR agonist alone. Purified splenic CD19+ B cells were incubated for 24 hr with phosphate-buffered saline (PBS), CpG or lipopolysaccharide (LPS) alone or combined with anti-CD40. Immature bone marrow-derived dendritic cells (DCs) were incubated for 24 hr with LPS alone or together with anti-CD40. After culture, supernatants were collected and assayed by enzyme-linked immunosorbent assay for IL-10, IL-6 and IL-12p70 production. The data are means of three individual experiments and error bars represent SD. *P < 0·05, TLR ligand alone versus TLR ligand + anti-CD40; ¶P < 0·001, CpG versus LPS; †P < 0·005, B cells versus DCs.
Activated B cells are efficiently cotransfected with a combination of mRNAs encoding six different molecules
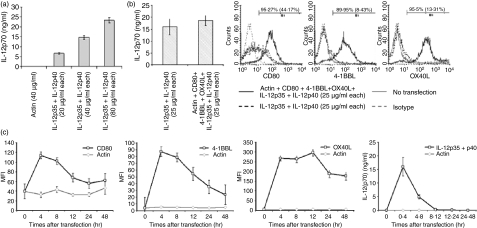
We and others have previously reported that transfection of tumour RNA by electroporation successfully loaded antigens onto MHC molecules in DCs16,22–24 and activated B cells.29 In the previous studies, one or two mRNAs were transfected but it is unclear how many different mRNAs can be cotransfected into a single cell. In the current study, we investigated whether activated B cells could be cotransfected with multiple mRNAs by electroporation. As shown in Fig. 4(a), IL-12p70 production of activated B cells was found to be proportionate to the amount of cotransfected mRNAs encoding IL-12p35 and IL-12p40 (r2 = 0·9774). Electroporation did not deteriorate cell viability unless activated B cells were electroporated with over 160 μg/ml mRNAs in 2 × 106 cells at a time (data not shown). B cells cotransfected with IL-12p35 and IL-12p40 mRNAs at a concentration of 25 μg/ml expressed amounts of IL-12p70 similar to those expressed by mature DCs (data not shown). Based on these results, 25 μg/ml was selected as a concentration of single mRNA for transfection. We next explored the efficiency of cotransfection with six different in-vitro-transcribed mRNAs, encoding IL-12p35, IL-12p40, CD80, OX40L, 4-1BBL and actin, into activated B cells. As shown in Fig. 4(b), no significant difference was observed in IL-12p70 production between activated B cells cotransfected with two mRNAs (those for IL-12p35 and IL-12p40) and the cells cotransfected with six different mRNAs (those for IL-12p35, IL-12p40, CD80, OX40L, 4-1BBL and actin). In addition, activated B cells cotransfected with the six different mRNAs expressed over 89% of CD80, OX40L and 4-1BBL along with IL-12p70 production (Fig. 4b). Interestingly, the peak protein expression of these molecules was observed at 4 hr after electroporation (Fig. 4c). Protein expression decreased 4–12 hr after electroporation but was detected until at least 48 hr after electroporation (Fig. 4c).
Figure 4.
Simultaneous electroporation of a mixture of mRNAs encoding CD80, 4-1BBL, OX40L, IL-12p35, IL-12p40 and actin into activated B cells. (a) B cells activated for 2 days with a combination of CpG and anti-CD40 were transfected with either actin mRNA (40 μg/ml) or a mixture of IL-12p35 and IL-12p40 mRNAs at various concentrations (20 μg/ml, 40 μg/ml or 80 μg/ml each). Culture supernatants were collected 24 hr after transfection and assayed by IL-12p70 enzyme-linked immunosorbent assay (ELISA). (b) Activated B cells were transfected with either a mixture of IL-12p35 and IL-12p40 mRNAs or a mixture of IL-12p35, IL-12p40, CD80, OX40L, 4-1BBL and actin mRNAs (each of them at 25 μg/ml). At 24 hr after transfection, production of IL-12p70 was measured by ELISA and expression of CD80, 4-1BBL and OX40L was analysed by flow cytometry. The numbers in the histograms are percentage of positive cells after transfection with a mixture of mRNAs encoding IL-12p35, IL-12p40, CD80, OX40L, 4-1BBL and actin and, in parentheses, percentage of positive cells after transfection with mixture of mRNAs encoding IL-12p35 and IL-12p40. (c) Activated B cells were transfected with either actin mRNAs (150 μg/ml; grey line) or a mixture of IL-12p35, IL-12p40, CD80, OX40L, 4-1BBL and actin (25 μg/ml each; black line). Amounts of CD80, 4-1BBL and OX40L were determined by flow cytometry at the indicated times after transfections. IL-12p70 production by the cells during the indicated culture periods after transfection was measured by ELISA. The data, except the histogram, are means of three experiments and the histograms are a representative of three experiments. Error bars represent SD.
Coexpression of OX40L, 4-1BBL and IL-12p70 on activated B cells synergistically enhances IL-2 and IFN-γ production by antigen-specific CD8+ T cells
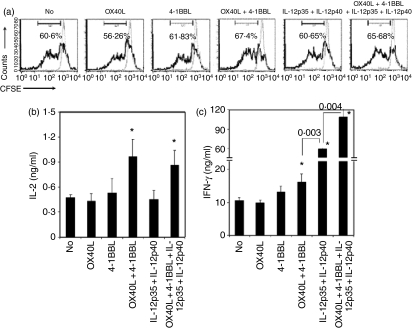
To explore the synergistic effects of OX40L, 4-1BBL and IL-12p70 on antigen-specific CD8+ T-cell stimulation, activated B cells were cotransfected with mRNAs encoding OVA, OX40L, 4-1BBL, IL-12p35 and IL-12p40. These transfected B cells were used as stimulators for induction of OVA-specific CD8+ T-cell responses. As shown in Fig. 5, the B cells cotransfected with mRNAs encoding OVA and OX40L did not significantly enhance proliferation and cytokine production by OVA-specific CD8+ T cells compared to cells transfected with OVA mRNA alone. Although activated B cells cotransfected with OVA and 4-1BBL mRNAs did not affect proliferation and IL-2 production by CD8+ T cells, they showed a trend of increasing IFN-γ production by the T cells but this was not statistically significant. Interestingly, activated B cells cotransfected with mRNAs encoding OX40L, 4-1BBL and OVA significantly enhanced proliferation (70·8 ± 3·1% versus 59·4 ± 1·2%, P = 0·042) and IL-2 and IFN-γ production by OVA-specific CD8+ T cell (P < 0·05).
Figure 5.
Coexpression of OX40L, 4-1BBL and IL-12p70 has a synergistic effect on interleukin-2 (IL-2) and interferon-γ (IFN-γ) production and proliferation of ovalbumin (OVA) -specific CD8+ T cells. B cells activated with CpG and anti-CD40 were cotransfected with actin or OVA mRNAs in addition to the indicated combination of IL-12p35, IL-12p40, OX40L and 4-1BBL mRNAs. These transfected B cells were used as stimulators. CD8+ T cells were isolated from spleens of OTI mice, labelled with carboxyfluorescein succinimidyl ester (CFSE) and used as responders. Stimulators were cocultured with 1 × 105 CFSE-labelled responders at a ratio of 1 : 16 (stimulator:responder). After 48 hr of coculture, cell pellets and culture supernatants were analysed for T-cell proliferation by analysis of CFSE reduction and cytokine production by enzyme-linked immunosorbent assay (ELISA), respectively. (a) Cells were stained with phycoerythrin-conjugated anti-CD8α. CFSE expression in CD8+ T cells was analysed by flow cytometry to quantify the proliferation of OVA-specific CD8+ T cells. Black and grey lines represent T cells stimulated with B cells transfected with OVA mRNAs and actin mRNAs, respectively. The data represent one of three experiments. (b, c) Interleukin-2 (IL-2) and interferon-γ (IFN-γ) production of OVA-specific CD8+ T cells was measured by ELISA. The amounts of cytokines presented in figures are means of three experiments after subtraction of amounts of cytokines produced by non-specific stimulation. The error bars represent SD. P values of paired groups are described. *P < 0·05 comparing stimulation of activated B cells transfected with OVA mRNA alone.
While cotransfection of IL-12p35, IL-12p40 and OVA mRNAs into activated B cells did not affect proliferation and IL-2 production by OVA-specific CD8+ T cells compared to B cells transfected with OVA mRNA alone, the cotransfection of these mRNAs into activated B cells dramatically enhanced IFN-γ production by the CD8+ T cells (P < 0·001) (Fig. 5). This IFN-γ production was further augmented when CD8+ T cells were stimulated with activated B cells simultaneously transfected with mRNAs encoding IL-12p35, IL-12p40, OX40L and 4-1BBL.
Activated B cells cotransfected with IL-12p35, IL-12p40, OX40L and 4-1BBL induced antigen-specific CTL responses as efficiently as mature DCs
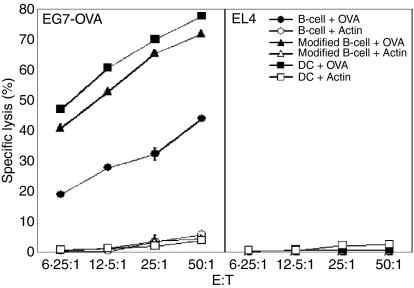
Next we investigated whether CD8+ T cells stimulated with activated B cells coexpressing IL-12p70, OX40L, 4-1BBL and antigen could enhance the induction of CTL responses against cells expressing the corresponding antigen. Activated B cells transfected with OVA mRNAs stimulated CTLs that lysed EG7-OVA, but not EL4 cells (Fig. 6). When activated B cells were cotransfected with OVA mRNAs along with IL-12p35, IL-12p40, OX40L and 4-1BBL mRNAs, they enhanced the activation of CTLs, which was consistent with our data on proliferation and cytokine production by antigen-specific CD8+ T cells (Fig. 5). Compared to mature DCs transfected with OVA mRNAs, activated B cells transfected with the same mRNAs induced approximately 10-fold lower CTL responses against EG7-OVA cells while activated B cells cotransfected with OVA, IL-12p35, IL-12p40, OX40L and 4-1BBL mRNAs induced antigen-specific CTL responses as efficiently as mature DCs (Fig. 6).
Figure 6.
Activated B cells transfected with a mixture of mRNAs encoding OX40L, 4-1BBL, IL-12p35, IL-12p40 and ovalbumin (OVA) induced OVA-specific cytotoxic T-lymphocyte (CTL) responses as efficiently as OVA mRNA-transfected mature dendritic cells (DCs). Splenic B cells were activated with CpG and anti-CD40 and transfected with OVA (B cell+OVA) or actin mRNAs (B cell+Actin) or cotransfected with a mixture of mRNAs encoding OX40L, 4-1BBL, IL-12p35, IL-12p40 and OVA (modified B cell+OVA) or actin (modified B cell+Actin). Immature bone-marrow-derived DCs were transfected with OVA (DC+OVA) or actin mRNAs (DC+Actin) and stimulated with lipopolysaccharide (LPS). Transfected B cells and mature DCs were used as stimulators in in vitro CTL induction; 2 × 105 cells/ml of stimulators were cocultured with 2 × 106 cells/ml of syngeneic splenocytes in 96-well, U-bottom plates. After 5 days of coculture, cells were used as effector cells in the in vitro CTL assay as described in the Materials and methods.
Activated B cells are inferior to mature DCs at priming CD8+ T cells in vivo
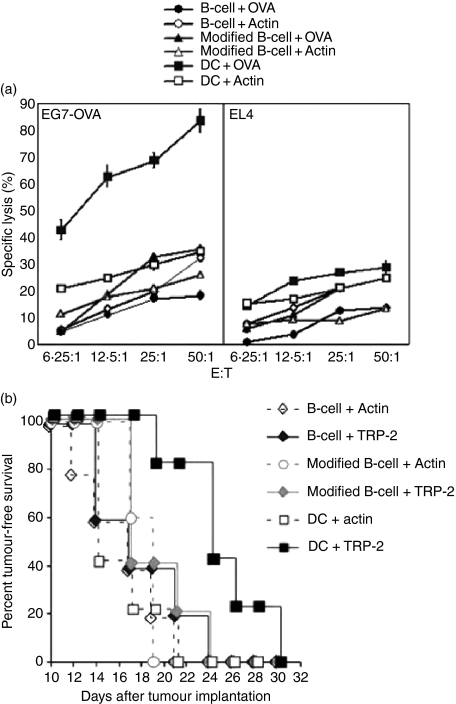
Finally, we asked whether activated B cells are comparable with mature DCs at eliciting antigen-specific T-cell responses in vivo. To address this question, we investigated in vivo CTL induction by RNA-modified B cells and mature DCs. Unexpectedly, immunization with RNA-modified B cells did not induce significant amounts of CTL activation in vivo while mature DCs did (Fig. 7a). Furthermore, immunization with DCs but not RNA-modified B cells prevented tumour growth in tumour-bearing mice (Fig. 7b).
Figure 7.
In vivo cytotoxic T-lymphocyte (CTL) induction and tumour immunotherapy. (a) Splenic B cells isolated from C57BL/6 (H-2b) were activated with CpG and anti-CD40 and transfected with ovalbumin (B cell+OVA) or actin mRNAs (B cell+Actin) or cotransfected with a mixture of mRNAs encoding OX40L, 4-1BBL, IL-12p35, IL-12p40, and OVA (modified B cell+OVA) or actin (modified B cell+Actin). Immature bone-marrow-derived dendritic cells (DCs) were transfected with OVA (DC+OVA) or actin mRNAs (DC+Actin) and stimulated with lipopolysaccharide (LPS). Mice were immunized intraperitoneally with 4 × 105 cells in 200 μl phosphate-buffered saline (PBS) as indicated in the figure. Splenocytes were isolated 1 week after immunization; 1 × 107 splenocytes were cocultured with 5 × 105γ-irradiated EG7-OVA in six-well plates. After a 5-day coculture, cells were used as effector cells in a 4-hr europium release CTL assay. EL4 (murine thymoma cell line; H-2b) and EG7-OVA (EL4 cells stably transduced with chicken OVA) were used as target cells. (b) C57BL/6 mice (n = 5 in each group) were implanted subcutaneously with 3 × 104 B16/F10.9 melanoma cells.22 On days 2 and 9 after tumour implantation mice were immunized with 4 × 105 B cells, modified B cells or DCs transfected with actin or melanoma-specific antigen, tyrosinase-related protein-2 (TRP-2) mRNA in 200 μl PBS as shown. Mice were monitored for appearance of palpable tumours.
Discussion
In the current study, we have shown that B cells activated with TLR agonists or anti-CD40 expressed costimulatory molecules (CD80, OX40L and 4-1BBL) less than mature DCs and produced IL-10 and IL-6 but negligible IL-12p70. Interestingly, compared to B cells activated with TLR agonists alone, the B cells activated with the combination of TLR agonist and anti-CD40 reduced IL-10 production and increased IL-6 production as well as stimulation of CD8+ T cells. Furthermore, we have demonstrated that a mixture of diverse mRNAs encoding costimulatory molecules (OX40L and 4-1BBL), cytokines (IL-12p35 and IL-12p40) and antigen (OVA) were efficiently cotransfected into activated B cells by electroporation. Using this technique, we found that coexpression of OX40L, 4-1BBL and IL-12p70 by activated B cells synergistically enhanced proliferation and cytokine production by CD8+ T cells in an antigen-specific manner. Finally, these RNA-modified activated B cells induced in vitro antigen-specific CTL responses as efficiently as mature DCs.
A growing body of evidence indicates that B cells activated ex vivo have the potential to serve as a cellular adjuvant in immunotherapy. From a practical perspective, immunotherapy based on such ex-vivo-activated B cells has significant advantages over a DC-based immunotherapy. However, several studies that compared the APC function of activated B cells to that of DCs have demonstrated that activated B cells are less effective stimulators than DCs.2,11,25 Consistent with these studies, our data demonstrate that activated B cells transfected with OVA mRNAs induced significantly less proliferation and IL-2 and IFN-γ production by CD8+ T cells compared to mature DCs. Furthermore, T cells stimulated with activated B cells transfected with OVA mRNAs induced lower CTL responses against EG.7-OVA cells than those stimulated with mature DCs transfected with OVA mRNAs.
It is uncertain why activated B cells induce less T-cell stimulation than DCs. One potential explanation is that lower amounts of costimulatory and adhesion molecules are expressed on activated B cells compared to mature DCs. Optimal stimulation of T cells requires several factors including T-cell receptor, costimulatory and integrin signals.30,31 Cassell et al.11 demonstrated that activated murine B cells expressed threefold less of the ligands (mostly CD80 and CD86) to bind cytotoxic T-lymphocyte antigen 4–immunoglobulin fusion proteins and twofold to threefold less CD54 than DCs. Subklewe et al.12 reported that human EBV-transformed B cells expressed twofold to threefold less CD86 and CD54 than mature DCs, which caused less efficient conjugation of EBV-transformed B cells and T cells than conjugation of DCs and T cells. In contrast to previous studies, we have shown that expression of CD86 and CD54 on the activated B cells was comparable to that on mature DCs while expression of CD80, 4-1BBL and OX40L was much less on B cells activated with CpG, LPS, agonistic anti-CD40 or combinations than on LPS-mature DCs. In our transfection study, although we did not see significant enhancement of CD8+ T-cell stimulation by activated B cells overexpressing either CD80 or OX40L, overexpression of 4-1BBL in activated B cells slightly enhanced IFN-γ production but not proliferation and IL-2 production by CD8+ T cells. Interestingly, proliferation and IFN-γ and IL-2 production by CD8+ T cells were significantly enhanced by activated B cells cotransfected with mRNAs encoding 4-1BBL and OX40L.
Both 4-1BB and OX40 are members of the tumour necrosis factor receptor superfamily and function as costimulatory receptors of T cells.30 Although 4-1BB and OX40 signalling predominantly influence CD8+ and CD4+ T cells, respectively, they can independently affect activation of both CD8+ and CD4+ T cells.30 Consistent with our data, it has also been shown that simultaneous costimulation through 4-1BB and OX40 synergistically enhances clonal expansion and IFN-γ production by antigen-specific CD8+ T cells.18 4-1BB and OX40 are transiently expressed on CD8+ T cells only after activation. In vitro, 4-1BB expression can be detected after 24 hr of primary CD8+ T-cell activation; peaks at 48 hr; and has declined by 4–5 days.32,33 In contrast, OX40 expression is not detectable after 24 hr of primary CD8+ T-cell activation, but can be detected at 48 hr.34 This temporal expression of 4-1BB and OX40 on CD8+ T cells probably explains why activated B cells overexpressing either 4-1BBL or OX40L alone did not significantly enhance the stimulation of CD8+ T cells in our study. Since 4-1BBL and OX40L expression on the activated B cells reached a maximum 4–12 hr after transfection of 4-1BBL or OX40L mRNAs, and declined at 12–24 hr (Fig. 4), amounts of 4-1BBL or OX40L on the mRNA-transfected activated B cells might be insufficient to provide optimal costimulatory signals for CD8+ T cells expressing 4-1BB and OX40.
Another explanation for the inefficient APC function of activated B cells is that activated B cells produce an unfavourable profile of cytokines for T-cell stimulation. The balance of immune stimulatory cytokines, e.g. IL-12p70, and immune regulatory cytokines, e.g. IL-10 and transforming growth factor-β (TGF-β,) expressed in APCs is a critical decision factor in the induction or regulation of cellular immunity.35,36 Barr et al.37 reported that B cells activated with various TLR agonists produced 1000-fold less IL-12p40 than LPS-mature DCs while the activated B cells, but not the mature DCs, produced a large amount of IL-10.37 In agreement with this, we have shown that activated B cells did not produce a significant amount of the immune stimulatory cytokine, IL-12p70, but did produce a large amount of the immune suppressive cytokine, IL-10, which is known to inhibit Th1 differentiation, induce regulatory T-cell differentiation and anergy of CD4+ T cells while suppressing proliferation and IFN-γ production by CD8+ T cells.38,39 Inoue et al. demonstrated that murine splenic B cells, after activation through CD40 signalling, produced a large amount of IL-10 that interfered with IFN-γ production by CD8+ T cells and inhibited anti-tumour immunity.40 It has also been shown that inhibition of IL-10 production through transfection of IL-10-targeting small interference RNA into human monocyte-derived DCs enhanced Th1 responses by increasing proliferation and IFN-γ production by T cells.41 In the current studies, B cells treated with a combination of TLR agonist and anti-CD40 produced twofold to threefold less IL-10 than cells treated with TLR agonist alone, which is inversely correlated with proliferation and cytokine production by CD8+ T cells.
In addition to IL-10, activated B cells have been shown to produce another immune suppressive cytokine, TGF-β, which downregulates cellular immune responses. Tian et al.42 demonstrated that expression of Fas ligand and TGF-β in LPS-activated murine B cells was correlated with inhibition of Th1 autoimmunity in nonobese diabetic mice. Furthermore, Parekh et al.43 reported that murine splenic B cells activated with LPS induced hyporesponsive CD8+ T cells, and this hyporesponsiveness could be rescued by treatment with anti-TGF-β neutralizing antibodies. It is therefore interesting to investigate whether cointroduction of small interference RNA targeting TGF-β and IL-10 into activated B cells would suppress the production of the corresponding cytokines, and consequently enhance CD8+ T-cell stimulation in a synergistic manner.
Unexpectedly, activated B cells failed to prime CD8+ T cells in vivo (Fig. 7). In secondary lymphoid organs, B cells are anatomically segregated from T cells (especially CD8+ T cells), which might prevent contact of antigen-presenting B cells and corresponding naïve CD8+ T cells. We believe that this spatial segregation of B cells and T cells possibly explains why immunization with activated B cells was unable to induce CD8+ T-cell activation in this study. Currently, we are investigating if CD8+ T cells can be stimulated in vivo by activated B cells expressing chemokines and chemokine receptors.
In summary, our findings demonstrate that electoroporation of multiple mRNAs are able to modify ex-vivo-activated B cells to coexpress a diverse array of immune stimulatory molecules. These RNA-modified activated B cells can be an optimal replacement of DCs in the induction of T-cell stimulation for adoptive T-cell immunotherapy against cancers. Ultimately, use of modified activated B cells as APCs may lead to the generation of potent and simple cellular vaccines against malignant cells in patients.
Acknowledgments
We would like to thank Mr Dave Snyder for animal care and bone marrow preparation and Dr Vidya Chandramohan for technical assistance.
References
- 1.Schultze JL, Grabbe S, von Bergwelt-Baildon MS. DCs and CD40-activated B cells: current and future avenues to cellular cancer immunotherapy. Trends Immunol. 2004;25:659–64. doi: 10.1016/j.it.2004.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Schultze JL, Michalak S, Seamon MJ, et al. CD40-activated human B cells: an alternative source of highly efficient antigen presenting cells to generate autologous antigen-specific T cells for adoptive immunotherapy. J Clin Invest. 1997;100:2757–65. doi: 10.1172/JCI119822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shirota H, Sano K, Hirasawa N, et al. B cells capturing antigen conjugated with CpG oligodeoxynucleotides induce Th1 cells by elaborating IL-12. J Immunol. 2002;169:787–94. doi: 10.4049/jimmunol.169.2.787. [DOI] [PubMed] [Google Scholar]
- 4.Kondo E, Topp MS, Kiem HP, et al. Efficient generation of antigen-specific cytotoxic T cells using retrovirally transduced CD40-activated B cells. J Immunol. 2002;169:2164–71. doi: 10.4049/jimmunol.169.4.2164. [DOI] [PubMed] [Google Scholar]
- 5.Lapointe R, Bellemare-Pelletier A, Housseau F, Thibodeau J, Hwu P. CD40-stimulated B lymphocytes pulsed with tumor antigens are effective antigen-presenting cells that can generate specific T cells. Cancer Res. 2003;63:2836–43. [PubMed] [Google Scholar]
- 6.Kondo E, Akatsuka Y, Kuzushima K, et al. Identification of novel CTL epitopes of CMV-pp65 presented by a variety of HLA alleles. Blood. 2004;103:630–8. doi: 10.1182/blood-2003-03-0824. [DOI] [PubMed] [Google Scholar]
- 7.Wagner M, Poeck H, Jahrsdoerfer B, et al. IL-12p70-dependent Th1 induction by human B cells requires combined activation with CD40 ligand and CpG DNA. J Immunol. 2004;172:954–63. doi: 10.4049/jimmunol.172.2.954. [DOI] [PubMed] [Google Scholar]
- 8.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172:1501–7. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 9.von Bergwelt-Baildon M, Shimabukuro-Vornhagen A, Popov A, et al. CD40-activated B cells express full lymph node homing triad and induce T-cell chemotaxis: potential as cellular adjuvants. Blood. 2006;107:2786–9. doi: 10.1182/blood-2004-01-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croft M, Duncan DD, Swain SL. Response of naive antigen-specific CD4+ T cells in vitro: characteristics and antigen-presenting cell requirements. J Exp Med. 1992;176:1431–7. doi: 10.1084/jem.176.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cassell DJ, Schwartz RH. A quantitative analysis of antigen-presenting cell function: activated B cells stimulate naive CD4 T cells but are inferior to dendritic cells in providing costimulation. J Exp Med. 1994;180:1829–40. doi: 10.1084/jem.180.5.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Subklewe M, Sebelin K, Block A, et al. Dendritic cells expand Epstein–Barr virus specific CD8+ T cell responses more efficiently than EBV transformed B cells. Hum Immunol. 2005;66:938–49. doi: 10.1016/j.humimm.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Ribas A. Genetically modified dendritic cells for cancer immunotherapy. Curr Gene Ther. 2005;5:619–28. doi: 10.2174/156652305774964758. [DOI] [PubMed] [Google Scholar]
- 14.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–73. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 15.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–51. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 16.Dannull J, Nair S, Su Z, et al. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood. 2005;105:3206–13. doi: 10.1182/blood-2004-10-3944. [DOI] [PubMed] [Google Scholar]
- 17.Serghides L, Bukczynski J, Wen T, et al. Evaluation of OX40 ligand as a costimulator of human antiviral memory CD8 T cell responses: comparison with B7.1 and 4-1BBL. J Immunol. 2005;175:6368–77. doi: 10.4049/jimmunol.175.10.6368. [DOI] [PubMed] [Google Scholar]
- 18.Lee SJ, Myers L, Muralimohan G, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–12. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 19.Maruo S, Oh-hora M, Ahn HJ, et al. B cells regulate CD40 ligand-induced IL-12 production in antigen-presenting cells (APC) during T cell/APC interactions. J Immunol. 1997;158:120–6. [PubMed] [Google Scholar]
- 20.Tatsumi T, Takehara T, Yamaguchi S, et al. Injection of IL-12 gene-transduced dendritic cells into mouse liver tumor lesions activates both innate and acquired immunity. Gene Ther. 2007;14:863–71. doi: 10.1038/sj.gt.3302941. [DOI] [PubMed] [Google Scholar]
- 21.Bontkes HJ, Kramer D, Ruizendaal JJ, et al. Dendritic cells transfected with interleukin-12 and tumor-associated antigen messenger RNA induce high avidity cytotoxic T cells. Gene Ther. 2007;14:366–75. doi: 10.1038/sj.gt.3302874. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Fassnacht M, Nair S, Boczkowski D, Gilboa E. Tumor immunotherapy targeting fibroblast activation protein, a product expressed in tumor-associated fibroblasts. Cancer Res. 2005;65:11156–63. doi: 10.1158/0008-5472.CAN-05-2805. [DOI] [PubMed] [Google Scholar]
- 23.Boczkowski D, Nair SK, Snyder D, Gilboa E. Dendritic cells pulsed with RNA are potent antigen-presenting cells in vitro and in vivo. J Exp Med. 1996;184:465–72. doi: 10.1084/jem.184.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nair S, Boczkowski D, Moeller B, Dewhirst M, Vieweg J, Gilboa E. Synergy between tumor immunotherapy and antiangiogenic therapy. Blood. 2003;102:964–71. doi: 10.1182/blood-2002-12-3738. [DOI] [PubMed] [Google Scholar]
- 25.Metlay JP, Pure E, Steinman RM. Distinct features of dendritic cells and anti-Ig activated B cells as stimulators of the primary mixed leukocyte reaction. J Exp Med. 1989;169:239–54. doi: 10.1084/jem.169.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–40. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- 27.Schultze JL, Michalak S, Lowne J, et al. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–12. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burdin N, Rousset F, Banchereau J. B-cell-derived IL-10: production and function. Methods. 1997;11:98–111. doi: 10.1006/meth.1996.0393. [DOI] [PubMed] [Google Scholar]
- 29.Coughlin CM, Vance BA, Grupp SA, Vonderheide RH. RNA-transfected CD40-activated B cells induce functional T-cell responses against viral and tumor antigen targets: implications for pediatric immunotherapy. Blood. 2004;103:2046–54. doi: 10.1182/blood-2003-07-2379. [DOI] [PubMed] [Google Scholar]
- 30.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 31.Kinashi T. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol. 2005;5:546–59. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 32.Vinay DS, Kwon BS. Role of 4-1BB in immune responses. Semin Immunol. 1998;10:481–9. doi: 10.1006/smim.1998.0157. [DOI] [PubMed] [Google Scholar]
- 33.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–24. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 34.Taraban VY, Rowley TF, O’Brien L, et al. Expression and costimulatory effects of the TNF receptor superfamily members CD134 (OX40) and CD137 (4-1BB), and their role in the generation of anti-tumor immune responses. Eur J Immunol. 2002;32:3617–27. doi: 10.1002/1521-4141(200212)32:12<3617::AID-IMMU3617>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 35.de Jong EC, Smits HH, Kapsenberg ML. Dendritic cell-mediated T cell polarization. Springer Semin Immunopathol. 2005;26:289–307. doi: 10.1007/s00281-004-0167-1. [DOI] [PubMed] [Google Scholar]
- 36.Becker Y. Molecular immunological approaches to biotherapy of human cancers – a review, hypothesis and implications. Anticancer Res. 2006;26:1113–34. [PubMed] [Google Scholar]
- 37.Barr TA, Brown S, Ryan G, Zhao J, Gray D. TLR-mediated stimulation of APC: distinct cytokine responses of B cells and dendritic cells. Eur J Immunol. 2007;37:3040–53. doi: 10.1002/eji.200636483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Endharti AT, Rifa IMs, Shi Z, et al. Cutting edge: CD8+CD122+ regulatory T cells produce IL-10 to suppress IFN-gamma production and proliferation of CD8+ T cells. J Immunol. 2005;175:7093–7. doi: 10.4049/jimmunol.175.11.7093. [DOI] [PubMed] [Google Scholar]
- 39.Mege JL, Meghari S, Honstettre A, Capo C, Raoult D. The two faces of interleukin 10 in human infectious diseases. Lancet Infect Dis. 2006;6:557–69. doi: 10.1016/S1473-3099(06)70577-1. [DOI] [PubMed] [Google Scholar]
- 40.Inoue S, Leitner WW, Golding B, Scott D. Inhibitory effects of B cells on antitumor immunity. Cancer Res. 2006;66:7741–7. doi: 10.1158/0008-5472.CAN-05-3766. [DOI] [PubMed] [Google Scholar]
- 41.Liu G, Ng H, Akasaki Y, et al. Small interference RNA modulation of IL-10 in human monocyte-derived dendritic cells enhances the Th1 response. Eur J Immunol. 2004;34:1680–7. doi: 10.1002/eji.200425081. [DOI] [PubMed] [Google Scholar]
- 42.Tian J, Zekzer D, Hanssen L, Lu Y, Olcott A, Kaufman DL. Lipopolysaccharide-activated B cells down-regulate Th1 immunity and prevent autoimmune diabetes in nonobese diabetic mice. J Immunol. 2001;167:1081–9. doi: 10.4049/jimmunol.167.2.1081. [DOI] [PubMed] [Google Scholar]
- 43.Parekh VV, Prasad DV, Banerjee PP, Joshi BN, Kumar A, Mishra GC. B cells activated by lipopolysaccharide, but not by anti-Ig and anti-CD40 antibody, induce anergy in CD8+ T cells: role of TGF-beta 1. J Immunol. 2003;170:5897–911. doi: 10.4049/jimmunol.170.12.5897. [DOI] [PubMed] [Google Scholar]