Abstract
Antimicrobial peptides like human β-defensin-2 (HBD-2) play an important role in the innate immune system protecting the intestinal mucosa against bacterial invasion. The dietary histone deacetylase (HDAC) inhibitors sulforaphane (SFN) and butyrate have received a great deal of attention because of their ability to simultaneously modulate multiple cellular targets involved in cellular protection. In this study the influence of SFN and butyrate on HBD-2 expression as well as the molecular pathways involved in SFN-mediated induction of HBD-2 were scrutinized. Treatment of Caco-2, HT-29 and SW480 cells with SFN led to a time- and dose-dependent upregulation of HBD-2 mRNA expression as determined by semi-quantitative reverse transcription–polymerase chain reaction. Moreover, HBD-2 protein production increased in response to SFN, measured by enzyme-linked immunosorbent assay. Induction of HBD-2 was also observed in response to butyrate. Immunofluorescence analysis revealed that the protein was localized in the cytosol. Coincubation of SFN with a vitamin D receptor (VDR), or an extracellular-regulated kinase 1/2 or a nuclear factor-κB inhibitor all reduced HBD-2 mRNA upregulation. In contrast, transfection of cells with a dominant-negative peroxisome proliferator-activated receptor γ (PPARγ) mutant vector to inhibit PPARγ wild-type action and inhibition of p38 mitogen-activated protein kinase (MAPK) signalling did not affect SFN-mediated upregulation of HBD-2 mRNA. Moreover, SFN induced the expression of VDR, PPARγ and phosphorylated ERK1/2 but did not affect p38 MAPK activation. The data clearly demonstrate for the first time that the dietary HDAC inhibitor SFN is able to induce antimicrobial peptides in colonocytes. In this process HBD-2 expression is regulated via VDR, mitogen-activated protein kinase kinase/extracellular-regulated kinase and nuclear factor-κB signalling.
Keywords: β-defensin-2, innate immunity, MEK/ERK signalling pathway, sulforaphane, vitamin D receptor
Introduction
The gastrointestinal tract is constantly in contact with the many commensal microorganisms residing in the colon and distal small intestine. Although the presence of this native flora in general is mutually beneficial, the host also requires protection against these microorganisms. The role of antimicrobial peptides of the defensin family mediating protective responses has been established.1–4 Disturbances in their expression in the intestinal tract have been linked to inflammatory bowel diseases.5–8
Defensins are small cationic peptides with a broad spectrum of antimicrobial activity having characteristic pairs of intramolecular disulphide bonds, a beta-sheet structure and a mass of 3000–5000.9,10 They are classified as α- and β-defensins based on the position of three disulphide bridges.11 The β-defensins are ubiquitously expressed throughout the gastrointestinal tract, including the colon.2 Among the β-defensins, human β-defensin-1 (HBD-1) is constitutively expressed,12,13 whereas HBD-2 is inducible in response to infection, proinflammatory mediators such as interleukin-1β, tumour necrosis factor-α, and probiotic bacteria.14,15 Permeabilization of target membranes is the crucial step in defensin-mediated antimicrobial activity and cytotoxicity.3 The positively charged defensin molecules are inserted into the bacterial membranes under the influence of cell-generated transmembrane potentials and local electrostatic fields, resulting in the cessation of RNA, DNA, and protein synthesis in bacteria.3,16 Besides their direct antimicrobial effects, defensins exert further functions related to host defence such as the induction of histamine release by mast cells and chemoattraction of various cells of the immune system including neutrophils and T cells.15,17 Moreover, they seem to be involved in carcinogenesis.18–20
The dietary histone deacetylase (HDAC) inhibitor sulforaphane (SFN) is one of the most biologically active phytochemicals in the human diet and is present at high concentrations in some cruciferous vegetables, especially broccoli (Brassica oleracea).21 Sulforaphane has received a great deal of attention because of its ability to simultaneously modulate multiple cellular targets involved in cellular protection.22,23 It is regarded as a highly promising dietary preventative agent because of its capacity to induce apoptosis and to inhibit cell proliferation in various tumour cells, including colorectal cancer.21,22 In addition, several studies indicate that SFN exhibits immunomodulatory capacities by interfering with the actions of the proinflammatory transcription factor nuclear factor-κB (NF-κB) and by the modulation of the production of proinflammatory and anti-inflammatory cytokines.24–26 Recent studies, including our own, report that HDAC inhibitors like butyrate and trichostatin A induce the expression of antimicrobial peptides in several colorectal cancer cells.27–30 The aim of this work was to explore a possible role for SFN in the induction of HBD-2 expression as well as to scrutinize the molecular pathways involved in this regulatory process.
Materials and methods
Cell culture
The human colorectal cancer cell lines Caco-2, HT-29 and SW480 were obtained from the European Collection of Cell Cultures (ECACC, Salisbury, UK). Cells were cultured in a humidified incubator at 37° in an atmosphere of 95% air and 5% CO2. Caco-2 and SW480 cells were grown in Dulbecco’s modified Eagle’s medium (DMEM), supplemented with 10% fetal calf serum (FCS), 1% non-essential amino acids, 1% sodium pyruvate and 1% penicillin/streptomycin. HT-29 cells were grown in McCoy’s 5A medium, supplemented with 10% FCS and 1% penicillin/streptomycin. Medium from the dominant-negative peroxisome proliferator-activated receptor γ (PPARγ) mutant and empty-vector HT-29 cells was supplied with 400 μg/ml Geneticin 418 sulphate (G418; Gibco-BRL, Eggenstein, Germany). Cells were regularly screened for Mycoplasma contamination using the VenorGem Mycoplasma detection kit (Minerva Biolabs, Berlin, Germany).
For experiments, cells were seeded in plastic cell culture wells and were cultivated in either DMEM or McCoy's 5A medium, as indicated above, until 80% confluency was reached. Media were then removed and replaced with media containing either the solvent, SFN (1–20 μm), butyrate (1–5 mm) or one of the combinations of SFN (10 μm) with the vitamin D receptor (VDR) inhibitor ZK191732 (10 μm), the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (20 μm), the extracellular signal-regulated kinase (ERK) 1/2 inhibitor PD98059 (40 μm), or the NF-κB inhibitor helenalin (20 μm). In these experiments, cells were pretreated with the inhibitors for 5 hr, followed by challenge with SFN for up to 48 hr. Sodium butyrate (Merck–Schuchardt, Hohenbrunn, Germany) was dissolved in phosphate buffered saline (PBS), SFN (Axxora, San Diego, CA) was rendered soluble in aqua ad iniectabilia (DeltaSelect, Pfufflingen, Germany). PD98059, SB203580 and helenalin (all from Calbiochem, Schwalbach/Taunus, Germany) were dissolved in dimethyl sulphoxide (Fluka; Sigma-Aldrich-Chemie, Steinheim, Germany), ZK191732 (supplied by the Department of Medicinal Chemistry at Schering AG, Berlin, Germany) was dissolved in ethanol. The maximum concentration of solvents in each medium was kept below 0·1% volume/volume and the media were changed every day. Cells were then harvested at the times indicated in the figure legends. Concentrations of SFN and butyrate used in our experiments correspond to appropriate physiological concentrations.31–33
Transfection assay
The following plasmids were used for transfection: pcDNA3 (Invitrogen, Karlsruhe, Germany), as an empty-vector for control transfection, and the plasmid pcDNA3-PPARγL468A/E471A, a dominant-negative PPARγ double-mutant, which was kindly provided by V.K. Chatterjee (Department of Medicine, University of Cambridge, Addenbrooke’s Hospital, Cambridge, UK).34 These constructs were transfected into subconfluent HT-29 cells with lipofectamine 2000 (Invitrogen) in serum-free conditions. After 6 hr, the cells were supplied with fresh medium containing 10% FCS. Twenty-four hours later, the cells were supplied with medium containing G418 (400 μg/ml) and this G418-supplemented culture medium was replaced twice a week. G418-resistant colonies were collected and used for further analysis. Successful transfection of the dominant-negative PPARγ cell system was recently demonstrated.35,36
Cytotoxicity
Cytotoxicity in all experiments was excluded by lactate dehydrogenase release assay using a commercial kit (LDH kit; Roche, Mannheim, Germany).
Messenger RNA isolation
Cells were cultivated in six-well plates and were treated at 80% confluency with SFN, butyrate, or the combination of SFN with one of the inhibitors for 24 and 48 hr, respectively. Total RNA was isolated from cells using an RNA isolation reagent (TRIR, Abgene, Epsom, UK), followed by phenol extraction and ethanol precipitation.
Semi-quantitative RT-PCR
Reverse transcription–polymerase chain reaction (RT-PCR) was conducted with the Gene Amp RNA PCR kit (Applied Biosystems, Branchburg, NJ) according to the manufacturer’s protocol, starting from 1 μg total RNA. All RNA samples were subjected to DNase-treatment during the RT-step to remove traces of genomic DNA (Shrimp Nuclease, Abgene). Amplification of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as control. From the results of preliminary experiments, 20 PCR cycles for GAPDH and 35 cycles for HBD-2 were selected as the optimal amplification conditions to produce a log-linear relationship between the amount of each messenger RNA (mRNA) and the intensity of the PCR product. The PCR contained 0·2 mm dNTPs (Invitrogen Corporation, Carlsbad, CA), 0·05 U/μl AmpliTaqGold DNA polymerase (Applied Biosystems), 1·5 mm Mg2Cl (Applied Biosystems) and 0·2 μmol/l of either of the primers of HBD-2 and GAPDH (Biospring, Frankfurt, Germany). The PCR conditions for HBD-2 were: initial denaturation at 94° for 1 min followed by annealing at 58° for 1 min, extension at 72° for 2 min and a final extension at 72° for 7 min after the last cycle. The respective annealing temperature for GAPDH was 45°. Primers for amplification were as follows: HBD-2 sense 5′-ggtggtataggcgatcctgtt-3′, HBD-2 antisense 5′-agggcaaaagactggatgaca-3′; GAPDH sense 5′-gcaccgtcaaggctgagaac-3′, GAPDH antisense 5′-ccaccaccctgttgctgtag-3′. The expected sizes of HBD-2 and GAPDH were 66 and 803 base pairs, respectively. Aliquots of the PCR mixtures (10 μl) were analysed by electrophoresis using a 1·5% agarose gel containing ethidium bromide. Gels were placed on a UV transilluminator and digitalized using the DocuGel V-System (Scananalytics, Biozym, Landgraaf, the Netherlands). For semi-quantitative analysis of amplified PCR products, the fluorescent dye PicoGreen (Molecular Probes, Eugene, OR) was used according to the manufacturer’s instructions. In brief, 2 μl of amplified DNA in 100 μl TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7·5) was mixed with an equal volume of diluted PicoGreen reagent (1 : 200, volume/volume in TE buffer). Samples were incubated for 5 min at room temperature and protected from light in a microtitre plate. The fluorescence was measured (λex = 485 nm; λem = 538 nm) in the fluorescence microplate reader Tecan SpectraFluor PLUS (MTX Lab Systems, Vienna, VA). A λ DNA linear standard curve was applied for each experiment.
Immunofluorescence assay
Caco-2 cells were grown on glass chamber slides (Lab-Tek; Nunc, Rochester, NY) and allowed to reach 80% confluency. Cells were fixed in ice-cold ethanol (95%) for 10 min and permeabilized by addition of 0·1% Triton X-100 in PBS for 10 min. After washing, unspecific binding of antibodies was blocked by incubating cells in 8% horse serum in PBS containing 0·1% Tween-20 for 1 hr at room temperature. HBD-2 antibody (Immundiagnostik, Bensheim, Germany) was diluted 1 : 25 in blocking buffer. After 1·5 hr of incubation, cells were washed three times with PBS before secondary antibody (cy3 conjugated rabbit–anti-goat; Sigma, St Louis, MO) was added for 1 hr. Following washing and air-drying, the cells were embedded in 4′,6-diamidino-2-phenylindol (DAPI) mounting medium (Vector Laboratories, Burlingame, CA) and evaluated by immunofluorescence microscopy (Eclipse E 600; Nikon, Tokyo, Japan) with a digital camera DX 20H (Kappa, Monrovia, CA).
β-defensin-2 enzyme-linked immunosorbent assay (ELISA)
Caco-2 cells were grown in six-well plates and were treated at 80% confluency with SFN (20 μm), butyrate (3 mm), or one of the combinations of SFN (20 μm) with the p38 MAPK inhibitor SB203580 (20 μm) or the ERK1/2 inhibitor PD98059 (40 μm). Cells were washed three times with ice-cold PBS and incubated with cell lysis buffer (Cell Signaling, Beverly, MA) containing multiple protease inhibitors (Complete, Roche, Mannheim, Germany) for 5 min at 4°. Protein extracts were obtained after sonication of cell lysates (twice for 5 seconds each time) and centrifugation at 7500 g at 4° (10 min). Protein content was determined using a colorimetric assay according to the method of Bradford (Bio-Rad Laboratories, Munich, Germany). Cell lysates were diluted 1 : 3 with PBS containing 0·1% bovine serum albumin. 100 μl was used for each reaction. The HBD-2 ELISA Kit (Immundiagnostik) was then used according to the manufacturer’s instructions. Protein concentration of HBD-2 was analysed and samples were normalized to equal protein concentrations.
Sodium dodecyl sulphate (SDS)–polyacrylamide gel electrophoresis and immunoblot analysis
Caco-2 and HT-29 cells were stimulated with SFN at 80% confluency. Cells were than harvested and protein content was determined using a Bio-Rad colorimetric assay (see β-defensin-2 ELISA section). Equal amounts of total protein lysates were separated on a 10% SDS–polyacrylamide gel for VDR, PPARγ and on a 12·5% SDS–polyacrylamide gel for ERK1/2, phospho-ERK1/2, p38 MAPK and phospho-p38 MAPK. Proteins were transferred onto a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany). Subsequently, membranes were blocked for 1 hr with 5% (weight/volume; wt/v) non-fat dried milk in Tris-buffered saline containing 0·05% Tween-20 (TBS-T). Membranes were then incubated overnight with a 1 : 1000 dilution of VDR, ERK1/2 and phospho-ERK1/2 antibodies (all from Santa Cruz Biotechnologies, Santa Cruz, CA) or with p38 MAPK and phospho-p38 MAPK antibodies (both from Cell Signaling), or with a 1 : 2000 dilution of PPARγ antibody (Calbiochem, LaJolla, CA) in 0·05% TBS-T and 5% (wt/v) non-fat dried milk. After washing, the blots were incubated for 30 min with the corresponding horseradish peroxidase-conjugated antibody (Vector Laboratories, dilution 1 : 2000) in 0·05% TBS-T and 5% (wt/v) non-fat dried milk. The washing steps were repeated, and subsequently enhanced chemoluminescence detection was performed according to the manufacturer’s instructions (Amersham Pharmacia Biotech, Buckinghamshire, UK) on Hyperfilm-MP (Amersham International plc, Buckinghamshire, UK). Blots were then reprobed with β-actin antibody (Sigma). For quantitative analysis, bands were detected by scanning densitometry, using a Desaga CabUVIS scanner and Desaga Provildoc software (Desaga, Wiesloch, Germany).
Statistics
All statistical analyses were performed using GraphPad Prism 4·01 (San Diego, CA). Analysis of variance was performed when more than two groups were compared and, when significant (P < 0·05), multiple comparisons were performed using Tukey’s test. Data are expressed as means ± SD from three independent experiments. A P value < 0·05 was considered to be significant.
Results
Sulforaphane is a direct inducer of HBD-2 mRNA expression in colonocytes
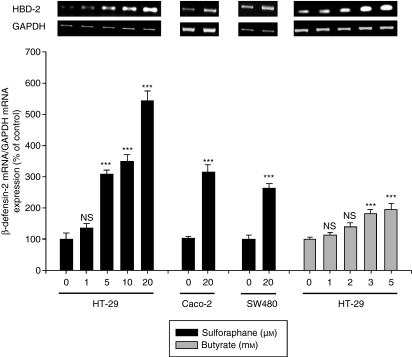
Challenge of HT-29 cells with increasing concentrations of SFN (1–20 μm) provoked a dose-dependent upregulation of HBD-2 mRNA after 24 hr (Fig. 1) and 48 hr of treatment (data not shown). Significant effects were seen at concentrations ≥ 5 μm. Increased expression of the HBD-2 gene in response to SFN (20 μm) was also obtained in the colon cancer cell lines Caco-2 and SW480 after 24 hr (Fig. 1), reflecting a common mechanism in colorectal cancer cells. Analogously, stimulation of HT-29 cells with increasing concentrations of the dietary HDAC inhibitor butyrate (1–5 mm) also resulted in a dose-dependent upregulation of HBD-2 mRNA compared to control cells (Fig. 1) after 24 hr. A similar upregulation was seen in the colorectal cancer cell line Caco-2 (data not shown).
Figure 1.
Dose-dependent effect of sulforaphane (1–20 μm) and butyrate (1–5 mm) on β-defensin-2 messenger RNA (mRNA) expression in HT-29 cells after 24 hr of treatment. Moreover the induction of β-defensin-2 mRNA expression in Caco-2 and SW480 cells in response to sulforaphane (20 μm) was demonstrated. The β-defensin-2 mRNA expression was measured by semi-quantitative reverse transcription–polymerase chain reaction using the fluorescent dye Pico Green. All values for mRNA levels are normalized to corresponding mRNA amounts for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). One representative gel of three independent experiments is shown. Induction of β-defensin-2 mRNA is displayed as relative percentage to solvent treated control cells. ***P < 0·001, NS, not significant.
Sulforaphane elevates HBD-2 protein expression in colonocytes
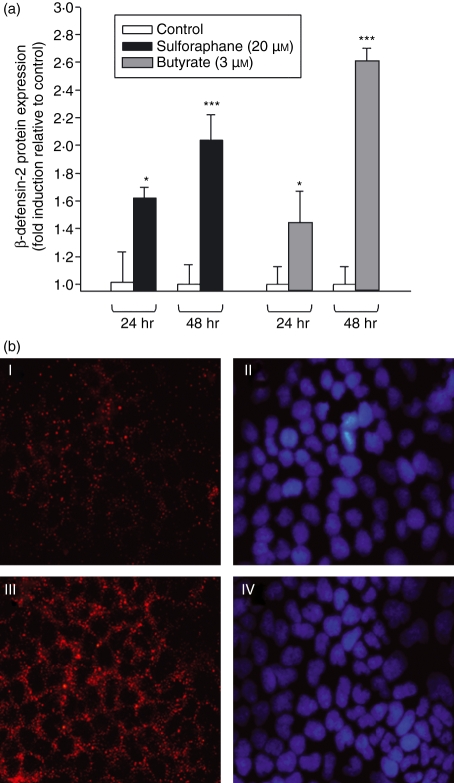
Upregulation of HBD-2 mRNA was accompanied by a similar increase in protein level as measured by ELISA. In Caco-2 cells, a time-dependent increase of HBD-2 protein level was observed after exposure to SFN (20 μm) or butyrate (3 mm) (Fig. 2a: SFN: 24 hr: + 62%, P < 0·05; 48 hr: + 104%, P < 0·001; butyrate: 24 hr: + 47%, P < 0·05; 48 hr: + 161%, P < 0·001). In addition, the subcellular localization of HBD-2 protein in Caco-2 cells was determined via immunofluorescence analysis (Fig. 2b). Caco-2 cells were treated with SFN (20 μm) up to 48 hr. Both control cells and SFN-treated cells showed strong staining for HBD-2 that was predominantly in the cytosol. Moreover, SFN challenge for 48 hr led to an increased expression of the HBD-2 protein, which is in line with the results obtained by ELISA. Similar results were obtained after challenging the cells with butyrate used at a concentration of 3 mm (data not shown).
Figure 2.
(a) β-defensin-2 protein expression in Caco-2 cells measured by enzyme-linked immunosorbent assay (ELISA). Cells were starved for 72 hr and then stimulated with sulforaphane (10–20 μm) or butyrate (3 mm) for 24 and 48 hr. Proteins were harvested and β-defensin-2 was measured by ELISA. The concentration range of all experiments was in ng/ml. Induction of β-defensin-2 protein is displayed as fold induction relative to solvent-treated control cells. ***P < 0·001, *P < 0·05. (b) Representative immunofluorescence assays for β-defensin-2 in Caco-2 cells. Confluent cells were challenged with sulforaphane (20 μm) for 48 hr (III) or grown in culture medium as controls (I). (II, IV) Nuclear counterstaining with 4′,6-diamidino-2-phenylindol (DAPI) mounting medium for each of the adjacent panels. Magnification, × 400.
The VDR but not the PPARγ is involved in SFN-induced HBD-2 mRNA expression
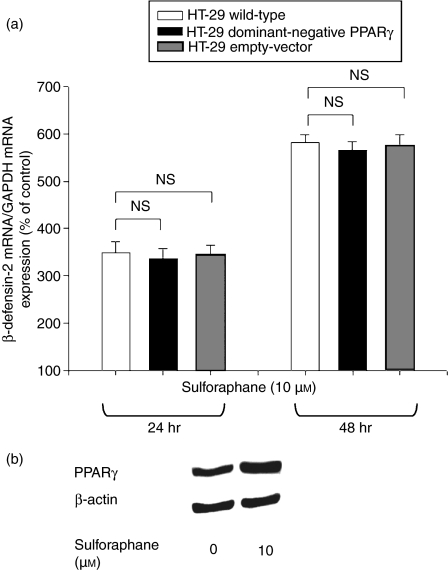
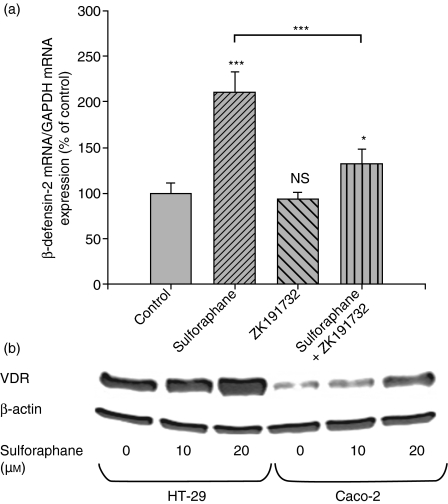
The nuclear receptors VDR and PPARγ are potent regulators of immune responses. To evaluate the involvement of PPARγ in SFN-induced HBD-2 mRNA expression, HT-29 cells transfected with a dominant-negative PPARγ mutant to inhibit wild-type receptor action were used (Fig. 3a). In these mutant cells, an equal time-dependent increase of HBD-2 mRNA compared to wild-type and empty-vector cells was observed in response to SFN, indicating that PPARγ activity is not required for HBD-2 regulation. However, stimulation of HT-29 wild-type cells with SFN (10 μm) for 48 hr caused significant upregulation of the expression of PPARγ (Fig. 3b), indicating that the receptor is involved in SFN-mediated signalling. Moreover, wild-type Caco-2 cells were treated with SFN (10 μm) in the presence of the VDR antagonist ZK191732. Inhibition of VDR significantly antagonized SFN-induced HBD-2 mRNA after 24 hr of incubation, demonstrating the pivotal role of the receptor in SFN-induced HBD-2 upregulation (− 71% versus control, P < 0·001, Fig. 4a). To reveal whether VDR is a target of the SFN signalling pathway, colonocytes were stimulated with SFN (10–20 μm) for 24 hr. As depicted in Fig. 4(b), SFN increased the production of VDR protein in HT-29 cells. A similar pattern was observed for the colorectal cancer cell line Caco-2 (Fig. 4b).
Figure 3.
(a) Time-dependent effect of sulforaphane (10 μm) on β-defensin-2 messenger RNA (mRNA) expression in wild-type, empty-vector and peroxisome proliferator-activated receptor γ (PPARγ) mutant HT-29 cells after 24 and 48 hr of treatment. β-Defensin-2 mRNA was measured by semi-quantitative reverse transcription polymerase chain reaction with the fluorescent dye Pico Green. All values for mRNA levels are normalized to corresponding mRNA amounts for glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Induction of β-defensin-2 mRNA is displayed as a relative percentage to solvent-treated control cells; NS, not significant. (b) Western blot for PPARγ expression after treatment of HT-29 wild-type cells with sulforaphane (10 μm) for 48 hr. One representative blot of three independent experiments is shown.
Figure 4.
(a) β-defensin-2 messenger RNA (mRNA) expression in Caco-2 cells. Medium was supplemented with the solvent, sulforaphane (10 μm), the vitamin D receptor (VDR) inhibitor ZK191732 (10 μm) or a combination of sulforaphane and ZK191732 for 24 hr. Semi-quantitative analysis of polymerase chain reaction products was performed using Pico Green. All values for mRNA levels are normalized to corresponding mRNA amounts of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Induction of β-defensin-2 mRNA is displayed as relative percentage to solvent-treated control cells. ***P < 0·001, *P < 0·05, NS, not significant. (b) Western blot for VDR expression after treatment of HT-29 and Caco-2 cells with sulforaphane (10–20 μm) for 24 hr. One representative blot of three independent experiments is shown.
The mitogen-activated protein kinase kinase (MEK)/ERK signalling pathway and NF-κB play a role in SFN-induced HBD-2 expression
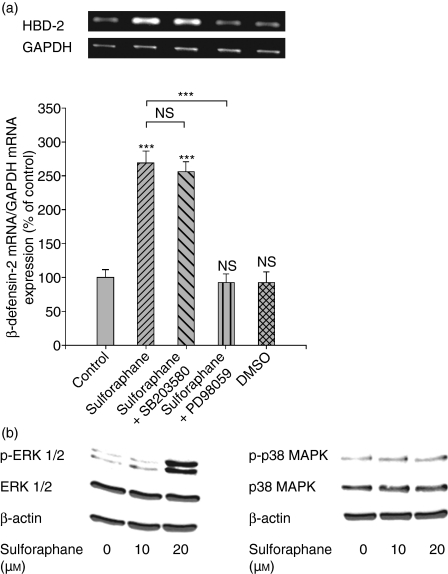
To determine the involvement of intracellular signal transduction pathways such as p38 MAPK and ERK1/2 in SFN-mediated HBD-2 expression, Caco-2 cells were preincubated for 5 hr with specific inhibitors before stimulation with SFN (10 μm). Combined treatment of SFN with the ERK1/2 inhibitor PD98059 reduced the induction of HBD-2 mRNA expression after 24 hr (Fig. 5a). In contrast, coincubation with the p38 MAPK inhibitor SB203580 did not affect the elevated HBD-2 mRNA expression caused by SFN at 24 hr (Fig. 5a). Similar to the observations on mRNA level, inhibition of the ERK1/2 pathway almost blocked SFN-induced HBD-2 protein expression (−87%, P < 0·001) in Caco-2 cells after 24 hr, as detected by ELISA, while inhibition of the p38 MAPK trail did not influence the induction of the peptide by the drug (−16%, not significant). Moreover, exposure of Caco-2 cells with SFN (10–20 μm) resulted in a rapid phosphorylation of ERK1/2 after 8 hr, while the amount of total ERK1/2 protein was not affected. The expressions of phospho-p38 MAPK and p38 MAPK proteins were unchanged by SFN in Caco-2 cells (Fig. 5b). To gain more insights into the signalling events of HBD-2 mRNA expression in response to SFN, the involvement of the NF-κB pathway in this regulation was scrutinized. Treatment with the NF-κB inhibitor helenalin partially reversed the induction of HBD-2 mRNA (−23%, P < 0·05, 24 hr) caused by SFN (10 μm) in Caco-2 cells.
Figure 5.
(a) Influence of the p38 mitogen-activated protein kinase (MAPK) inhibitor SB203580 (20 μm) and the extracellular signal-regulated kinase (ERK) 1/2 inhibitor PD98059 (40 μm) on sulforaphane-induced (10 μm) β-defensin-2 messenger RNA (mRNA) expression after 24 hr of treatment in Caco-2 cells. β-defensin-2 expression was analysed by semi-quantitative reverse transcription–polymerase chain reaction with the fluorescent dye Pico Green. All values for mRNA levels are normalized to corresponding mRNA amounts of glyceraldehyde-3-phosphate dehydrogenase (GAPDH). One representative gel of three independent experiments is shown. Induction of β-defensin-2 mRNA is displayed as relative percentage to solvent-treated control cells. ***P < 0·001, NS, not significant. (b) Western blot for ERK1/2, phospho-ERK1/2, p38 MAPK and phospho-p38 MAPK expression after treatment of Caco-2 cells with sulforaphane (10–20 μm) for 8 hr. One representative blot of three independent experiments is shown.
Discussion
The discovery of antimicrobial peptides such as defensins has extended our knowledge of non-specific defence mechanisms. A deficiency in the antimicrobial defence system of defensins may be a reasonable and plausible explanation for the breakdown of barrier functions with increased invasion of infectious pathogens, leading to inflammation or deleterious immune responses. Abnormalities in defensin expression have not only been observed in inflammatory bowel diseases8,16 but also in cystic fibrosis, where the inactivation of the defensins as the result of high salt concentrations has been linked to recurrent bronchopulmonary infections.37 In contrast to ulcerative colitis and ileal Crohn’s disease, colonic Crohn’s disease is characterized by an impaired induction of the epithelial HBD-2.6,38,39 This defective regulation could be explained by the lower HBD-2 gene copy number in the HBD-2 locus in colonic Crohn's disease.39 A normal HBD-2 copy number distribution was seen in ileal Crohn's disease and also in ulcerative colitis.39 Eventually, the lack of HBD-2 induction may contribute to a defective antimicrobial barrier leading to chronic inflammation in Crohn's disease affecting the large bowel.39 Detailed knowledge of HBD-2 expression and regulation is still lacking. The aim of this study was to determine a possible role for the dietary HDAC inhibitor SFN in the regulation of HBD-2. Similar experiments were accomplished with the dietary HDAC inhibitor butyrate. In this context, the involvement of the nuclear receptors VDR and PPARγ as well as the MEK/ERK and NF-κB pathways in SFN-mediated induction of HBD-2 were examined.
Accumulating evidence suggests that SFN is a highly promising dietary preventive agent as the result of its ability to exert immunomodulatory effects via multiple mechanisms of action including anti-inflammatory actions.24,40 The anti-inflammatory effects of SFN and butyrate may be achieved via different pathways: The agents can affect the expression of a diverse array of genes potentially involved in immune modulation through their ability to inhibit HDAC activity via histone hyperacetylation.40 This results in chromatin relaxation and increased transcription of target genes.40 SFN and butyrate are also able to block tumour necrosis factor-α-induced and lipopolysaccharide-induced NF-κB translocation resulting in reduced expression of proinflammatory cytokines.24,25,41 Moreover, the involvement of HDAC inhibitors such as butyrate and trichostatin A in the modulation of the intestinal antimicrobial peptides make these peptides possible targets for the actions of SFN.27,30,42 Indeed, in a variety of human colon cancer cells, including HT-29 cells, and in a mouse model, SFN, when used under the same experimental conditions as in our study, inhibited HDAC activity accompanied by a global increase in histone H3 and H4 acetylation.40,43,44 Similarly, inhibition of HDAC activity and histone hyperacetylation have also been demonstrated for the dietary HDAC inhibitors diallyl disulphide and butyrate in the colon cancer cell lines Caco-2 and HT-29.29,45,46 However, the exact mechanism by which HDAC inhibitors induce the release of antimicrobial peptides is not completely understood. Recently, a novel approach was provided by the study of Schauber et al., which demonstrated that VDR coactivators are important for HDAC-inhibitor-induced acetylation of histone proteins.47 Increased histone acetylation caused by butyrate intensified the induction of the antimicrobial peptide cathelicidin in response to 1,25-dihydroxyvitamin D3. For this regulation, correct functioning of both the VDR and of the steroid receptor coactivator 3 (SRC3)/p300, which possesses inherent histone acetyltransferase activity, were required.47
The present study demonstrated for the first time that the dietary HDAC inhibitor SFN is a direct inducer of HBD-2 mRNA and protein in colonocytes. Similar results were obtained after butyrate treatment. Immunofluorescence experiments showed that the peptide exhibits a primarily cytoplasmic localization, as demonstrated by previous groups in colon cancer cells.12,48 In our in vitro setting, butyrate has only a minor effect on HBD-2 transcription compared to SFN, but has a much stronger effect on HBD-2 peptide expression. The different amount of HBD-2 protein can be explained by variations in the modulation of intracellular signalling initiated by each HDAC inhibitor, concerning stability of mRNA transcripts as well as protein translation, e.g. via interaction with initiation factors. In the present study, SFN activates ERK1/2, whereas butyrate mainly induces p38 MAPK activation.36 Recently it was demonstrated that ERK can regulate translation initiation via dephosphorylation of the initiation factor elF2α.49 Moreover, downstream substrates of p38 MAPK were shown to regulate mRNA stability and also translation.50 Activation of different mechanisms may therefore contribute to the observed diversity in HBD-2 protein expression compared to mRNA transcription.
Both PPARγ and VDR are highly expressed in the colonic epithelium, indicating that both receptors are important agents in the physiology of the human colon.36,51,52 The receptors partly mediate their anti-inflammatory actions through negative interference with proinflammatory transcription factors such as NF-κB.35,53,54 Moreover, there is a great influx of information that PPARγ ligands may influence the inflammatory response in inflammatory bowel diseases and colon cancer.55,56 Similarly, the active form of vitamin D, 1,25-dihydroxyvitamin D3, was shown to inhibit the development of various autoimmune diseases, including inflammatory bowel diseases.57 In addition, a VDR gene polymorphism has been associated with susceptibility to Crohn’s disease.58 All these features imply the possible involvement of both receptors in SFN-mediated HBD-2 regulation. Using the VDR inhibitor ZK191732 and a Caco-2 dominant-negative PPARγ mutant cell line in our experiments, we demonstrated that VDR activity, but not PPARγ activity, is required for HBD-2 expression induced by SFN. The properties of the VDR inhibitor ZK191732 and the functional successful transfection of the dominant-negative PPARγ mutant cell system have been described recently.35 Our findings are supported by the presence of a consensus vitamin D response element in the promoter of the human HBD-2 gene.59 Accordingly, stimulation of several human cell types with 1,25-dihydroxyvitamin D3 resulted in increased expression of HBD-2 mRNA.59 Moreover, our in vitro model demonstrates for the first time the upregulation of VDR and PPARγ after SFN stimulation in colonocytes, not only corroborating the involvement of both receptors in SFN-mediated signalling but also making them attractive targets involved in the HBD-2 pathway. A similar increase of the receptors was observed after butyrate treatment.60–62
Stimulation with SFN may activate different MAPKs in colorectal cancer cells.31,63,64 Kinase pathways act as signal sorters and conduct a variety of upstream signals to the nucleus of the eukaryotic cell, where transcription of specific target genes will be affected.65 To gain insight into the regulatory pathways by which SFN augments HBD-2 expression in Caco-2 cells, our in vitro model focused on two major MAPKs: p38 MAPK and ERK1/2. These MAPKs are involved in a large variety of cellular activities, including cell survival, proliferation and inflammatory responses.65–68 Increased phosphorylation of ERK1/2 by SFN in various cell lines, including human colon adenocarcinoma Caco-2 cells, has been demonstrated by several groups, although the amount of ERK1/2 protein was not affected. In contrast, SFN treatment had no impact on p38 MAPK activation.31,63 These observations are confirmed by our experiments. Moreover, inhibition of the ERK1/2 pathway in this study prevented SFN-mediated induction of HBD-2 mRNA expression in Caco-2 cells, while blocking of the p38 MAPK trail did not affect HBD-2 levels. Our data are in accordance with observations in middle-ear epithelial cells, demonstrating transcriptional activation of the HBD-2 gene caused by interleukin-1α which is mediated through a Raf-MEK1/2-ERK1/2 signalling pathway.69 Conflicting results were obtained in the lung epithelial cell line A549, in which interleukin-1β-induced upregulation of HBD-2 was partly attenuated by inhibiting the p38 MAPK but not by the ERK1/2 signalling pathway.70 These different observations indicate a cell-specific and stimulus-dependent kinase pathway leading to induction of HBD-2. However, all these studies underscore the importance of the MAPK signalling pathways in innate immunity through the regulation of HBD-2 levels.
Nuclear factor-κB plays a key role in regulating the transcription of several members of a proinflammatory gene programme in intestinal epithelial cells that is induced in response to inflammation, especially in inflammatory bowel diseases, or to infection with enteroinvasive bacteria.12 The NF-κB pathway has been described as an important regulator of HBD-2 induction in colon epithelial cells.12,14,71 Moreover, this pathway has been shown to participate in SFN signalling.24,25 Our data demonstrate that pretreatment of Caco-2 cells with the NF-κB inhibitor helenalin slightly diminished HBD-2 upregulation caused by SFN. This finding is not only supported by former studies demonstrating the necessity of NF-κB in the induction of HBD-2 by inhibitor experiments, but also by the identification of a NF-κB consensus sequence in the proximal promoter of the hBD-2 gene, which appears to be necessary for optimal HBD-2 gene expression.12,14,71,72 Nevertheless, we suggest that the induction of HBD-2 in response to SFN is more complex than if it were based solely on the actions of VDR, ERK1/2 and NF-κB. Since several mechanisms of action have been identified as modulating HBD-2 signalling, for instance the pathways of Ap1, protein kinase C, phosphatidylinositol-3-kinase and cJun N-terminal kinase, and because these pathways are also targets of SFN in colonocytes, multiple signalling pathways may be involved in the upregulation of the peptide.14,31,70,71,73,74
In summary, we have demonstrated for the first time that SFN induces the expression of the antimicrobial peptide HBD-2 at both mRNA and protein levels in colorectal cancer cells. Similar effects were obtained after butyrate treatment. Furthermore, we revealed that SFN-mediated induction of HBD-2 is modulated via VDR, MEK/ERK and the NF-κB signalling pathway. These data support the potential usefulness of dietary HDAC inhibitors in the therapy of colonic Crohn's disease. Further in vivo studies are required to establish the relevance of these findings.
Acknowledgments
The authors would like to thank Nada Povse for excellent technical assistance. This work was supported by an unrestricted research grant from the Else Kröner–Fresenius–Foundation, Bad Homburg, Germany. Markus Schwab is supported by the Frankfurt International Research Graduate School for Translational Biomedicine (FIRST).
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindol
- DMEM
Dulbecco's modified Eagle's medium
- ECACC
European Collection of Cell Cultures
- ERK
extracellular signal-regulated kinase
- FCS
fetal calf serum
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HBD-2
human β-defensin-2
- HDAC
histone deacetylase
- JNK
cJun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MEK/ERK
mitogen-activated protein kinase kinase/extracellular-regulated kinase
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- PPARγ
peroxisome proliferator-activated receptor γ
- SCFA
short-chain fatty acid
- SFN
sulforaphane
- VDR
vitamin D receptor
References
- 1.Wehkamp J, Stange EF. Paneth cells and the innate immune response. Curr Opin Gastroenterol. 2006;22:644–50. doi: 10.1097/01.mog.0000245541.95408.86. [DOI] [PubMed] [Google Scholar]
- 2.Sansonetti PJ. War and peace at mucosal surfaces. Nat Rev Immunol. 2004;4:953–64. doi: 10.1038/nri1499. [DOI] [PubMed] [Google Scholar]
- 3.Ganz T. Defensins: antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3:710–20. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 4.Zasloff M. Antimicrobial peptides in health and disease. N Engl J Med. 2002;347:1199–200. doi: 10.1056/NEJMe020106. [DOI] [PubMed] [Google Scholar]
- 5.Wehkamp J, Schmid M, Fellermann K, Stange EF. Defensin deficiency, intestinal microbes, and the clinical phenotypes of Crohn's disease. J Leukoc Biol. 2005;77:460–5. doi: 10.1189/jlb.0904543. [DOI] [PubMed] [Google Scholar]
- 6.Wehkamp J, Fellermann K, Stange EF. Human defensins in Crohn's disease. Chem Immunol Allergy. 2005;86:42–54. doi: 10.1159/000086672. [DOI] [PubMed] [Google Scholar]
- 7.Wehkamp J, Salzman NH, Porter E, et al. Reduced Paneth cell alpha-defensins in ileal Crohn's disease. Proc Natl Acad Sci U S A. 2005;102:18129–34. doi: 10.1073/pnas.0505256102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zasloff M. Defending the epithelium. Nat Med. 2006;12:607–8. doi: 10.1038/nm0606-607. [DOI] [PubMed] [Google Scholar]
- 9.Eckmann L. Innate immunity and mucosal bacterial interactions in the intestine. Curr Opin Gastroenterol. 2004;20:82–8. doi: 10.1097/00001574-200403000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Bevins CL, Martin-Porter E, Ganz T. Defensins and innate host defence of the gastrointestinal tract. Gut. 1999;45:911–5. doi: 10.1136/gut.45.6.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eckmann L. Defence molecules in intestinal innate immunity against bacterial infections. Curr Opin Gastroenterol. 2005;21:147–51. doi: 10.1097/01.mog.0000153311.97832.8c. [DOI] [PubMed] [Google Scholar]
- 12.O'Neil DA, Porter EM, Elewaut D, Anderson GM, Eckmann L, Ganz T, Kagnoff MF. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–24. [PubMed] [Google Scholar]
- 13.Wehkamp J, Harder J, Weichenthal M, Mueller O, Herrlinger KR, Fellermann K, Schroeder JM, Stange EF. Inducible and constitutive beta-defensins are differentially expressed in Crohn's disease and ulcerative colitis. Inflamm Bowel Dis. 2003;9:215–23. doi: 10.1097/00054725-200307000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Wehkamp J, Harder J, Wehkamp K, et al. NF-kappaB- and AP-1-mediated induction of human beta defensin-2 in intestinal epithelial cells by Escherichia coli Nissle 1917: a novel effect of a probiotic bacterium. Infect Immun. 2004;72:5750–8. doi: 10.1128/IAI.72.10.5750-5758.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Smet K, Contreras R. Human antimicrobial peptides: defensins, cathelicidins and histatins. Biotechnol Lett. 2005;27:1337–47. doi: 10.1007/s10529-005-0936-5. [DOI] [PubMed] [Google Scholar]
- 16.Wehkamp J, Fellermann K, Herrlinger KR, Bevins CL, Stange EF. Mechanisms of disease: defensins in gastrointestinal diseases. Nat Clin Pract Gastroenterol Hepatol. 2005;2:406–15. doi: 10.1038/ncpgasthep0265. [DOI] [PubMed] [Google Scholar]
- 17.Befus AD, Mowat C, Gilchrist M, Hu J, Solomon S, Bateman A. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J Immunol. 1999;163:947–53. [PubMed] [Google Scholar]
- 18.Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci. 2005;62:784–90. doi: 10.1007/s00018-005-4560-2. [DOI] [PubMed] [Google Scholar]
- 19.Markeeva N, Lisovskiy I, Lyzogubov V, et al. Expression of beta-defensin-2 in human gastric tumors: a pilot study. Exp Oncol. 2005;27:130–5. [PubMed] [Google Scholar]
- 20.Bullard RS, Gibson W, Bose SK, et al. Functional analysis of the host defense peptide Human Beta Defensin-1: new insight into its potential role in cancer. Mol Immunol. 2008;45:839–48. doi: 10.1016/j.molimm.2006.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fimognari C, Hrelia P. Sulforaphane as a promising molecule for fighting cancer. Mutat Res. 2007;635:90–104. doi: 10.1016/j.mrrev.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 22.Myzak MC, Dashwood RH. Histone deacetylases as targets for dietary cancer preventive agents: lessons learned with butyrate, diallyl disulfide, and sulforaphane. Curr Drug Targets. 2006;7:443–52. doi: 10.2174/138945006776359467. [DOI] [PubMed] [Google Scholar]
- 23.Myzak MC, Ho E, Dashwood RH. Dietary agents as histone deacetylase inhibitors. Mol Carcinog. 2006;45:443–6. doi: 10.1002/mc.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thejass P, Kuttan G. Immunomodulatory activity of Sulforaphane, a naturally occurring isothiocyanate from broccoli (Brassica oleracea) Phytomedicine. 2007;14:538–45. doi: 10.1016/j.phymed.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 25.Heiss E, Herhaus C, Klimo K, Bartsch H, Gerhauser C. Nuclear factor kappa B is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–15. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 26.Bertl E, Bartsch H, Gerhauser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–85. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 27.Schwab M, Reynders V, Shastri Y, Loitsch S, Stein J, Schroder O. Role of nuclear hormone receptors in butyrate-mediated up-regulation of the antimicrobial peptide cathelicidin in epithelial colorectal cells. Mol Immunol. 2007;44:2107–14. doi: 10.1016/j.molimm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 28.Hase K, Eckmann L, Leopard JD, Varki N, Kagnoff MF. Cell differentiation is a key determinant of cathelicidin LL-37/human cationic antimicrobial protein 18 expression by human colon epithelium. Infect Immun. 2002;70:953–63. doi: 10.1128/iai.70.2.953-963.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schauber J, Iffland K, Frisch S, et al. Histone-deacetylase inhibitors induce the cathelicidin LL-37 in gastrointestinal cells. Mol Immunol. 2004;41:847–54. doi: 10.1016/j.molimm.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 30.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–19. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jakubikova J, Sedlak J, Mithen R, Bao Y. Role of PI3K/Akt and MEK/ERK signaling pathways in sulforaphane- and erucin-induced phase II enzymes and MRP2 transcription, G2/M arrest and cell death in Caco-2 cells. Biochem Pharmacol. 2005;69:1543–52. doi: 10.1016/j.bcp.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 32.Traka M, Gasper AV, Smith JA, Hawkey CJ, Bao Y, Mithen RF. Transcriptome analysis of human colon Caco-2 cells exposed to sulforaphane. J Nutr. 2005;135:1865–72. doi: 10.1093/jn/135.8.1865. [DOI] [PubMed] [Google Scholar]
- 33.Wachtershauser A, Stein J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur J Nutr. 2000;39:164–71. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- 34.Gurnell M, Wentworth JM, Agostini M, et al. A dominant-negative peroxisome proliferator-activated receptor gamma (PPARgamma) mutant is a constitutive repressor and inhibits PPARgamma-mediated adipogenesis. J Biol Chem. 2000;275:5754–9. doi: 10.1074/jbc.275.8.5754. [DOI] [PubMed] [Google Scholar]
- 35.Schwab M, Reynders V, Loitsch S, Steinhilber D, Stein J, Schroder O. Involvement of different nuclear hormone receptors in butyrate-mediated inhibition of inducible NFkappaB signalling. Mol Immunol. 2007;44:3625–32. doi: 10.1016/j.molimm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 36.Schwab M, Reynders V, Ulrich S, Zahn N, Stein J, Schroder O. PPARgamma is a key target of butyrate-induced caspase-3 activation in the colorectal cancer cell line Caco-2. Apoptosis. 2006;11:1801–11. doi: 10.1007/s10495-006-9788-2. [DOI] [PubMed] [Google Scholar]
- 37.Goldman MJ, Anderson GM, Stolzenberg ED, Kari UP, Zasloff M, Wilson JM. Human beta-defensin-1 is a salt-sensitive antibiotic in lung that is inactivated in cystic fibrosis. Cell. 1997;88:553–60. doi: 10.1016/s0092-8674(00)81895-4. [DOI] [PubMed] [Google Scholar]
- 38.Wehkamp J, Fellermann K, Herrlinger KR, et al. Human beta-defensin 2 but not beta-defensin 1 is expressed preferentially in colonic mucosa of inflammatory bowel disease. Eur J Gastroenterol Hepatol. 2002;14:745–52. doi: 10.1097/00042737-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Fellermann K, Stange DE, Schaeffeler E, et al. A chromosome 8 gene-cluster polymorphism with low human Beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79:439–48. doi: 10.1086/505915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dashwood RH, Myzak MC, Ho E. Dietary HDAC inhibitors: time to rethink weak ligands in cancer chemoprevention? Carcinogenesis. 2006;27:344–9. doi: 10.1093/carcin/bgi253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Place RF, Noonan EJ, Giardina C. HDAC inhibition prevents NF-kappa B activation by suppressing proteasome activity: down-regulation of proteasome subunit expression stabilizes I kappa B alpha. Biochem Pharmacol. 2005;70:394–406. doi: 10.1016/j.bcp.2005.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Schauber J, Svanholm C, Termen S, et al. Expression of the cathelicidin LL-37 is modulated by short chain fatty acids in colonocytes: relevance of signalling pathways. Gut. 2003;52:735–41. doi: 10.1136/gut.52.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Myzak MC, Karplus PA, Chung FL, Dashwood RH. A novel mechanism of chemoprotection by sulforaphane: inhibition of histone deacetylase. Cancer Res. 2004;64:5767–74. doi: 10.1158/0008-5472.CAN-04-1326. [DOI] [PubMed] [Google Scholar]
- 44.Myzak MC, Dashwood WM, Orner GA, Ho E, Dashwood RH. Sulforaphane inhibits histone deacetylase in vivo and suppresses tumorigenesis in Apc-minus mice. FASEB J. 2006;20:506–8. doi: 10.1096/fj.05-4785fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Druesne N, Pagniez A, Mayeur C, Thomas M, Cherbuy C, Duee PH, Martel P, Chaumontet C. Diallyl disulfide (DADS) increases histone acetylation and p21(waf1/cip1) expression in human colon tumor cell lines. Carcinogenesis. 2004;25:1227–36. doi: 10.1093/carcin/bgh123. [DOI] [PubMed] [Google Scholar]
- 46.Waldecker M, Kautenburger T, Daumann H, Busch C, Schrenk D. Inhibition of histone-deacetylase activity by short-chain fatty acids and some polyphenol metabolites formed in the colon. J Nutr Biochem. 2007 doi: 10.1016/j.jnutbio.2007.08.002. doi 10.1016/j.jnutbio.2007.08.002 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 47.Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, Bikle DD, Gallo RL. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D(3) J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5701102. doi 10.1038/sj.jid.5701102 Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 48.Rahman A, Fahlgren A, Sitohy B, Baranov V, Zirakzadeh A, Hammarstrom S, Danielsson A, Hammarstrom ML. Beta-defensin production by human colonic plasma cells: a new look at plasma cells in ulcerative colitis. Inflamm Bowel Dis. 2007;13:847–55. doi: 10.1002/ibd.20141. [DOI] [PubMed] [Google Scholar]
- 49.Monick MM, Powers LS, Gross TJ, Flaherty DM, Barrett CW, Hunninghake GW. Active ERK contributes to protein translation by preventing JNK-dependent inhibition of protein phosphatase 1. J Immunol. 2006;177:1636–45. doi: 10.4049/jimmunol.177.3.1636. [DOI] [PubMed] [Google Scholar]
- 50.Schindler JF, Monahan JB, Smith WG. p38 pathway kinases as anti-inflammatory drug targets. J Dent Res. 2007;86:800–11. doi: 10.1177/154405910708600902. [DOI] [PubMed] [Google Scholar]
- 51.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26:662–87. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 52.Desvergne B, Wahli W. Peroxisome proliferator-activated receptors: nuclear control of metabolism. Endocr Rev. 1999;20:649–88. doi: 10.1210/edrv.20.5.0380. [DOI] [PubMed] [Google Scholar]
- 53.Sun J, Kong J, Duan Y, Szeto FL, Liao A, Madara JL, Li YC. Increased NF-kappaB activity in fibroblasts lacking the vitamin D receptor. Am J Physiol Endocrinol Metab. 2006;291:E315–22. doi: 10.1152/ajpendo.00590.2005. [DOI] [PubMed] [Google Scholar]
- 54.Delerive P, Fruchart JC, Staels B. Peroxisome proliferator-activated receptors in inflammation control. J Endocrinol. 2001;169:453–9. doi: 10.1677/joe.0.1690453. [DOI] [PubMed] [Google Scholar]
- 55.Bull AW. The role of peroxisome proliferator-activated receptor gamma in colon cancer and inflammatory bowel disease. Arch Pathol Lab Med. 2003;127:1121–3. doi: 10.5858/2003-127-1121-TROPPR. [DOI] [PubMed] [Google Scholar]
- 56.Auwerx J. Nuclear receptors. I. PPAR gamma in the gastrointestinal tract: gain or pain? Am J Physiol Gastrointest Liver Physiol. 2002;282:G581–5. doi: 10.1152/ajpgi.00508.2001. [DOI] [PubMed] [Google Scholar]
- 57.Cantorna MT, Zhu Y, Froicu M, Wittke A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am J Clin Nutr. 2004;80:1717S–20S. doi: 10.1093/ajcn/80.6.1717S. [DOI] [PubMed] [Google Scholar]
- 58.Simmons JD, Mullighan C, Welsh KI, Jewell DP. Vitamin D receptor gene polymorphism: association with Crohn's disease susceptibility. Gut. 2000;47:211–4. doi: 10.1136/gut.47.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang TT, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–12. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 60.Wachtershauser A, Loitsch SM, Stein J. PPAR-gamma is selectively upregulated in Caco-2 cells by butyrate. Biochem Biophys Res Commun. 2000;272:380–5. doi: 10.1006/bbrc.2000.2793. [DOI] [PubMed] [Google Scholar]
- 61.Gaschott T, Stein J. Short-chain fatty acids and colon cancer cells: the vitamin D receptor – butyrate connection. Recent Results Cancer Res. 2003;164:247–57. doi: 10.1007/978-3-642-55580-0_18. [DOI] [PubMed] [Google Scholar]
- 62.Gaschott T, Werz O, Steinmeyer A, Steinhilber D, Stein J. Butyrate-induced differentiation of Caco-2 cells is mediated by vitamin D receptor. Biochem Biophys Res Commun. 2001;288:690–6. doi: 10.1006/bbrc.2001.5832. [DOI] [PubMed] [Google Scholar]
- 63.Keum YS, Yu S, Chang PP, et al. Mechanism of action of sulforaphane: inhibition of p38 mitogen-activated protein kinase isoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006;66:8804–13. doi: 10.1158/0008-5472.CAN-05-3513. [DOI] [PubMed] [Google Scholar]
- 64.Shen G, Xu C, Chen C, Hebbar V, Kong AN. p53-independent G1 cell cycle arrest of human colon carcinoma cells HT-29 by sulforaphane is associated with induction of p21CIP1 and inhibition of expression of cyclin D1. Cancer Chemother Pharmacol. 2006;57:317–27. doi: 10.1007/s00280-005-0050-3. [DOI] [PubMed] [Google Scholar]
- 65.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- 66.Ballif BA, Blenis J. Molecular mechanisms mediating mammalian mitogen-activated protein kinase (MAPK) kinase (MEK)-MAPK cell survival signals. Cell Growth Differ. 2001;12:397–408. [PubMed] [Google Scholar]
- 67.Chang L, Karin M. Mammalian MAP kinase signalling cascades. Nature. 2001;410:37–40. doi: 10.1038/35065000. [DOI] [PubMed] [Google Scholar]
- 68.Nebreda AR, Porras A. p38 MAP kinases: beyond the stress response. Trends Biochem Sci. 2000;25:257–60. doi: 10.1016/s0968-0004(00)01595-4. [DOI] [PubMed] [Google Scholar]
- 69.Moon SK, Lee HY, Li JD, et al. Activation of a Src-dependent Raf-MEK1/2-ERK signaling pathway is required for IL-1alpha-induced upregulation of beta-defensin 2 in human middle ear epithelial cells. Biochim Biophys Acta. 2002;1590:41–51. doi: 10.1016/s0167-4889(02)00196-9. [DOI] [PubMed] [Google Scholar]
- 70.Jang BC, Lim KJ, Paik JH, et al. Up-regulation of human beta-defensin 2 by interleukin-1beta in A549 cells: involvement of PI3K, PKC, p38 MAPK, JNK, and NF-kappaB. Biochem Biophys Res Commun. 2004;320:1026–33. doi: 10.1016/j.bbrc.2004.06.049. [DOI] [PubMed] [Google Scholar]
- 71.Schlee M, Wehkamp J, Altenhoefer A, Oelschlaeger TA, Stange EF, Fellermann K. Induction of human beta-defensin 2 by the probiotic Escherichia coli Nissle 1917 is mediated through flagellin. Infect Immun. 2007;75:2399–407. doi: 10.1128/IAI.01563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harder J, Siebert R, Zhang Y, Matthiesen P, Christophers E, Schlegelberger B, Schroder JM. Mapping of the gene encoding human beta-defensin-2 (DEFB2) to chromosome region 8p22-p23.1. Genomics. 1997;46:472–5. doi: 10.1006/geno.1997.5074. [DOI] [PubMed] [Google Scholar]
- 73.Jeong WS, Kim IW, Hu R, Kong AN. Modulatory properties of various natural chemopreventive agents on the activation of NF-kappaB signaling pathway. Pharm Res. 2004;21:661–70. doi: 10.1023/b:pham.0000022413.43212.cf. [DOI] [PubMed] [Google Scholar]
- 74.Jeong WS, Kim IW, Hu R, Kong AN. Modulation of AP-1 by natural chemopreventive compounds in human colon HT-29 cancer cell line. Pharm Res. 2004;21:649–60. doi: 10.1023/b:pham.0000022412.69380.d7. [DOI] [PubMed] [Google Scholar]