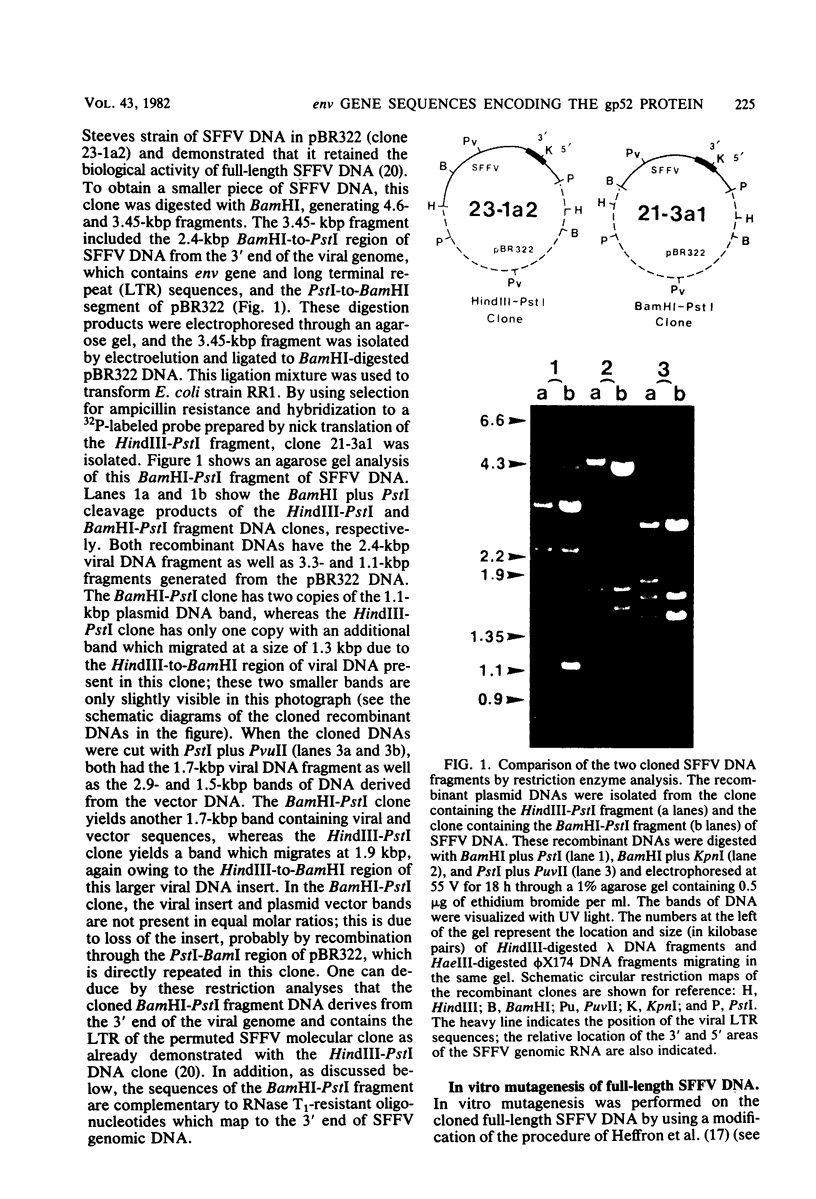
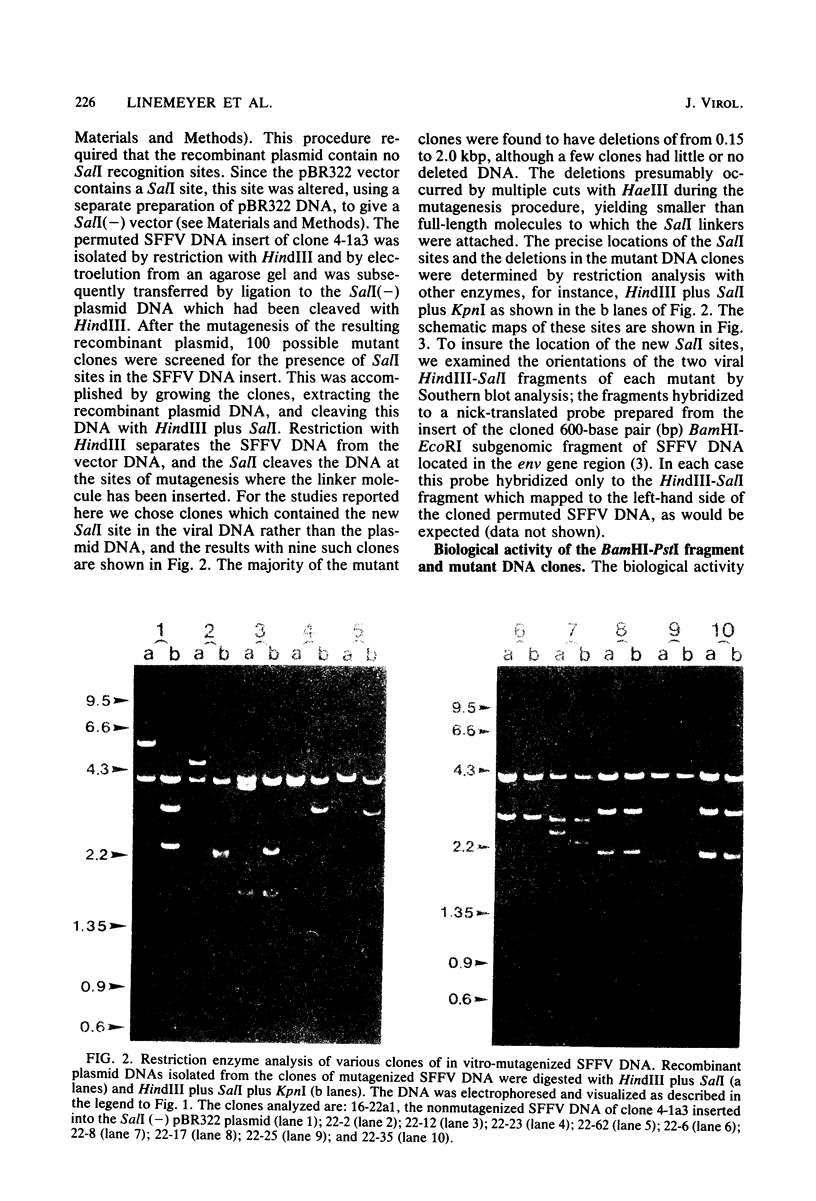
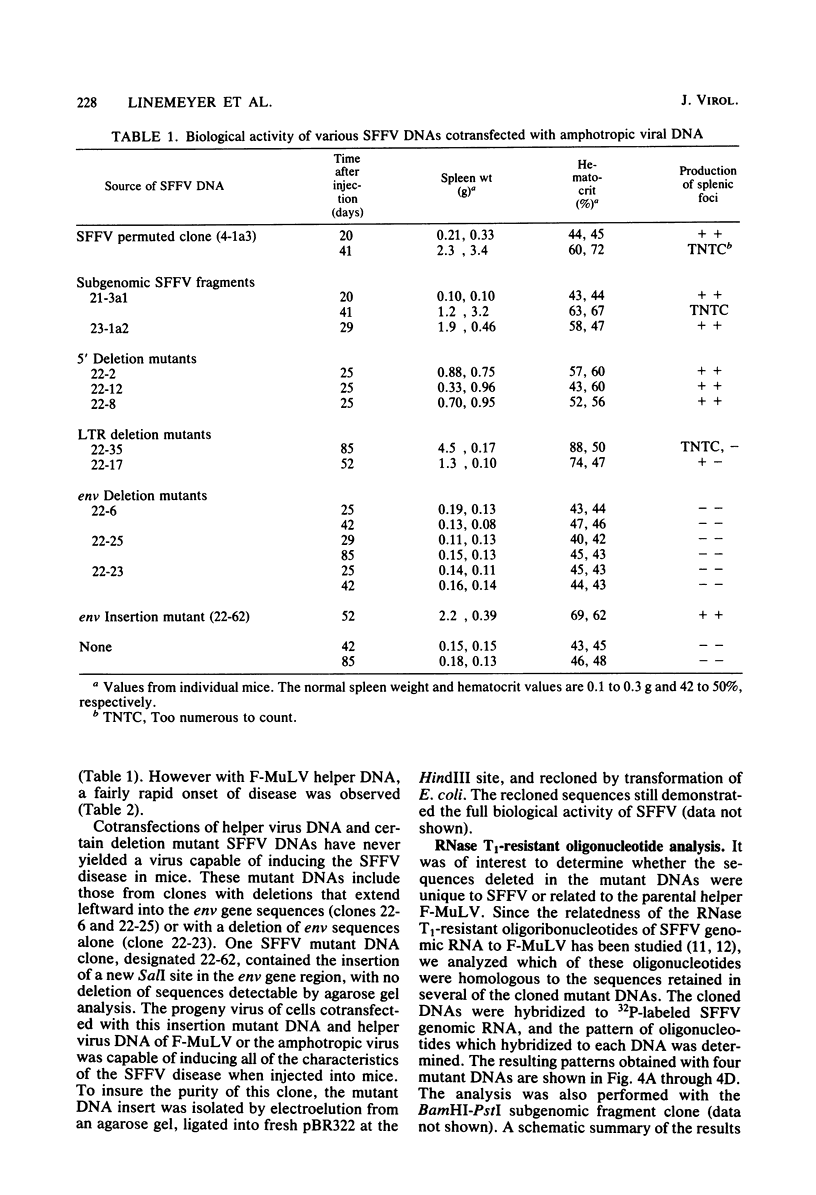
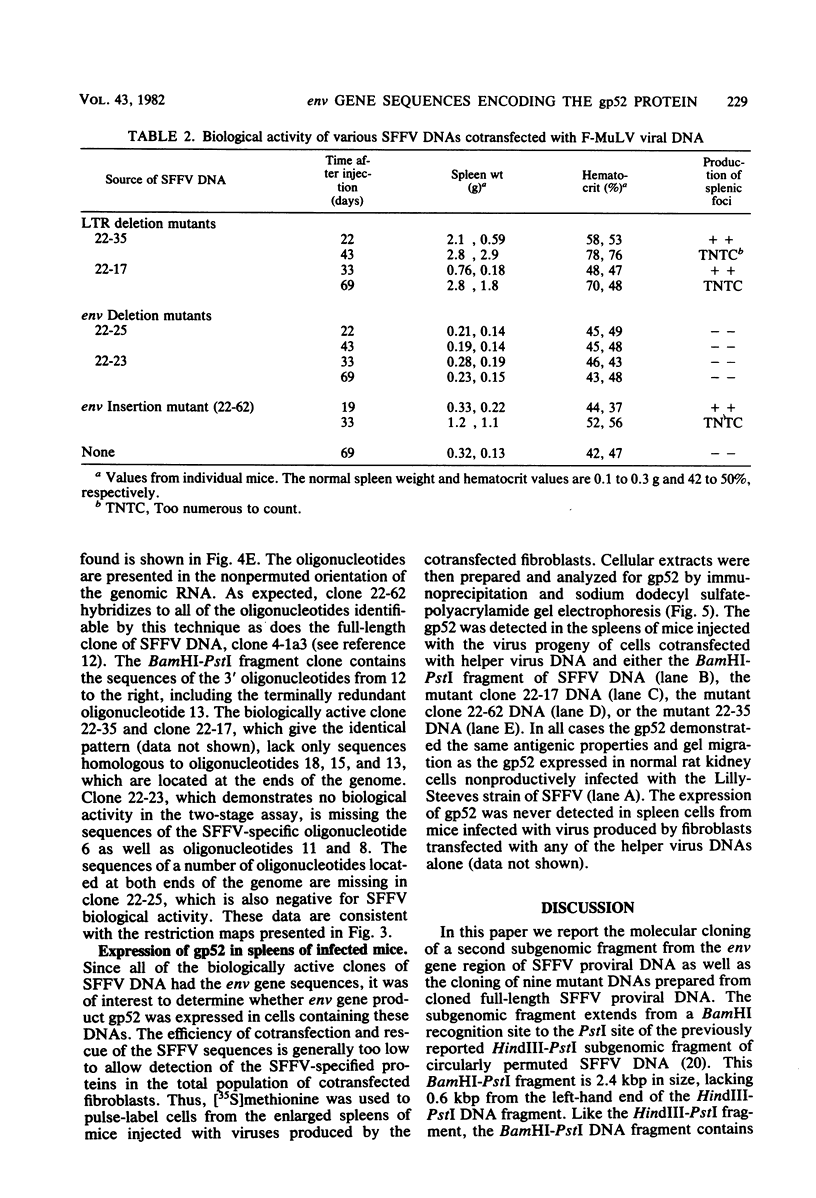
Abstract
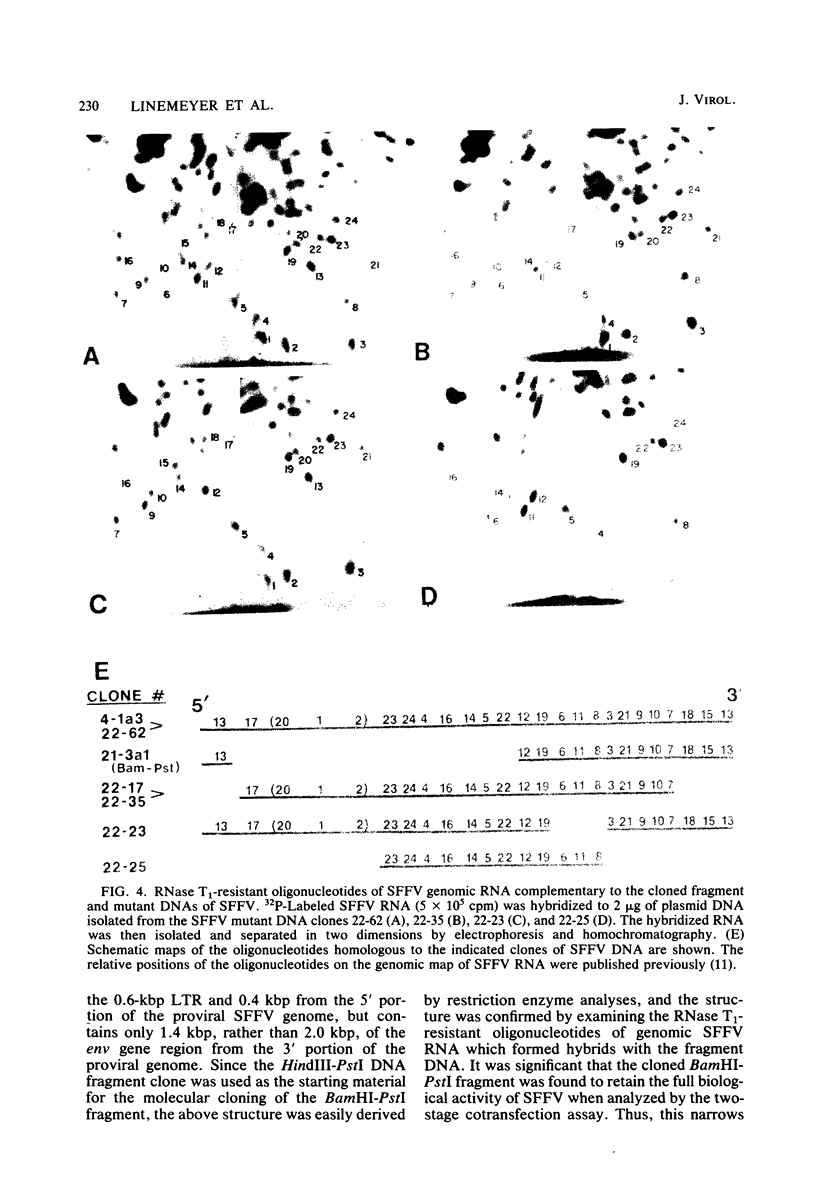
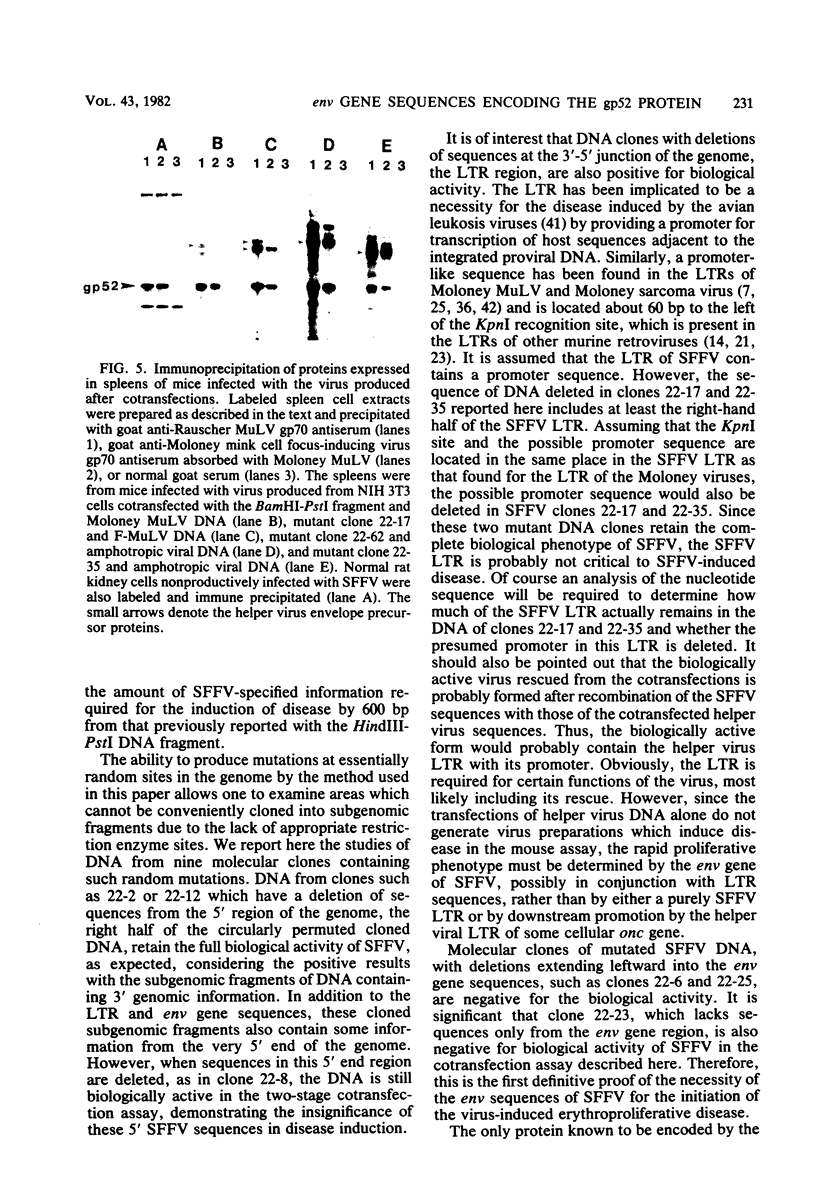
A series of insertion-deletion mutants was constructed in a molecularly cloned DNA copy of the Friend strain of spleen focus-forming virus (SFFV). The mutants were produced by inserting a synthetic oligonucleotide linker containing the recognition sequence of SalI endonuclease into several different locations of the SFFV DNA. Three classes of mutants were isolated: insertion-deletion mutants in the 5' half of the SFFV genome, in the long terminal repeat of the SFFV genome, and in the env gene of the SFFV genome. The env gene mutant has a deletion of sequences shared in common between the env gene of SFFV and the env genes of mink cell focus-inducing murine leukemia viruses. From analyses of the biological activity of the various mutants and a biologically active subgenomic SFFV DNA fragment described herein, we can deduce that the coding sequence encompassing the env gene of SFFV is required for the biological activity. This region, required for the pathogenic phenotype, cannot be larger than 1.5 kilobase pairs, a size only slightly more than that sufficient to encode the nonglycosylated precursor of the gp52 env gene product.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Troxler D. H., Scolnick E. M., Aaronson S. A. Analysis of translational products of Friend strain of spleen focus-forming virus. J Virol. 1978 Sep;27(3):826–830. doi: 10.1128/jvi.27.3.826-830.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Cloyd M. W., Linemeyer D. L., Lander M. R., Rands E., Lowy D. R. Cellular origin and role of mink cell focus-forming viruses in murine thymic lymphomas. Nature. 1982 Jan 7;295(5844):25–31. doi: 10.1038/295025a0. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Oliff A. I., Linemeyer D. L., Lander M. R., Lowy D. R. Genomes of murine leukemia viruses isolated from wild mice. J Virol. 1981 Sep;39(3):777–791. doi: 10.1128/jvi.39.3.777-791.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar R., McClements W. L., Enquist L. W., Vande Woude G. F. Nucleotide sequences of integrated Moloney sarcoma provirus long terminal repeats and their host and viral junctions. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3937–3941. doi: 10.1073/pnas.77.7.3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresler S., Ruta M., Murray M. J., Kabat D. Glycoprotein encoded by the Friend spleen focus-forming virus. J Virol. 1979 May;30(2):564–575. doi: 10.1128/jvi.30.2.564-575.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H. Transforming genes of retroviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):13–29. doi: 10.1101/sqb.1980.044.01.005. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1633–1637. doi: 10.1073/pnas.76.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L. H., Duesberg P. H., Troxler D. H., Scolnick E. M. Spleen focus-forming Friend virus: identification of genomic RNA and its relationship to helper virus RNA. J Virol. 1979 Jul;31(1):133–146. doi: 10.1128/jvi.31.1.133-146.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans L., Nunn M., Duesberg P. H., Troxler D., Scolnick E. RNAs of defective and nondefective components of Friend anemia and polycythemia virus strains identified and compared. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):823–835. doi: 10.1101/sqb.1980.044.01.087. [DOI] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Naturally occurring murine leukemia viruses in wild mice: characterization of a new "amphotropic" class. J Virol. 1976 Jul;19(1):19–25. doi: 10.1128/jvi.19.1.19-25.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayman M. J., Royer-Pokora B., Graf T. Defectiveness of avian erythroblastosis virus: synthesis of a 75K gag-related protein. Virology. 1979 Jan 15;92(1):31–45. doi: 10.1016/0042-6822(79)90212-5. [DOI] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F., Steeves R. A. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV). Virology. 1973 Oct;55(2):363–370. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- Linemeyer D. L., Ruscetti S. K., Scolnick E. M., Evans L. H., Duesberg P. H. Biological activity of the spleen focus-forming virus is encoded by a molecularly cloned subgenomic fragment of spleen focus-forming virus DNA. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1401–1405. doi: 10.1073/pnas.78.3.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirand E. A., Steeves R. A., Avila L., Grace J. T., Jr Spleen focus formation by polycythemic strains of Friend leukemia virus. Proc Soc Exp Biol Med. 1968 Mar;127(3):900–904. doi: 10.3181/00379727-127-32831. [DOI] [PubMed] [Google Scholar]
- Oliff A. I., Hager G. L., Chang E. H., Scolnick E. M., Chan H. W., Lowy D. R. Transfection of molecularly cloned Friend murine leukemia virus DNA yields a highly leukemogenic helper-independent type C virus. J Virol. 1980 Jan;33(1):475–486. doi: 10.1128/jvi.33.1.475-486.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostertag W., Vehmeyer K., Fagg B., Pragnell I. B., Paetz W., Le Bousse M. C., Smadja-Joffe F., Klein B., Jasmin C., Eisen H. Myeloproliferative virus, a cloned murine sarcoma virus with spleen focus-forming properties in adult mice. J Virol. 1980 Feb;33(2):573–582. doi: 10.1128/jvi.33.2.573-582.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy E. P., Smith M. J., Aaronson S. A. Complete nucleotide sequence and organization of the Moloney murine sarcoma virus genome. Science. 1981 Oct 23;214(4519):445–450. doi: 10.1126/science.6170110. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Ruscetti S. K., Linemeyer D., Feild J., Troxler D., Scolnick E. M. Characterization of a protein found in cells infected with the spleen focus-forming virus that shares immunological cross-reactivity with the gp70 found in mink cell focus-inducing virus particles. J Virol. 1979 Jun;30(3):787–798. doi: 10.1128/jvi.30.3.787-798.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruscetti S., Troxler D., Linemeyer D., Scolnick E. Three laboratory strains of spleen focus-forming virus: comparison of their genomes and translational products. J Virol. 1980 Jan;33(1):140–151. doi: 10.1128/jvi.33.1.140-151.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabran J. L., Hsu T. W., Yeater C., Kaji A., Mason W. S., Taylor J. M. Analysis of integrated avian RNA tumor virus DNA in transformed chicken, duck and quail fibroblasts. J Virol. 1979 Jan;29(1):170–178. doi: 10.1128/jvi.29.1.170-178.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Scolnick E. M., Siegler R. Induction of erythroid leukaemia by Harvey and Kirsten sarcoma viruses. Nature. 1975 Jul 17;256(5514):225–226. doi: 10.1038/256225a0. [DOI] [PubMed] [Google Scholar]
- Schultz A. M., Ruscetti S. K., Scolnick E. M., Oroszlan S. The env-gene of the spleen focus-forming virus lacks expression of p15(E) determinants. Virology. 1980 Dec;107(2):537–542. doi: 10.1016/0042-6822(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scholnick E. M. Identification of a sarcoma virus-coded phosphoprotein in nonproducer cells transformed by Kirsten or Harvey murine sarcoma virus. Virology. 1979 Jul 15;96(1):64–79. doi: 10.1016/0042-6822(79)90173-9. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Young H. A., Scolnick E. M. p21 of Kirsten murine sarcoma virus is thermolabile in a viral mutant temperature sensitive for the maintenance of transformation. J Virol. 1979 Aug;31(2):546–546. doi: 10.1128/jvi.31.2.546-546.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih T. Y., Williams D. R., Weeks M. O., Maryak J. M., Vass W. C., Scolnick E. M. Comparison of the genomic organization of Kirsten and Harvey sarcoma viruses. J Virol. 1978 Jul;27(1):45–55. doi: 10.1128/jvi.27.1.45-55.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinnick T. M., Lerner R. A., Sutcliffe J. G. Nucleotide sequence of Moloney murine leukaemia virus. Nature. 1981 Oct 15;293(5833):543–548. doi: 10.1038/293543a0. [DOI] [PubMed] [Google Scholar]
- Steeves R. A. Editorial: Spleen focus-forming virus in Friend and Rauscher leukemia virus preparations. J Natl Cancer Inst. 1975 Feb;54(2):289–297. doi: 10.1093/jnci/54.2.289. [DOI] [PubMed] [Google Scholar]
- Troxler D. H., Boyars J. K., Parks W. P., Scolnick E. M. Friend strain of spleen focus-forming virus: a recombinant between mouse type C ecotropic viral sequences and sequences related to xenotropic virus. J Virol. 1977 May;22(2):361–372. doi: 10.1128/jvi.22.2.361-372.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Lowy D., Howk R., Young H., Scolnick E. M. Friend strain of spleen focus-forming virus is a recombinant between ecotropic murine type C virus and the env gene region of xenotropic type C virus. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4671–4675. doi: 10.1073/pnas.74.10.4671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troxler D. H., Parks W. P., Vass W. C., Scolnick E. M. Isolation of a fibroblast nonproducer cell line containing the Friend strain of the spleen focus-forming virus. Virology. 1977 Feb;76(2):602–615. doi: 10.1016/0042-6822(77)90242-2. [DOI] [PubMed] [Google Scholar]
- Tsichlis P. N., Coffin J. M. Role of the C region in relative growth rates of endogenous and exogenous avian oncoviruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1123–1132. doi: 10.1101/sqb.1980.044.01.121. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., Goddard J. G., Berns A., Verma I. M. Structure of Moloney murine leukemia viral DNA: nucleotide sequence of the 5' long terminal repeat and adjacent cellular sequences. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3307–3311. doi: 10.1073/pnas.77.6.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waneck G. L., Rosenberg N. Abelson leukemia virus induces lymphoid and erythroid colonies in infected fetal cell cultures. Cell. 1981 Oct;26(1 Pt 1):79–89. doi: 10.1016/0092-8674(81)90035-0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y., Gamble C. L., Clark S. P., Joyner A., Shibuya T., MacDonald M. E., Mager D., Bernstein A., Mak T. W. Clonal analysis of early and late stages of erythroleukemia induced by molecular clones of integrated spleen focus-forming virus. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6893–6897. doi: 10.1073/pnas.78.11.6893. [DOI] [PMC free article] [PubMed] [Google Scholar]