Abstract
Autoreactive T cells are thought to play an essential role in the pathogenesis of multiple sclerosis (MS). We examined the stimulatory effect of human myelin basic protein (MBP) on mononuclear cell (MNC) cultures from 22 patients with MS and 22 sex-matched and age-matched healthy individuals, and related the patient responses to disease activity, as indicated by magnetic resonance imaging. The MBP induced a dose-dependent release of interferon-γ (IFN-γ), tumour necrosis factor-α (TNF-α) and interleukin-10 (IL-10) by patient-derived MNCs. The patients’ cells produced higher amounts of IFN-γ and TNF-α, and lower amounts of IL-10, than cells from healthy controls (P < 0·03 to P < 0·04). Five patients with MS and no controls, displayed MBP-induced CD4+ T-cell proliferation. These high-responders exhibited enhanced production of IL-17, IFN-γ, IL-5 and IL-4 upon challenge with MBP, as compared with the remaining patients and the healthy controls (P < 0·002 to P < 0·01). A strong correlation was found between the MBP-induced CD4+ T-cell proliferation and production of IL-17, IFN-γ, IL-5 and IL-4 (P < 0·0001 to P < 0·01) within the patient group, and the production of IL-17 and IL-5 correlated with the number of active plaques on magnetic resonance images (P = 0·04 and P = 0·007). These data suggest that autoantigen-driven CD4+ T-cell proliferation and release of IL-17 and IL-5 may be associated with disease activity. Larger studies are needed to confirm this.
Keywords: CD4+ T cells, interleukin-17, multiple sclerosis, myelin basic protein, T helper type 17 cells
Introduction
Autoreactive T cells play a central role in multiple sclerosis (MS) (reviewed in ref. 1). Their attack on the white matter of the central nervous system leads to multiple demyelinating lesions. Myelin basic protein (MBP) is considered to be a self-antigen of major importance in this process. The initiating event in the activation of autoreactive CD4+ T cells is the presentation of self-peptides by antigen-presenting cells. Traditionally, dendritic cells and macrophages, both of which are derived from monocytes, are viewed as the key players in this respect (reviewed in ref. 1). However, B cells may also play an important role as antigen-presenting cells in MS, as demonstrated in experimental autoimmune encephalomyelitis (EAE) in both rats2,3 and mice.4
Naive CD4+ T helper (Th) cells may develop into different committed helper cell subsets characterized by distinct cytokine profiles,5 interferon-γ (IFN-γ) and interleukin-4 (IL-4) being the signature cytokines of Th1 and Th2 cells, respectively. Moreover, a subset of memory CD4+ T cells (Th17 cells) producing IL-17 under the influence of IL-6, IL-23 and transforming growth factor-β (TGF-β) has been described in mice.6,7 Recently, human Th17 cells have been characterized,8 and it has been shown that human naïve T cells differentiate into IL-17-producing cells under the influence of IL-1β and IL-6, while TGF-β apparently suppresses Th17 differentiation in humans.9 On the other hand, the existence of CD4+ T-cell subsets with suppressive activity is now well established. These include inducible type 1 regulatory T cells (Tr1) producing IL-10 (reviewed in ref. 10).
The Th1 cytokines play a pathogenic role in MS by driving the recruitment and activation of immune cells or by having toxic or proapoptotic effects on oligodendrocytes (reviewed in ref. 1). A detrimental role of IFN-γ in the pathogenesis of MS was demonstrated when exacerbation of the disease was observed in seven of 18 patients treated with IFN-γ.11 Moreover, the IFN-γ responses to MBP-derived peptides in vitro correlate with clinical disease progression.12 Elevated levels of IL-17 have been demonstrated in the cerebrospinal fluid and in brain lesions of patients with MS,13 but the pathogenic role of Th17 cells and the manner in which they are activated remain unresolved. In adoptive transfer experiments, T cells producing IL-17 induce EAE, while T cells producing IFN-γ do not.7,14 Accordingly, administration of antibodies to IL-17 prevents the development of EAE and delays the onset of paralysis once EAE has been induced.7,15 The source of IL-17 production in wild-type mice with EAE appears to be almost exclusively Th17 cells, the presence of which has been demonstrated in the central nervous system as well as in the superficial lymph nodes.16
IL-10 is thought to protect against disruption of the blood–brain barrier17 and the development of EAE in mice.18–20 In proteolipid protein-stimulated cultures, a higher production of IL-10 was observed in cultures from patients whose MS was in remission, compared with cultures from either patients undergoing acute MS attacks or control subjects.21 IL-4 also plays a protective role in autoimmune disease, usually in concert with IL-10.19,22 In accordance, T-cell clones from patients with MS exacerbations have been shown to respond to challenge with proteolipid protein with decreased production of IL-4, compared with clones from patients in remission or healthy controls.21
The existence of circulating T cells reacting with MBP has been demonstrated in healthy individuals as well as in patients with MS.23–26 Some investigators have used MBP-derived peptides for activation of these T cells23–25 and others have used bovine MBP.23,24 However, pulsing of antigen-presenting cells with peptides may not represent the physiological manner of antigen presentation, and xenogenic differences may account for some of the reactivity with bovine MBP. Other studies have shown that circulating T cells from all patients with MS and from healthy controls can be stimulated for proliferation by human MBP, but these responses required addition of exogenous IL-2.26
Here, we examine the CD4+ T-cell proliferation and cytokine production upon stimulation of mononuclear cells (MNCs) from 22 patients with MS and 22 matched controls with purified human MBP under near-physiological circumstances, i.e. in the presence of relatively high concentrations of autologous serum and in the absence of exogenous cytokines. We identify a subset of patients with MS who display a distinct response to stimulation with MBP, including CD4+ T-cell proliferation and the production of IL-4, IL-5, IL-17 and relatively large amounts of IFN-γ. The CD4+ T-cell proliferation and production of IL-17, as well as IL-5, correlated with disease activity, as determined by active brain lesions detectable by magnetic resonance imaging (MRI). A mixed Th1/Th2/Th17 response to MBP seems to be a characteristic of active MS.
Materials and methods
Subjects
Blood samples were collected from 22 untreated patients with MS (Table 1; 14 women and eight men, median age 35 years, range 25–46 years). Twenty of the 22 patients were diagnosed with relapsing-remitting MS, and the remaining two patients were diagnosed with a clinically isolated syndrome. None of the patients had been treated with glucocorticoids within 4 weeks of study entry, and none of the patients had ever been treated with immunosuppressive drugs such as azathioprine, methotrexate, cyclophosphamide or mitoxantrone. Twenty-two healthy blood donors (14 women and eight men, median age 35 years, range 23–48 years) were used as controls. The patients with MS were human leucocyte antigen-typed by single specific primer–polymerase chain reaction (Genovision, Qiagen, Ballerup, Denmark). The informed consent of all participating subjects was obtained and the study was approved by the local ethics committee.
Table 1.
Patient characteristics
| Patient # | Gender | Age | Disease duration (years) | Diagnosis | MBP-induced CD4+ T-cell proliferation1 | HLA-DR2 | HLA-DQ2 | MRI3 | EDSS4 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 38 | New | RRMS | + | 4, 13 | 3, 6 | 6 | 0 |
| 2 | F | 40 | New | CIS | − | 14, 15 | 5, 6 | 2 | 1 |
| 3 | F | 39 | New | RRMS | − | 4, 15 | 3, 6 | 3 | 1 |
| 4 | M | 30 | 1 | RRMS | − | 3, 7 | 2 | 0 | 1 |
| 5 | F | 25 | 1 | RRMS | + | 15, 16 | 5, 6 | 12 | 0 |
| 6 | F | 28 | 1 | RRMS | + | 14, 15 | 5, 6 | n.d. | 2·5 |
| 7 | M | 40 | 2 | RRMS | − | 1, 15 | 5, 6 | 1 | 2 |
| 8 | F | 46 | 2 | RRMS | − | 3, 13 | 2, 6 | 0 | 2 |
| 9 | F | 29 | 2 | RRMS | − | 15 | 6 | 0 | 5 |
| 10 | F | 36 | 12 | RRMS | − | 3, 13 | 2, 3 | n.d. | 2 |
| 11 | M | 25 | 3 | RRMS | − | 4, 13 | 3, 6 | 0 | 0 |
| 12 | F | 35 | 3 | RRMS | − | 8, 15 | 4, 6 | 2 | 0 |
| 13 | F | 27 | 3 | CIS | − | 8, 15 | 4, 6 | 2 | 0 |
| 14 | F | 37 | 3 | RRMS | − | 13, 15 | 6 | 0 | 6 |
| 15 | M | 25 | 2 | RRMS | − | 4, 15 | 3, 6 | n.d. | 1·5 |
| 16 | M | 35 | 4 | RRMS | − | 3, 15 | 2, 6 | 0 | 1 |
| 17 | M | 41 | 7 | RRMS | − | 4, 7 | 2, 3 | 0 | 0 |
| 18 | M | 29 | 9 | RRMS | + | 1, 15 | 5, 6 | 2 | 2 |
| 19 | F | 44 | 9 | RRMS | − | 3, 15 | 2, 6 | 0 | 3 |
| 20 | F | 29 | 12 | RRMS | + | 3, 15 | 2, 6 | 0 | 1 |
| 21 | F | 35 | 2 | RRMS | − | 7, 15 | 2, 6 | n.d. | 0 |
| 22 | M | 39 | 18 | RRMS | − | 1, 3 | 2, 5 | 0 | 1 |
RRMS, relapsing–remitting multiple sclerosis; CIS, clinically isolated syndrome; EDSS, Expanded Disability Status Scale; n.d, not determined.
Positive CD4+ T-cell proliferation (net proliferation > 1%).
Human leucocyte antigen (HLA) typing was carried out in-house using low-resolution technology.
Number of active lesions as determined by magnetic resonance imaging.
EDSS44 was measured at the time of blood sampling.
Magnetic resonance imaging
Scanning was performed using a 3·0 Tesla whole body scanner (Trio, Siemens, Erlangen, Germany). A single three-dimensional MPRAGE (Magnetization Prepared RApid Gradient Echo) structural imaging sequence was acquired approximately 15 min after the administration of gadolinium contrast (0·2 mmol/kg body weight of Magnevist; Schering AG, Berlin, Germany). Lesions that enhance after the administration of gadolinium are active MS lesions with blood–brain barrier disruption. Enhancing lesions were detected and counted by an experienced technician using siftware developed in-house. The MRI was performed in 18 of the 22 patients. Fourteen patients were scanned up to 1 week before blood sampling. Two patients were scanned the day after blood sampling, and one was scanned 1 week after sampling. Four patients were not scanned as the result of a variety of technical problems.
Cells and serum
Cell samples were collected in lithium-heparin tubes (BD Bioscience, Brondby, Denmark), and MNCs were isolated by density centrifugation (Ficoll–Hypaque; Lymphoprep, Nycomed, Oslo, Norway). Serum was collected in dry Vacutainer tubes (BD Bioscience) and isolated after 1 hr by spinning at 814 g for 30 min. The MNCs were incubated with 5,6-carboxyfluorescein-diacetate-succinimidyl-ester (CFSE) dye (Molecular Probes, Poortgebouw, the Netherlands) at a final concentration of 2 μm, for 10 min at 37°, and were thereafter washed and resuspended in RPMI-1640 (Biological Industries, Kibbutz Beit Haemek, Israel) containing 50 μg/ml gentamycin (Gibco, Paisley, UK), 2 mm glutamine (Gibco) and 30% (v/v) serum. The labelled MNCs were distributed into 96-well Nunclon™ flat-bottomed MicroWell™ Plates (Life Technologies, Roskilde, Denmark) at 5 × 105 cells/well in a final volume of 150 μl. The cells were grown for 10 days at 37°, in 5% CO2, in the absence of antigen (negative control) or in the presence of either MBP or tetanus toxoid. Eighty-five-microlitre samples were taken at days 1 and 7 for assessment of cytokine content, and 100 μl RPMI-1640 was added at these time-points. The cells were washed in phosphate-buffered saline and assessed for CFSE content using a FACSCalibur (Becton Dickinson, Brondby, Denmark), at a band pass filter wavelength of 530 nm. CD4+ T cells were identified by staining with peridinin chlorophyll protein (PerCP) -conjugated anti-CD4.
Antigens
Purified human MBP was purchased from Insight Biotechnology Ltd. (Wembley, UK) and used for stimulation of MNC cultures at a concentration of 30 μg/ml, unless otherwise stated. A secondary, foreign control antigen, tetanus toxoid (Statens Serum Institute, Copenhagen, Denmark), was used for stimulation at 10 μg/ml.
Measurement of proliferation
Measurement of CD4+ T-cell proliferation was carried out as described.27 In brief, the CFSE content of the MNCs was measured at day 10 using a FACScalibur flow cytometer (BD Bioscience), at a band pass filter wavelength of 530 nm. CD4+ T cells were identified on the basis of staining with PerCP–anti-CD4 (BD Bioscience). Cell divisions were tracked on the basis of the CFSE content, which is halved upon each cell division, so cells with less than half the fluorescence intensity of the major peak (undivided cells) were considered to be undergoing proliferation. High-responders to MBP were defined as individuals with > 1% proliferating CD4+ T cells at this time-point, after allowance for the background proliferation occurring in the absence of stimulating antigen (< 0·5%). The interassay variability was 21%.
Measurement of cytokines in culture supernatants:
Cytokines [IL-2, IL-4, IL-5, IL-10, tumour necrosis factor-α (TNF-α) and IFN-γ] were measured by flow cytometry using the cytometric bead array Th1/Th2 kit and the corresponding software (BD Bioscience), according to the manufacturer’s protocol. The intra-assay variability was 2–5%, and the interassay variability was 5–10%. Interleukin-17 was measured using an IL-17 singleplex kit from Biosource (Invitrogen, Biosource, Taastrup, Denmark) using a Luminex 100 IS (Luminex corp., Austin, TX). The starstation version 2·0 software (Applied Cytometry Systems, Sheffield, UK) was employed for analysis. All cytokines were measured at day 1 and day 7, and the values reported here are those measured at the time of peak production, day 1 for TNF-α and IL-10, and day 7 for the remaining cytokines.
Statistics
Prism 4 (GraphPad, San Diego, CA) was used for statistical analysis. The Mann–Whitney U-test was employed to compare data from two groups or subgroups. Spearman’s rank sum correlation coefficient (Rs) was used to assess whether two parameters correlated. P-values < 0·05 were considered significant.
Results
MBP-induced production of IFN-γ, TNF-α, IL-10 and IL-2
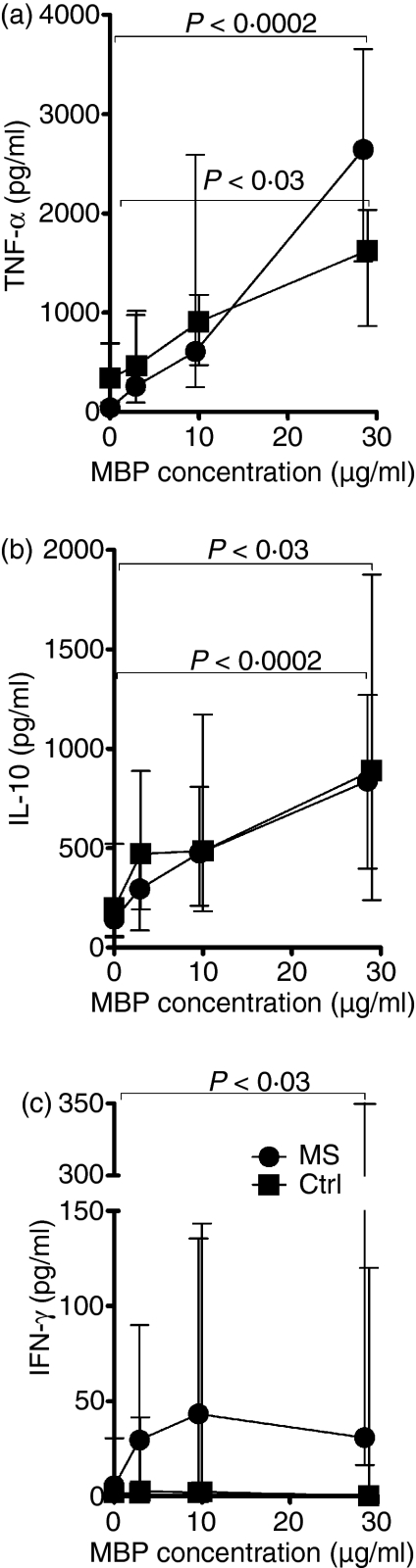
Stimulation with MBP elicited dose-dependent IL-10, TNF-α and IFN-γ responses in MNC cultures from patients with MS and healthy individuals (Fig. 1).
Figure 1.
Dose-dependent cytokine responses to myelin basic protein (MBP). Mononuclear cells from 13 patients with multiple sclerosis (MS; circles) and six healthy controls (Ctrl; squares) were grown in medium containing autologous serum (30% v/v) and increasing concentrations of purified human MBP. The levels of tumour necrosis factor-α (a) and interleukin-10 (b) in the culture supernatants after 1 day of incubation, and the levels of interferon-γ after 7 days of incubation (c) are shown as median and interquartile ranges.
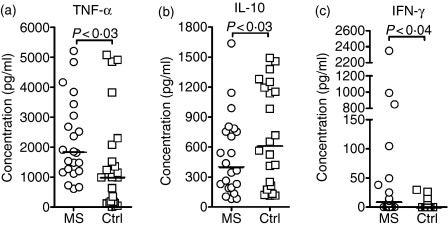
The MBP-elicited production of TNF-α and IL-10 was seen in all cultures after 1 day of stimulation (Fig. 2). Production of TNF-α was higher in the patient group than in the control group (Fig. 2a), while the opposite was true for IL-10 (Fig. 2b), in accordance with these cytokines' supposed detrimental and protective effects in MS, respectively.1
Figure 2.
Myelin basic protein (MBP)-elicited cytokine production by patient and control cells. Mononuclear cells from 22 untreated patients with multiple sclerosis [MS; 20 with relapsing–remitting MS (RRMS); two with clinically isolated syndrome (CIS)] and 22 healthy controls (Ctrl) were grown in the presence of human MBP (30 μg/ml) or no antigen. The resulting net production of tumour necrosis factor-α (TNF-α; a) and interleukin-10 (IL-10; b) at day 1, and interferon-γ at day 7 (IFN-γ; c), after subtraction of the background production in the absence of stimulating antigen is shown. The backround production was similar in patients and controls, < 34 pg/ml for TNF-α, < 60 pg/ml for IL-10 and < 3 pg/ml for IFN-γ. Horizontal bars represent median values.
When it occurred, MBP-elicited IFN-γ production increased during the observation period and was detectable (> 10 pg/ml) at day 7 in 11 of 22 MNC cultures derived from patients with MS, and in five of 22 MNC cultures from healthy controls (Fig. 2c). Overall, the IFN-γ responses were significantly higher in MNC cultures from patients with MS than in cultures from healthy controls.
Detectable production of IL-2 was observed at day 7 in three cultures from patients only (data not shown).
MBP-elicited CD4+ T-cell proliferation and production of IL-17, IFN-γ, IL-4 and IL-5 by a subgroup of high-responding patients
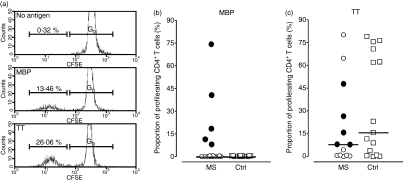
We identified five patients with MS, referred to as high-responders (HRs) in the following text, in whom CD4+ T cells exhibited a proliferative response to challenge with MBP (Table 1, Fig. 3a,b). None of the healthy controls exhibited a similar response. After 10 days of expansion, the MBP-reactive CD4+ T-cell subset in the cultures derived from the HRs had undergone 3·6–6·3 divisions, and their progeny constituted 8–74% of the total CD4+ T-cell population (Fig. 3b). The enhanced response of CD4+ T cells from the HRs to MBP did not appear to be the result of a generalized T-cell hyperreactivity because their response to a control antigen, tetanus toxoid, tended to be lower, rather than higher, than that of healthy controls (Fig. 3c).
Figure 3.
Myelin basic protein (MBP)-elicited CD4+ T-cell proliferation. Carboxyfluorescein-diacetate-succinimidyl-ester (CFSE)-labelled mononuclear cells were incubated for 10 days with human MBP (30 μg/ml), or no antigen, and divisions of CD4+ T cells were tracked by flow cytometry. (a) Histograms showing undivided CD4+ T cells (G0) and cells having undergone more than one division, as identified by a reduction in fluorescence intensity. The upper panel shows unstimulated cells, the middle panel shows cells stimulated with MBP, and the lower panel shows tetanus toxoid (TT)-stimulated cells. (b) The proportion of divided CD4+ T cells from 22 patients with multiple sclerosis (MS) and 22 sex-matched and age-matched healthy controls (Ctrl) after 10 days of stimulation with MBP is shown. (c) The corresponding proportion of divided CD4+ T cells from 13 randomly selected patients with multiple sclerosis (MS) and 15 healthy controls (Ctrl) after 10 days of stimulation with a control antigen, TT. Closed circles in (b) and (c) represent the five high-responders to MBP. In all cases, the proportion of cells in division was less than 0.5% in the absence of antigenic stimulation.
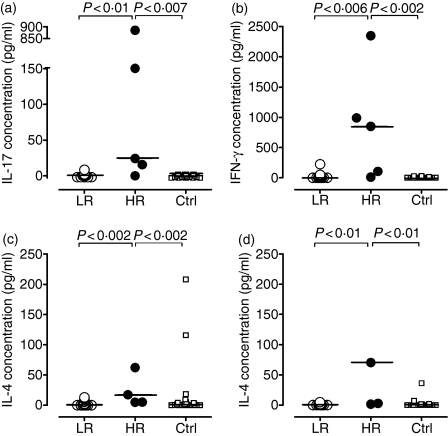
Detectable MBP-elicited secretion of IL-17 (> 10 pg/ml per well) was observed in four of the five cultures derived from HRs, but not in any cultures derived from the remaining patients, or from the healthy controls (Fig. 4a). Likewise, the HRs exhibited significantly higher production of IFN-γ, IL-4 and IL-5 than the remaining patients and the healthy controls (Fig. 4b–d). The MBP-elicited production of IL-17, IFN-γ, IL-5 and IL-4 all correlated with the MBP-induced CD4+ T-cell proliferation (P < 0·001, P < 0·002, P < 0·0001 and P < 0·01, respectively; data not shown).
Figure 4.
Myelin basic protein (MBP)-induced production of interleukin-17 (IL-17), interferon-γ (IFN-γ), IL-4 and IL-5. Mononuclear cells (MNCs) from patients with multiple sclerosis (MS), 17 low-responders (LR) and five high-responders (HR), and from 22 healthy, sex-matched and age-matched controls (Ctrl) were incubated with MBP for 7 days. The resulting net production of IL-17 (a), IFN-γ (b), IL-4 (c) and IL-5 (d), after subtraction of the background production in the absence of stimulating antigen, is shown. The background production was similar in the patient and control groups, being 0 pg/ml for IL-17, < 3 pg/ml for IFN-γ, < 3 pg/ml for IL-4 and < 2 pg/ml for IL-5.
Association between clinical activity and MBP-induced cytokine production
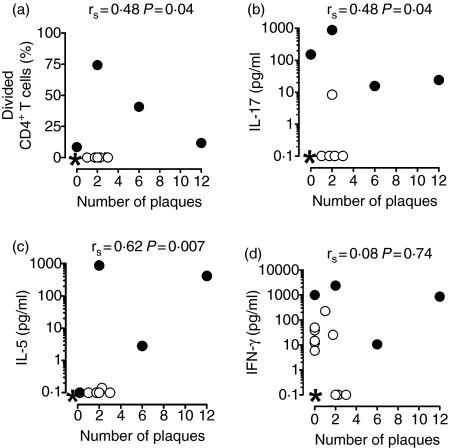
Within 1 week before or after blood sampling, the disease activity of 18 of the 22 patients with MS was assessed by MRI. Eight of these patients exhibited active brain lesions (Table 1). The number of active lesions correlated with both CD4+ T-cell proliferation (Fig. 5a) and the MBP-induced production of IL-17 (Fig. 5b) and IL-5 (Fig. 5c), which was characteristic of HRs, but somewhat surprisingly it was not correlated with the production of IFN-γ (Fig. 5d). No correlations were found for IL-4 (P < 0·77) or for the two cytokines produced by all individuals, TNF-α (P < 0·29) and IL-10 (P < 0·35) (data not shown). The data suggest that IL-17 and IL-5 responses are associated with active disease, but given the low number of patients with strong cytokine responses and the consequently low levels of significance, the results should be interpreted with caution.
Figure 5.
Correlation between myelin basic protein (MBP)-induced T-cell proliferation, interleukin-17 (IL-17) production and disease activity. Mononuclear cell (MNC) cultures from 18 patients with multiple sclerosis (MS) were incubated with human MBP for 10 days. The proportion of divided CD4+ T cells at day 10 (a), and the content of IL-17 (b), IL-5 (c), and interferon-γ (IFN-γ) (d) in the culture supernatants at day 7 correlated with the number of brain plaques, as assessed by magnetic resonance imaging. Closed circles represent the high-responders who were scanned. *Represents multiple values of 0, 0 [nine in (a–c), six in (d)].
Influence of tissue types on the observed responses to MBP
We found no difference in human leucocyte antigen phenotypes between HRs and the remaining patients without MBP-induced CD4+ T-cell proliferation (Table 1).
Discussion
T cells are known to play a critical role in the pathogenesis of MS and are found in active lesions in the central nervous system.28 Here we have compared T-cell responses to MBP, in terms of CD4+ T-cell proliferation and the production of a series of cytokines; in MNC cultures derived from patients with MS and from sex-matched and age-matched healthy individuals.
The CD4+ T-cell proliferation induced by MBP was observed in none of the cultures derived from healthy controls, but was found in the cultures of five patients (approximately 23%). Imaging was performed in four of these patients, and the finding of active lesions on MRI in three of them indicates that MBP-elicited proliferation of peripheral CD4+ T cells is a marker of active disease. Using a similar assay, Crawford and colleagues found CD4+ T-cell proliferation induced by bovine MBP in MNC cultures from as many as 63% of healthy individuals;24 this compares with none in the present study. This discrepancy is probably caused by xenogenic differences in antigenicity between bovine MBP and the human MBP, purified from brain tissue, that was used in the present study.
Pette and colleagues isolated MBP-reactive T cells from all tested patients and controls upon stimulation with purified human MBP (30 μg/ml as in the present study) in the presence of exogenous IL-2.26 The MBP-reactive T-cell lines isolated from patients with MS and from healthy controls were CD4+ CD8− T cells and mainly CD4+ CD8+ T cells, respectively. A likely explanation for the discrepancy between their results and ours is that we did not add IL-2 to the MNC cultures. The production of IL-2 was low in our experiments: MBP-stimulated cultures from just three patients and from no controls contained detectable levels (detection limit ∼ 15 pg/ml), and only at day 7. This may also be a result of rapid consumption of IL-2, however. Although MBP-specific CD4+ T cells may exist in all individuals, our data indicate that the balance between their activation and suppression is tipped in favour of activation in a subset of patients with active disease.
Cells from both patients and controls responded to MBP with the production of TNF-α and IL-10, but the patients showed increased production of the proinflammatory cytokine TNF-α, in accordance with previous findings of elevated numbers of blood cells producing this cytokine in patients with MS.29,30 By contrast, cells from the healthy controls produced higher amounts of IL-10 than cells from patients with MS. Indeed, de Jong and colleagues found that genetically determined low production of IL-10 and high production of TNF were associated with a marked increased risk of developing typical MS.31,32 Using proteolipid protein as stimulating antigen, Correale and colleagues found that T-cell clones isolated from patients in remission secreted more IL-10 than clones from patients with acute attacks, supporting a protective role for IL-10.21 However, they found no difference between the IL-10 production by clones from patients with acute attacks, on the one hand, and clones from healthy controls, on the other. Our data suggest that MNCs from healthy individuals are generally capable of mounting a higher production of IL-10 upon challenge with MBP than cells from patients with MS. We have not determined the source of the TNF-α and IL-10 production in this study, but it is likely that their production by T cells, monocytes and B cells is orchestrated by Th133 and Tr1 cells,10 respectively. The rapid onset of IL-10 production observed here suggests that MBP-experienced Tr1 or B cells exist in the periphery, or that IL-10 production by monocytes may be rapidly initiated by alternative T-cell signals. Interleukin-10 is thought to inhibit the production of Th1 cytokines22 via an effect on costimulatory molecules on antigen-presenting cells,34,35 and to protect against disruption of the blood–brain barrier.17
Interferon-γ stimulates a variety of cells of the immune system, including T cells and dendritic cells,36 and may be detrimental in MS by enhancing endothelial permeability17,37 and by being toxic for oligodendrocytes.1 The detrimental effect of IFN-γ in MS has clearly been demonstrated in a clinical trial.11 Moldovan and associates found that MNCs from patients with MS as well as from controls responded to MBP-peptides with the production of IFN-γ, but that a twofold higher production occurred in patients.12 Moreover, the increased IFN-γ production correlated with worsening disability.12 In the present study, the MS group, as a whole, exhibited significantly higher MBP-induced production of IFN-γ than the healthy controls. Detectable IFN-γ production was elicited by intact MBP in 11 out of 22 patients with MS and four of 22 controls, but was considerably increased in patients with concomitant CD4+ T-cell proliferation. The patients with CD4+ T-cell responses also showed significantly higher IL-4, IL-5 and, notably, IL-17 production in the late-phase response to MBP (i.e. after 7 days) than those of the remaining patients, and of the healthy controls, i.e. a mixed Th1/Th2/Th17 response.
Importantly, the MBP-elicited CD4+ T-cell proliferation, as well as the production of IL-17 and IL-5, correlated with active disease, as shown by active brain lesions on MRI. The association between IL-17 production and active brain lesions strongly supports an important role for IL-17 in the pathogenesis of MS, as previously suggested by the findings that IL-17 induces EAE in animals,38 and that the level of IL-17 is elevated in the cerebrospinal fluid and in brain lesions of patients with MS.13 Our data suggest that MBP-reactive Th17 cells are present in the circulation of a subset of patients with MS but not in healthy donors. The mechanisms by which these autoreactive cells escape central and/or peripheral regulation remain to be elucidated. While both CD4+ and CD8+ T cells may have contributed to the production of IFN-γ observed here, it is likely that IL-17 was produced almost exclusively by CD4+ T cells, as it is in mice.16
We only observed IL-5 production in patients with two or more active brain lesions. It is possible that IL-5 plays a protective role in MS because the levels are increased during treatment with IFN-β and, especially, with glatiramer acetate.39–41 The production of IL-5 by some of the patients with high cytokine responses to MBP may therefore reflect a protective, compensatory mechanism, but further studies with a larger number of patients are required to clarify this.
The IL-4 production by T cells from the HRs accords with the finding of high numbers of IL-4-producing cells in blood and cerebrospinal fluid of patients with MS,42 and with the increases in the levels of messenger RNA for IL-4 (along with IL-2, IL-4, IL-6, IFN-γ and IL-1α) in spinal cord samples from mice during the acute phase of EAE.43 By contrast, a decrease in the IL-4 production by proteolipid protein-stimulated T-cell clones from patients with MS during acute attacks has also been reported.21 However, as a result of rapid consumption, the levels of IL-4 in culture supernatants should be interpreted with caution.
In summary, we have demonstrated that MBP induces increased production of TNF-α and decreased production of IL-10 by circulating MNCs from patients with MS compared with MNCs from healthy individuals. We found a strong correlation between MBP-elicited CD4+ T-cell proliferation and production of IL-17, both of which occurred in a subgroup of high-responding patients. Notably, the proliferative T-cell responses as well as the production of IL-17 and IL-5 induced by MBP correlated with disease activity in vivo, as measured by active brain lesions on MRI scans. These data suggest that a mixed Th17/Th2 response to MBP plays an important role in active relapsing–remitting MS. Studies in larger cohorts of patients are needed to confirm this.
Acknowledgments
C.J.H. received funding from The Lundbeck Foundation, The Warwara Larsen Foundation, The Danish Multiple Sclerosis Society, Former Chief Manager Leo Nielsen and Spouse Karen Margrethe Nielsens Grant for basic medical science, Merchant L.F. Foght's Foundation, and the Augustinus Foundation. We thank W. Hansen for expert technical assistance.
Abbreviations
- CD
cluster of differentiation
- CFSE
5,6-carboxyfluorescein-diacetate-succinimidyl-ester
- EAE
experimental autoimmune encephalomyelitis
- EDSS
Expanded Disability Status Scale
- HR
high-responder
- IFN-γ
interferon-γ
- IL
interleukin
- MBP
myelin basic protein
- MNC
mononuclear cell
- MRI
magnetic resonance imaging
- MS
multiple sclerosis
- PLP
proteolipid protein
- TGF-β
transforming growth factor-β
- TNF
tumour necrosis factor
- Tr1
type 1 regulatory cells
References
- 1.Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 2.Gausas J, Paterson PY, Day ED, Dal Canto MC. Intact B-cell activity is essential for complete expression of experimental allergic encephalomyelitis in Lewis rats. Cell Immunol. 1982;72:360–6. doi: 10.1016/0008-8749(82)90484-1. [DOI] [PubMed] [Google Scholar]
- 3.Willenborg DO, Prowse SJ. Immunoglobulin-deficient rats fail to develop experimental allergic encephalomyelitis. J Neuroimmunol. 1983;5:99–109. doi: 10.1016/0165-5728(83)90001-2. [DOI] [PubMed] [Google Scholar]
- 4.Myers KJ, Sprent J, Dougherty JP, Ron Y. Synergy between encephalitogenic T cells and myelin basic protein-specific antibodies in the induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 1992;41:1–8. doi: 10.1016/0165-5728(92)90188-q. [DOI] [PubMed] [Google Scholar]
- 5.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- 6.Aggarwal S, Ghilardi N, Xie MH, de Sauvage FJ, Gurney AL. Interleukin-23 promotes a distinct CD4 T cell activation state characterized by the production of interleukin-17. J Biol Chem. 2003;278:1910–4. doi: 10.1074/jbc.M207577200. [DOI] [PubMed] [Google Scholar]
- 7.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Acosta-Rodriguez EV, Napolitani G, Lanzavecchia A, Sallusto F. Interleukins 1beta and 6 but not transforming growth factor-beta are essential for the differentiation of interleukin 17-producing human T helper cells. Nat Immunol. 2007;8:942–9. doi: 10.1038/ni1496. [DOI] [PubMed] [Google Scholar]
- 10.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 11.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–5. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 12.Moldovan IR, Rudick RA, Cotleur AC, Born SE, Lee JC, Karafa MT, Pelfrey CM. Interferon gamma responses to myelin peptides in multiple sclerosis correlate with a new clinical measure of disease progression. J Neuroimmunol. 2003;141:132–40. doi: 10.1016/s0165-5728(03)00221-2. [DOI] [PubMed] [Google Scholar]
- 13.Lock C, Hermans G, Pedotti R, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–8. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 14.Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–45. doi: 10.1038/nm1551. [DOI] [PubMed] [Google Scholar]
- 15.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–41. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss HA, Millward JM, Owens T. CD8+ T cells in inflammatory demyelinating disease. J Neuroimmunol. 2007;191:79–85. doi: 10.1016/j.jneuroim.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Minagar A, Alexander JS. Blood–brain barrier disruption in multiple sclerosis. Mult Scler. 2003;9:540–9. doi: 10.1191/1352458503ms965oa. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Koldzic DN, Izikson L, Reddy J, Nazareno RF, Sakaguchi S, Kuchroo VK, Weiner HL. IL-10 is involved in the suppression of experimental autoimmune encephalomyelitis by CD25+CD4+ regulatory T cells. Int Immunol. 2004;16:249–56. doi: 10.1093/intimm/dxh029. [DOI] [PubMed] [Google Scholar]
- 19.Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–306. [PubMed] [Google Scholar]
- 20.Samoilova EB, Horton JL, Chen Y. Acceleration of experimental autoimmune encephalomyelitis in interleukin-10-deficient mice: roles of interleukin-10 in disease progression and recovery. Cell Immunol. 1998;188:118–24. doi: 10.1006/cimm.1998.1365. [DOI] [PubMed] [Google Scholar]
- 21.Correale J, Gilmore W, McMillan M, Li S, McCarthy K, Le T, Weiner LP. Patterns of cytokine secretion by autoreactive proteolipid protein-specific T cell clones during the course of multiple sclerosis. J Immunol. 1995;154:2959–68. [PubMed] [Google Scholar]
- 22.Skapenko A, Niedobitek GU, Kalden JR, Lipsky PE, Schulze-Koops H. Generation and regulation of human Th1-biased immune responses in vivo: a critical role for IL-4 and IL-10. J Immunol. 2004;172:6427–34. doi: 10.4049/jimmunol.172.10.6427. [DOI] [PubMed] [Google Scholar]
- 23.Arbour N, Holz A, Sipe JC, Naniche D, Romine JS, Zyroff J, Oldstone MB. A new approach for evaluating antigen-specific T cell responses to myelin antigens during the course of multiple sclerosis. J Neuroimmunol. 2003;137:197–209. doi: 10.1016/s0165-5728(03)00080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crawford MP, Yan SX, Ortega SB, et al. High prevalence of autoreactive, neuroantigen-specific CD8+ T cells in multiple sclerosis revealed by novel flow cytometric assay. Blood. 2004;103:4222–31. doi: 10.1182/blood-2003-11-4025. [DOI] [PubMed] [Google Scholar]
- 25.Ota K, Matsui M, Milford EL, Mackin GA, Weiner HL, Hafler DA. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature. 1990;346:183–7. doi: 10.1038/346183a0. [DOI] [PubMed] [Google Scholar]
- 26.Pette M, Fujita K, Kitze B, Whitaker JN, Albert E, Kappos L, Wekerle H. Myelin basic protein-specific T lymphocyte lines from MS patients and healthy individuals. Neurology. 1990;40:1770–6. doi: 10.1212/wnl.40.11.1770. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen CH, Leslie RG, Jepsen BS, Kazatchkine MD, Kaveri SV, Fischer E. Natural autoantibodies and complement promote the uptake of a self antigen, human thyroglobulin, by B cells and the proliferation of thyroglobulin-reactive CD4(+) T cells in healthy individuals. Eur J Immunol. 2001;31:2660–8. doi: 10.1002/1521-4141(200109)31:9<2660::aid-immu2660>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 28.Traugott U, Reinherz EL, Raine CS. Multiple sclerosis: distribution of T cell subsets within active chronic lesions. Science. 1983;219:308–10. doi: 10.1126/science.6217550. [DOI] [PubMed] [Google Scholar]
- 29.Ozenci V, Kouwenhoven M, Huang YM, Kivisakk P, Link H. Multiple sclerosis is associated with an imbalance between tumour necrosis factor-alpha (TNF-alpha)- and IL-10-secreting blood cells that is corrected by interferon-beta (IFN-beta) treatment. Clin Exp Immunol. 2000;120:147–53. doi: 10.1046/j.1365-2249.2000.01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hohnoki K, Inoue A, Koh CS. Elevated serum levels of IFN-gamma, IL-4 and TNF-alpha/unelevated serum levels of IL-10 in patients with demyelinating diseases during the acute stage. J Neuroimmunol. 1998;87:27–32. doi: 10.1016/s0165-5728(98)00053-8. [DOI] [PubMed] [Google Scholar]
- 31.de Jong BA, Schrijver HM, Huizinga TW, et al. Innate production of interleukin-10 and tumor necrosis factor affects the risk of multiple sclerosis. Ann Neurol. 2000;48:641–6. doi: 10.1002/1531-8249(200010)48:4<641::aid-ana11>3.3.co;2-q. [DOI] [PubMed] [Google Scholar]
- 32.de Jong BA, Westendorp RG, Eskdale J, Uitdehaag BM, Huizinga TW. Frequency of functional interleukin-10 promoter polymorphism is different between relapse-onset and primary progressive multiple sclerosis. Hum Immunol. 2002;63:281–5. doi: 10.1016/s0198-8859(02)00369-5. [DOI] [PubMed] [Google Scholar]
- 33.Burger D, Dayer JM. The role of human T-lymphocyte–monocyte contact in inflammation and tissue destruction. Arthritis Res. 2002;4(Suppl. 3):S169–76. doi: 10.1186/ar558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. J Immunol. 1993;151:1224–34. [PubMed] [Google Scholar]
- 35.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O'Garra A. IL-10 inhibits cytokine production by activated macrophages. J Immunol. 1991;147:3815–22. [PubMed] [Google Scholar]
- 36.Pestka S, Krause CD, Walter MR. Interferons, interferon-like cytokines, and their receptors. Immunol Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 37.Minagar A, Long A, Ma T, et al. Interferon (IFN)-beta 1a and IFN-beta 1b block IFN-gamma-induced disintegration of endothelial junction integrity and barrier. Endothelium. 2003;10:299–307. doi: 10.1080/10623320390272299. [DOI] [PubMed] [Google Scholar]
- 38.Steinman L. Optic neuritis, a new variant of experimental encephalomyelitis, a durable model for all seasons, now in its seventieth year. J Exp Med. 2003;197:1065–71. doi: 10.1084/jem.20030320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wiesemann E, Klatt J, Sonmez D, Blasczyk R, Heidenreich F, Windhagen A. Glatiramer acetate (GA) induces IL-13/IL-5 secretion in naive T cells. J Neuroimmunol. 2001;119:137–44. doi: 10.1016/s0165-5728(01)00379-4. [DOI] [PubMed] [Google Scholar]
- 40.Chen M, Gran B, Costello K, Johnson K, Martin R, Dhib-Jalbut S. Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS. Mult Scler. 2001;7:209–19. doi: 10.1177/135245850100700401. [DOI] [PubMed] [Google Scholar]
- 41.Sanna A, Fois ML, Arru G, Huang YM, Link H, Pugliatti M, Rosati G, Sotgiu S. Glatiramer acetate reduces lymphocyte proliferation and enhances IL-5 and IL-13 production through modulation of monocyte-derived dendritic cells in multiple sclerosis. Clin Exp Immunol. 2006;143:357–62. doi: 10.1111/j.1365-2249.2006.02997.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Link J, Soderstrom M, Olsson T, Hojeberg B, Ljungdahl A, Link H. Increased transforming growth factor-beta, interleukin-4, and interferon-gamma in multiple sclerosis. Ann Neurol. 1994;36:379–86. doi: 10.1002/ana.410360309. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–505. [PubMed] [Google Scholar]
- 44.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]