Abstract
Trehalose preserves lipid bilayers during dehydration and rehydration by replacing water to form hydrogen bonds between its own OH groups and lipid headgroups. We compare the lipid conformation and dynamics between trehalose-protected lyophilized membranes and hydrated membranes, to assess the suitability of the trehalose-containing membrane as a matrix for membrane protein structure determination. 31P spectra indicate that the lipid headgroup of trehalose-protected dry POPC membrane (TRE-POPC) have an effective phase transition temperature that is ~50 K higher than that of the hydrated POPC membrane. In contrast, the acyl chains have similar transition temperatures in the two membranes. Intramolecular lipid 13C’-31P distances are the same in TRE-POPC and crystalline POPC, indicating that the lipid headgroup and glycerol backbone conformation is unaffected by trehalose incorporation. Intermolecular 13C-31P distances between a membrane peptide and the lipid headgroups are 10% longer in the hydrated membrane at 226 K than in the trehalose-protected dry membrane at 253 K. This is attributed to residual motions in the hydrated membrane, manifested by the reduced 31P chemical shift anisotropy, even at the low temperature of 226 K. Thus, trehalose lyoprotection facilitates the study of membrane protein structure by allowing experiments to be conducted at higher temperatures than possible with the hydrated membranes.
Keywords: trehalose, lipid bilayers, 13C-31P distances, membrane peptide structure, solid-state NMR
1. Introduction
Trehalose (TRE), a non-reducing disaccharide of glucose, is known to stabilize lipid bilayers and proteins during dehydration and rehydration. It is found at concentrations as much as 20 wt% of the dry weight of anhydrobiotic organisms [1]. Generally, the protection efficiency is proportional to the concentration of trehalose, but the full protection can be achieved when the concentration of trehalose reaches a threshold, which is ~100 mM in the preservation of proteins and 0.3 g/g of lipid in the preservation of phospholipid bilayers during drying [2, 3]. The mechanism of trehalose stabilization of cell membranes is proposed to be a depression of the gel to liquid-crystalline (LC) phase transition temperature (Tm) of the dry membrane, so that membrane disruption, which normally occurs during phase transition, is prevented during rehydration [4]. On a molecular level, trehalose is believed to replace the hydrogen bonds between water and the lipid phosphate group with hydrogen bonds between its own OH groups and the phosphate, thus maintaining membrane integrity in the absence of water. This “lyoprotecting” property of trehalose can be, and indeed has been [5], exploited in solid-state NMR studies of the depth of insertion of membrane proteins. The depth information can be obtained from distance measurements between 13C labels in the protein and the 31P spin of the lipid headgroup. This requires the motions that are abundant in hydrated lipid bilayers to be frozen to yield rigid-limit distance-dependent dipolar couplings. Freezing lipid motions requires temperatures of at least 40 K below Tm, often over an extended period of time to allow signal averaging of the lipid-diluted peptides. These are challenging conditions for NMR experiments. We show here that trehalose-containing dry lipid membranes preserve the lipid bilayer structure while removing the headgroup and glycerol backbone motions at higher temperatures than the hydrated membranes, thus facilitating the measurement of protein-lipid distances. While the phase properties of trehalose-DPPC mixtures had been investigated by NMR before [6, 7], a comparison of the conformation of the hydrated and trehalose-containing membranes for protein structure determination has not been reported.
2. Results and discussion
We first characterize the dynamic structure of trehalose-containing lipid membranes by static 31P and 2H NMR. POPC is chosen as a model system because its chain lengths (16 and 18 carbons) are dominant in biological membranes and the choline headgroup is common in eukaryotic cell membranes. Hydrated POPC bilayers have a relatively low Tm of 271 K, thus necessitating low temperatures of ~220 K or below to freeze the lipid motion. We compared the mobility of the lipid headgroup and the acyl chain between the hydrated POPC and trehalose-protected lyophilized POPC membrane (TRE-POPC) at various temperatures. Fig. 1 shows the 31P spectra of hydrated POPC bilayers with 35 wt% water (a) and lyophilized POPC membrane containing 20 wt% trehalose (b). The transition temperature for the headgroup region of the TRE-POPC membrane is ~323 K, which is ~50 K higher than that of the hydrated POPC membrane. At 273 K, the 31P spectrum of hydrated POPC (a) shows a small chemical shift anisotropy (CSA), δ = δZZ-δiso, of 30 ppm and an asymmetry parameter η of 0, characteristic of uniaxially mobile lipids in Lα-phase membrane. In contrast, TRE-POPC reaches a similarly narrow CSA and uniaxial lineshape only at ~323 K. To obtain the rigid-limit 31P CSA of ~110 ppm [8], a low temperature of 233 K is required for hydrated POPC while T = 263 K is sufficient for TRE-POPC. Fig. 1(c, d) plots 31P CSA δ and η as a function of temperature for the two samples. Compared to the hydrated POPC membrane, trehalose increases the lipid-headgroup phase transition temperature by ~50 K. This does not contradict the fact that trehalose suppresses the Tm of dry POPC, which is ~340 K [9]. Based on the observed temperature at which 31P CSA is motionally narrowed (323 K), the addition of 20% trehalose decreased the Tm of dry POPC by ~15 K.
Fig. 1.
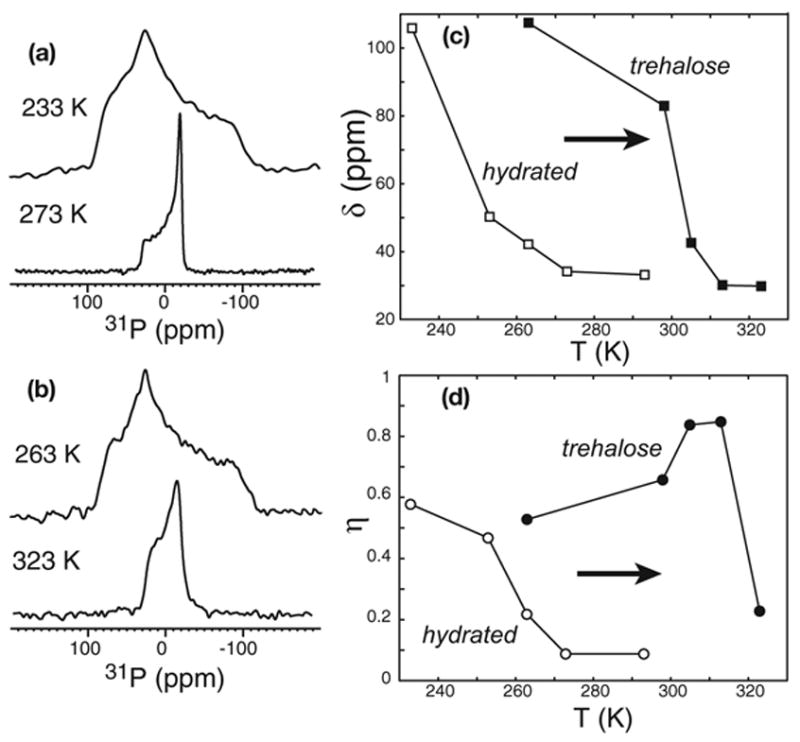
Static 31P spectra of (a) hydrated POPC and (b) lyophilized POPC with 20% trehalose. (c) Temperature dependence of the 31P chemical shift anisotropy parameter δ for hydrated POPC (open squares) and TRE-POPC (filled squares). (d) Temperature dependence of the 31P chemical shift asymmetry parameter η for hydrated POPC (open circles) and TRE-POPC (filled circles).
In comparison, the acyl chain region exhibits a much smaller difference between the transition temperatures of the hydrated POPC membrane and TRE-POPC membrane. The 2H quadrupolar couplings of d31-POPC (Fig. 2) indicate that at the highest temperature at which the lipid headgroups are rigid, the chains in the hydrated membrane are frozen (233 K) while the chains in the TRE-POPC membrane remain partly mobile (263 K). The deferred freezing of the acyl chains compared to the headgroup was also observed in TRE-DPPC membrane [6]. At the same temperature, the lipid chain dynamics is similar between the hydrated and the trehalose-protected POPC membranes, as seen, for example, in the 2H spectra at 263 K (Fig. 2). Thus, trehalose specifically restricts the motion of the lipid headgroups while largely preserving the acyl chain mobility. For the peptide-lipid headgroup distance measurement, it is sufficient that the headgroup is rigid.
Fig. 2.
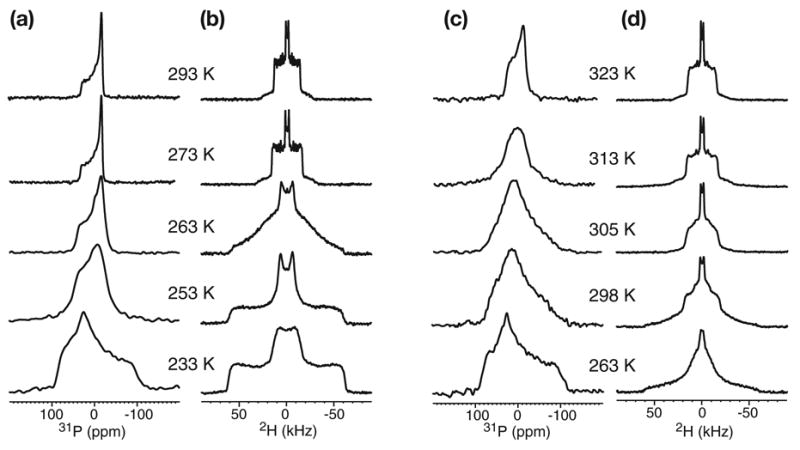
Static 31P (a, c) and 2H (b, d) spectra of d31-POPC in the hydrated membrane (a, b) and in the lyophilized membrane with 20% trehalose (c, d). The lipid headgroup transition temperature is ~273 K for the hydrated POPC but ~323 K for TRE-POPC. However, the acyl chain mobilities are similar between the two membranes.
To assess if the addition of trehalose affects the headgroup and glycerol backbone conformation of the lipid, we measured the intramolecular distances between the two carboxyl (C’) carbons and 31P in the 13C’-labeled crystalline POPC and TRE-POPC. The rotational-echo double resonance (REDOR) experiment [10] was used to measure the heteronuclear distance. Crystalline POPC was used to represent the structure of frozen hydrated POPC, since crystal structures of other phosphocholine lipids show well-defined lamellar structures in which the headgroup and backbone conformation of the molecules is believed to represent the main conformation of the hydrated lipid [11]. Fig. 3a shows 13C’-31P REDOR S/S0 values of 13C’-labeled crystalline POPC obtained at room temperature. Four 13C’ peaks are resolved, indicating the high degree of order of the sample. These are assigned to two unique molecules in the unit cell, each with two distinct 13C’ sites, sn-1 and sn-2 C’. The presence of two inequivalent molecules in the unit cell is inferred from the crystal structure of the analogous DMPC lipid [11], since the POPC crystal structure is not known. The two downfield 13C’ peaks exhibit faster REDOR decays indicative of a distance of 5.3 Å, while the two upfield peaks give longer distances of 6.2 Å and 6.7 Å (Fig. 3a). The crystal structure of DMPC [11] shows that the sn-2 13C’-31P distances are shorter than the sn-1 13C’-31P distances by 1.4 Å and 2.4 Å (Table 1). Thus, we assigned the downfield peaks to sn-2 13C’. Fig. 3b shows the REDOR data of TRE-POPC acquired at 263 K. While the resolution is reduced compared to the crystalline sample, the differential dephasing remains clear (inset). TRE-POPC also exhibits a short distance, 5.3 ± 0.6 Å, for the downfield signal and longer distances, 5.8 ± 1.0 Å and 6.8 ± 1.2 Å, for the two upfield peaks. The distances have significant distributions, reflected by the need for a Gaussian distribution function to simulate the REDOR curves, with the half-width-at-half-maximum of the Gaussian reported as the uncertainty. This distance distribution is consistent with the observed peak broadening, indicating increased conformational heterogeneity of the lyophilized TRE-POPC membrane compared to the crystalline POPC lipid. Despite this heterogeneity, the average distances are similar between the crystalline POPC and TRE-POPC, indicating that trehalose preserves the lipid headgroup and glycerol backbone conformation. To emphasize the innate disorder and distance distribution in lipid bilayers, we also show the 13C’-31P distances from MD simulations of hydrated POPC bilayers (Fig. 3c-d). The average distances and their standard deviations are 5.5 ± 0.5 Å (sn-2) and 6.2 ± 0.5 Å (sn-1) for the Lα phase (http://persweb.wabash.edu/facstaff/fellers/), and 4.7 ± 0.6 Å (sn-2) and 6.7 ± 0.6 Å (sn-1) for the gel phase [12]. The measured REDOR distances agree well with these values within experimental uncertainty, except for the 5.8 Å distance in the TRE-POPC sample.
Fig. 3.
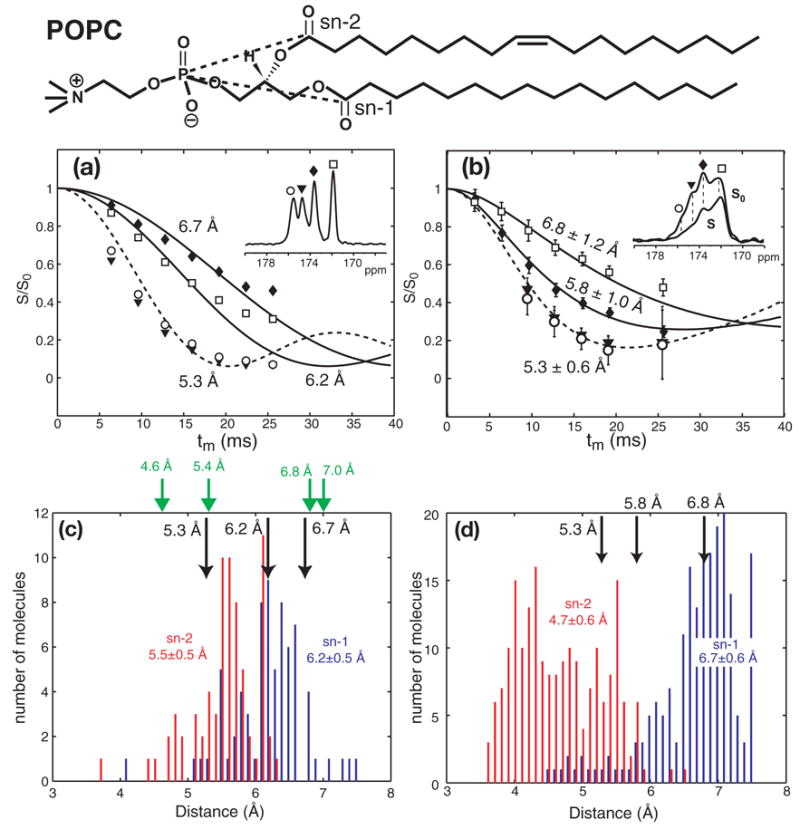
13C’{31P} REDOR data of (a) crystalline POPC at 293 K and (b) TRE-POPC at 263 K. The chemical structure of POPC is shown at the top, with the dashed lines indicating the distances measured here. (a) Best-fit distances for crystalline POPC, whose 13C’ spectrum is shown in the inset, are 5.3 Å (dashed line) for the two downfield peaks, and 6.2 Å and 6.7 Å (solid lines) for the two upfield peaks. (b) Inset is the S0 (solid line) and S (dashed line) spectra of TRE-POPC at tm = 12.8 ms. Best-fit distances are 5.3 ± 0.6 Å for the downfield peaks and 5.8 ± 1.0 Å and 6.8 ± 1.2 Å for the upfield peaks. Simulations in (a, b) used an intensity scaling factor of 90% to account for the effects of pulse imperfection [15]. (c) 13C’-31P distance distribution in liquid-crystalline POPC bilayers from MD simulations. The average sn-1 13C’ - 31P distance (blue) is 6.2 Å with a standard deviation of 0.5 Å. The average sn-2 13C’-31P distance (red) is 5.5 ± 0.5 Å. Black arrows: REDOR-extracted 13C’-31P distances in crystalline POPC. Green arrows: 13C’-31P distances in the DMPC crystal structure [11]. (d) 13C’-31P distance distribution in gel-phase POPC bilayers from MD simulations [12]. The average sn-1 13C’-31P distance (blue) is 6.7 ± 0.6 Å. The average sn-2 13C’-31P distance (red bars) is 4.7 ± 0.6 Å. Black arrows: measured 13C’-31P distances in the TRE-POPC membrane.
Table 1.
Comparison of intramolecular 13C’-31P distances in crystalline and trehalose-protected POPC lipids from REDOR, MD simulations and X-ray crystallography.
| Sites | Chemical shift (ppm) | 13C’- P distances (Å) 31 | ||||
|---|---|---|---|---|---|---|
| Crystalline POPC | TRE-POPC | MD | DMPC crystal structure | |||
| Lα phase a | Gel phase b | |||||
| sn-1 | 172.0 | 6.2 | 6.8 ± 1.2 | 6.2 ± 0.5 | 6.7 ± 0.6 | 6.8 |
| 173.5 | 6.7 | 5.8 ± 1.0 | 7.0 | |||
| sn-2 | 174.6 | 5.3 | 5.3 ± 0.6 | 5.5 ± 0.5 | 4.7 ± 0.6 | 5.4 |
| 175.5 | 5.3 | 5.3 ± 0.6 | 4.6 | |||
Obtained from: http://persweb.wabash.edu/facstaff/fellers/coordinates/popc.pdb.
Obtained from http://www.lrz-muenchen.de/~heller/membrane/gel.pdb [12].
We show an example of trehalose lyoprotection for facilitating structural investigation of membrane proteins using protegrin-1 (PG-1). PG-1 is a disulfide-stabilized 18-residue β-hairpin antimicrobial peptide that kills microbial cells by disrupting their cell membranes [13]. To determine the depth of insertion of PG-1 in the lipid bilayer, we measured the distance between 13C’-labeled Val16 of PG-1 and the lipid 31P. Fig. 4a shows 13C{31P} REDOR decays of Val16 13C’ in the dry TRE-POPE/POPG membrane (filled squares), acquired at 253 K, and in the hydrated POPE/POPG membrane (open circles), acquired at 226 K. The former gave a distance of 6.5 Å while the latter gave a longer distance of 7.2 Å. Although we cannot rule out the possibility of subtle differences in the PG-1 depth of insertion between the two membranes, the 13CO linewidth (3.5 ppm) and chemical shift (172 ppm) of Val16 are the same between the frozen hydrated membrane and the trehalose-protected membrane, indicating that the peptide conformation, and by inference its binding, is unchanged. At the same time, 31P NMR spectra show that there is residual motion in the hydrated membrane even at the low temperature of 226 K. The 31P CSA principle values are (82.9 ppm, 21.4 ppm, −110.0 ppm) for the TRE-POPE/POPG membrane (black) at 253 K (Fig. 4b), giving δ = 108.1 ppm, while the hydrated POPE/POPG membrane (red) has a smaller CSA of δ = 104.7 ppm (80.1 ppm, 21.1 ppm, −106.4 ppm). Thus, the hydrated lipid bilayer exhibits small-amplitude motion of the headgroups even at 226 K [8]. This motion is faster than the 31P CSA interaction of ~18 kHz. Since the measured 13C-31P dipolar coupling is about 35 Hz, there are likely additional slower headgroup motions on the 10−5 – 10−2 s timescale that further average the peptide-lipid dipolar coupling. Thus, we attribute the observed 0.7 Å longer distance or a dipolar order parameter of 0.74 (S = ω̄d/ωd ) to this residual motion.
Fig. 4.

(a) REDOR curves of PG-1 Val16 13CO after natural abundance correction in the TRE-POPE/POPG membrane (filled squares) and in hydrated POPE/POPG membrane (open circles). The experiments were conducted at 253 K for the lyophilized sample and 226 K for the hydrated sample. Best-fit distances are 6.5 Å for the former and 7.2 Å for the latter. (b) 31P static spectra of the TRE-POPE/POPG membrane (black) at 253 K and the hydrated POPE/POPG membrane (red) at 226 K. Both contain PG-1 at P/L (mole) = 1:12.5.
The above REDOR distances were extracted using two-spin simulations (one 13C and one 31P). Geometric constraints indicate that at most two 31P spins can be simultaneously close to any carbon in the middle of the peptide chains. Using 3-spin simulations changes the individual distances by up to 20% for short distances of < 4.5 Å and up to 10% for distances longer than 6.5 Å, and thus does not affect the structural conclusion significantly. A full report of the PG-1 peptide-lipid distance study will be given elsewhere.
3. Conclusion
In conclusion, we find that trehalose-protected dry lipid membrane gives rise to a more immobilized matrix in the headgroup and glycerol backbone region, while not significantly affecting the lipid chain mobility compared to the hydrated membrane. Importantly, the membrane interface immobilization by trehalose occurs without changing the lipid headgroup and backbone conformation, as shown by intramolecular 13C’-31P distances. This suggests that the rigid-limit membrane-bound structure of proteins should be very similar between hydrated and trehalose-protected dry membranes. A peptide-lipid 13C-31P distance measurement shows that the hydrated membrane sample gives a motionally averaged coupling even at 226 K while the trehalose-protected dry sample gives rigid-limit dipolar coupling already at 253 K. Thus, trehalose lyoprotection should facilitate the determination of membrane protein distances to lipid headgroups as well as membrane protein conformation itself by enabling these experiments at conveniently accessible mild low temperatures.
4. Materials and methods
Unlabeled and 13C’ labeled 1-palmitoyl-2-oleoyl-sn-glycerol-3-phosphatidylcholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanolamine (POPE), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylglycerol (POPG) were purchased from Avanti Polar Lipids (Alabaster, AL). PG-1 (NH2-RGGRLCYCRRRFCVCVGR-CONH2) was synthesized using Fmoc solid-phase protocols as previously described [14].
Membrane sample preparation
Six different membrane samples were prepared. Hydrated POPC and trehalose-protected dry POPC were used to measure the 31P chemical shift anisotropy and 2H quadrupolar couplings of the perdeuterated palmitoyl chains. 13C’-labeled POPC in the crystalline form and the trehalose-mixed form was used to measure intramolecular 13C’-31P distances. Two PG-1 containing membrane samples were compared with respect to their intermolecular 13C-31P distances: a trehalose-containing dry POPE/POPG membrane, and a hydrated POPE/POPG membrane.
Crystalline 13C’-labeled POPC was taken directly from the Avanti bottle. The high degree of structural order is evident from the fact that the 31P spectrum gives the rigid-limit CSA at ambient temperatures (not shown), and that four 13CO peaks are well resolved in a 4 ppm range, with linewidths (FWHM) of 0.4–0.6 ppm.
Hydrated POPC membrane was prepared by adding 35 wt% water directly to the dry lipid powder. The trehalose-containing dry POPC membrane was prepared by mixing trehalose (20% of dry lipid mass) and POPC in water, freeze-thawing the suspension three times, then lyophilizing the mixture. The dried mixture was packed into a 4 mm MAS rotor, then further lyophilized in the rotor to remove the moisture from packing.
To prepare the PG-1-containing membrane sample, POPE and POPG (3:1 molar ratio) were mixed in chloroform and blown dry, then redissolved in cyclohexane and lyophilized. The lipids were redissolved in water and subjected to five cycles of freeze-thawing to form homogeneous vesicles. The peptide and lipid solutions were mixed, incubated at 303 K for 12 hours, then ultracentrifuged at 55,000 rpm for 2 hours. For the hydrated POPE/POPG membrane sample, the pellet was directly packed into a 4 mm MAS rotor. For the trehalose-protected dry membrane sample, the pellet was resuspended in water, and an amount of trehalose equivalent to 20% of the dry weight of the lipids and peptide was added. The suspension was subjected to three more cycles of freeze-thawing, lyophilized, packed into a 4 mm MAS rotor, then lyophilized again to remove residual moisture from packing.
Solid-state NMR experiments
NMR experiments were carried out on a Bruker DSX-400 spectrometer operating at a resonance frequency of 400.49 MHz for 1H, 162.12 MHz for 31P, 61.48 MHz for 2H and 100.70 MHz for 13C. A double-resonance static probe equipped with a 5-mm diameter solenoid coil was used for static 2H and 31P experiments. A triple-resonance MAS probe with a 4 mm spinning module was used for the 13C{31P} REDOR experiments. Low temperatures were achieved using a Kinetics Thermal Systems XR air-jet sample cooler (Stone Ridge, NY). The temperature was maintained within ±1 K of the reported value, and the spinning speed was regulated to within ±3 Hz. Typical 90° pulse lengths were 4 – 5 μs for all nuclei. 1H decoupling field strengths of 50–80 kHz were used. 1H-13C cross-polarization (CP) contact times were 0.5 ms. 13C chemical shifts were referenced externally to the α-Gly 13C’ signal at 176.49 ppm on the TMS scale. The 31P chemical shift was referenced externally to 85% phosphoric acid at 0 ppm.
A modified REDOR pulse sequence containing composite 31P π pulses, 90°180°90°, and exorcycled 13C π pulse was used [15]. This composite-pulse sequence reduces the effect of the pulse flip angle errors, thus improving the distance accuracy. Two experiments were conducted for each tm, a control experiment (S0) where all the 31P pulses are turned off, and a dephasing experiment (S) where the 31P pulses are on. The normalized dephasing, S/S0, as a function of tm gives the dipolar coupling without the T2 relaxation effect of the 13C spin. Typical 180° pulse lengths were 10 μs for 13C and 8–10 μs for 31P. The spinning speed was 5 kHz for the pure lipid samples and 4.5 kHz for the peptide-containing membrane samples. REDOR data were simulated using an in-house Fortran program.
Acknowledgments
This work is supported by NIH grants GM-066976 to M. H. and AI-22839 and AI-37945 to A.J.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crowe JH, Crowe LM, Chapman D. Preservation of Membranes in Anhydrobiotic Organisms - the Role of Trehalose. Science. 1984;223:701–703. doi: 10.1126/science.223.4637.701. [DOI] [PubMed] [Google Scholar]
- 2.Crowe JH, Crowe LM, Carpenter JF, Wistrom CA. Stabilization of Dry Phospholipid-Bilayers and Proteins by Sugars. Biochem J. 1987;242:1–10. doi: 10.1042/bj2420001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anchordoquy TJ, Izutsu KI, Randolph TW, Carpenter JF. Maintenance of quaternary structure in the frozen state stabilizes lactate dehydrogenase during freeze-drying. Arch Biochem Biophys. 2001;390:35–41. doi: 10.1006/abbi.2001.2351. [DOI] [PubMed] [Google Scholar]
- 4.Crowe JH, Carpenter JF, Crowe LM. The role of vitrification in anhydrobiosis. Annu Rev Physiol. 1998;60:73–103. doi: 10.1146/annurev.physiol.60.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Toke O, Maloy WL, Kim SJ, Blazyk J, Schaefer J. Secondary structure and lipid contact of a peptide antibiotic in phospholipid bilayers by REDOR. Biophys J. 2004;87:662–674. doi: 10.1529/biophysj.103.032706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CW, Das Gupta SK, Mattai J, Shipley GG, Abdel-Mageed OH, Makriyannis A, Griffin RG. Characterization of the L lambda phase in trehalose-stabilized dry membranes by solid-state NMR and X-ray diffraction. Biochemistry. 1989;28:5000–5009. doi: 10.1021/bi00438a015. [DOI] [PubMed] [Google Scholar]
- 7.Lee CW, Waugh JS, Griffin RG. Solid-state NMR study of trehalose/1,2-dipalmitoyl-sn-phosphatidylcholine interactions. Biochemistry. 1986;25:3737–3742. doi: 10.1021/bi00361a001. [DOI] [PubMed] [Google Scholar]
- 8.Kohler SJ, Klein MP. Orientation and Dynamics of Phospholipid Head Groups in Bilayers and Membranes Determined from P-31 Nuclear Magnetic-Resonance Chemical Shielding Tensors. Biochemistry. 1977;16:519–526. doi: 10.1021/bi00622a028. [DOI] [PubMed] [Google Scholar]
- 9.Koster KL, Webb MS, Bryant G, Lynch DV. Interactions between soluble sugars and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration: vitrification of sugars alters the phase behavior of the phospholipid. Biochim Biophys Acta. 1994;1193:143–150. doi: 10.1016/0005-2736(94)90343-3. [DOI] [PubMed] [Google Scholar]
- 10.Gullion T, Schaefer J. Rotational echo double resonance NMR. J Magn Reson. 1989;81:196–200. doi: 10.1016/j.jmr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Pearson RH, Pascher I. The molecular structure of lecithin dihydrate. Nature. 1979;281:499–501. doi: 10.1038/281499a0. [DOI] [PubMed] [Google Scholar]
- 12.Heller H, Schaefer M, Schulten K. Molecular-Dynamics Simulation of a Bilayer of 200 Lipids in the Gel and in the Liquid-Crystal Phases. J Phys Chem. 1993;97:8343–8360. [Google Scholar]
- 13.Bellm L, Lehrer RI, Ganz T. Protegrins: new antibiotics of mammalian origin. Exp Opin Invest Drugs. 2000;9:1731–1742. doi: 10.1517/13543784.9.8.1731. [DOI] [PubMed] [Google Scholar]
- 14.Yamaguchi S, Hong T, Waring A, Lehrer RI, Hong M. Solid-state NMR investigations of peptide-lipid interaction and orientation of a beta-sheet antimicrobial peptide, protegrin. Biochemistry. 2002;41:9852–9862. doi: 10.1021/bi0257991. [DOI] [PubMed] [Google Scholar]
- 15.Sinha N, Schmidt-Rohr K, Hong M. Compensation for pulse imperfections in rotational-echo double-resonance NMR by composite pulses and EXORCYCLE. J Magn Reson. 2004;168:358–365. doi: 10.1016/j.jmr.2004.03.025. [DOI] [PubMed] [Google Scholar]


