Abstract
Transgenic antisense tobacco plants with a range of reductions in sedoheptulose-1,7-bisphosphatase (SBPase) activity were used to investigate the role of photosynthesis in stomatal opening responses. High resolution chlorophyll a fluorescence imaging showed that the quantum efficiency of photosystem II electron transport (Fq′/Fm′) was decreased similarly in both guard and mesophyll cells of the SBPase antisense plants compared to the wild-type plants. This demonstrated for the first time that photosynthetic operating efficiency in the guard cells responds to changes in the regeneration capacity of the Calvin cycle. The rate of stomatal opening in response to a 30 min, 10-fold step increase in red photon flux density in the leaves from the SBPase antisense plants was significantly greater than wild-type plants. Final stomatal conductance under red and mixed blue/red irradiance was greater in the antisense plants than in the wild-type control plants despite lower CO2 assimilation rates and higher internal CO2 concentrations. Increasing CO2 concentration resulted in a similar stomatal closing response in wild-type and antisense plants when measured in red light. However, in the antisense plants with small reductions in SBPase activity greater stomatal conductances were observed at all Ci levels. Together, these data suggest that the primary light-induced opening or CO2-dependent closing response of stomata is not dependent upon guard or mesophyll cell photosynthetic capacity, but that photosynthetic electron transport, or its end-products, regulate the control of stomatal responses to light and CO2.
Keywords: CO2 concentration, guard cell photosynthesis, light response, photosynthetic electron transport, SBPase, stomata, stomatal conductance
Introduction
Stomatal aperture controls the flux of CO2 and H2O between plant and atmosphere and responds to several environmental variables. Many authors have suggested that part of the mechanisms responsible for sensing these environmental variables must be located in the epidermis or the guard cells themselves because guard cells in epidermal peels respond similarly to those in intact leaf tissue (Willmer and Fricker, 1996; Frechilla et al., 2002). Chloroplasts are a notable feature of guard cells in almost all plant species examined, although the role they play in stomatal function and the response to CO2 concentration remains unclear (Vavasseur and Raghavendra, 2005). However, guard cell chloroplasts are believed to be involved in several different light transduction pathways (Zeiger et al., 2002). The photosynthesis-independent, blue light response has been shown to saturate at low fluence rates and is often associated with rapid stomatal opening (Zeiger et al., 2002). Stomata also open in response to higher intensities of light within the photosynthetically active radiation (PAR) waveband (Willmer and Fricker, 1996). This response saturates at high fluence rates similar to those that saturate guard cell and mesophyll photosynthesis and is inhibited by DCMU, indicating that it is photosynthesis-dependent (Kuiper, 1964; Sharkey and Raschke, 1981; Tominaga et al., 2001; Zeiger et al., 2002; Olsen et al., 2002; Messinger et al., 2006). Under PAR illumination, guard cell chloroplasts could provide a significant energy source for H+ extrusion and ion transport (Wu and Assmann, 1993; Tominaga et al., 2001), or they could provide sucrose through photosynthetic carbon assimilation, although the extent of this is still controversial (Talbott and Zeiger, 1998; Zeiger et al., 2002; Outlaw, 2003; Vavasseur and Ragavendra, 2005). Furthermore, guard cells show O2 and CO2 modulation of PSII electron transport, indicating Rubisco-mediated carbon assimilation (Lawson et al., 2002, 2003). However, Roelfsema and Hedrich (2005) have returned to the earlier suggestion that the opening response to PAR is only due to intracellular or intercellular CO2 depletion. Thus, the involvement of guard cell photosynthesis in light- and CO2-modulated stomatal behaviour remains to be resolved and the use of transgenic plants has offered a different approach (von Caemmerer et al., 2004; Baroli et al., 2008).
Antisense technology has been exploited extensively to study the control of photosynthetic carbon flux through the Calvin cycle (Stitt and Schulze, 1994; Raines, 2003). Analyses of transgenic plants with reduced levels of a number of individual Calvin cycle enzymes have usually shown that CO2 assimilation rate (A) was decreased and internal CO2 concentration (Ci) increased. Stomata usually respond to increased Ci by reducing aperture (Morison, 1987; Mott, 1990) so a reduction in stomatal conductance (gs) would be expected in these transgenic plants. However, as most studies report no difference in gs between wild-type (WT) and transgenic plants, this suggests that stomata do not respond to either the increase in Ci or the reduced guard cell or mesophyll photosynthesis (Muschak et al., 1999; Quick et al., 1991; Hudson et al., 1992). Using antisense Rubisco tobacco plants, von Caemmerer et al. (2004) have shown that, despite similar reductions in guard and mesophyll cell photosynthetic operating efficiency (Fq′/Fm′), stomatal responses to a step increase in mixed red and blue photon flux density (PFD) and to changes in Ci were similar to wild-type plants. These results suggested that the light-induced opening and Ci-mediated closing responses of the stomatal pores are not dependent on the photosynthetic capacity of the guard or mesophyll cells. Further support for this has come from a more recent study using antisense cytochrome b6f complex plants that showed, despite large differences in photosynthetic electron transport rates, stomatal opening was essentially the same as wild-type (Baroli et al., 2008).
In this study, tobacco plants with a range of reduced levels of sedoheptulose 1,7-bisphosphatase (SBPase) have been used. There are several reasons for using these plants in comparison with the earlier work on antisense Rubisco plants (von Caemmerer et al., 2004). Firstly, SBPase controls the regeneration of ribulose-1,5-bisphosphate (RuBP) to drive off the Calvin cycle (regeneration capacity) in contrast to Rubisco which controls carboxylation. Secondly, the Rubisco transgenic plants used had severe reductions in enzyme activity (∼80%), and therefore photosynthetic capacity, so that plants were grown in controlled environment chambers at CO2 concentrations of 800 μmol mol−1. However, growth CO2 concentration (Lodge et al., 2001), water stress (Raschke, 1975), and differences between environmental chambers and greenhouse conditions have been shown to influence guard cell sensitivity to CO2 severely (Talbott et al., 1996; Frechilla et al., 2002, 2004; Zeiger et al., 2002). Using the antisense SBPase tobacco plants such potential complications were avoided by growing plants in normal air under glasshouse conditions. In addition, plants could be assessed with a range of reductions in photosynthesis capacity and therefore possible dose-dependent responses could be determined. Previously it has been shown that photosynthetic carbon fixation rates are sensitive to reductions in SBPase activity (Harrison et al., 1998). In plants with small reductions in SBPase activity (20–35%) a reduction in the regeneration capacity of the Calvin cycle was evident, but the rate of carboxylation remained the same as in the WT plants (Harrison et al., 2001; Raines, 2003). Therefore, these transgenic plants provide an opportunity to determine both the impact of a range of decreases in photosynthesis on stomatal responses and also the possible contribution of regeneration capacity to guard cell function. These plants were used to investigate (i) the relationship between CO2 assimilation rate and stomatal conductance in response to step changes in normal mixed blue/red light, and red light alone (to avoid non-photosynthetic blue light responses), and (ii) the influence of reduced Calvin cycle regeneration capacity on stomatal responses to CO2 concentration and photon flux density.
Materials and methods
Growth of plants for physiological analysis
Wild-type and transgenic T2 progeny tobacco (Nicotiana tabacum L. cv. Samsun) seeds were germinated on sterile MS medium containing 1% (w/v) sucrose (Murashige and Skoog, 1962). For transgenic seedlings the medium was supplemented with kanamycin (300 μg ml−1). Three-week-old seedlings were transferred to a peat and loam-based compost (F2, Levington Horticulture Ltd., Ipswich, UK) and acclimatized in a propagator before transfer to 18 cm pots. Plants were grown in a heated glasshouse, where temperature was maintained above 20 °C at night and rarely exceeded 30 °C during the day. The plants were kept well-watered throughout. Supplementary lighting (PFD of 350 μmol m−2 s−1) was provided from 07.00 h to 19.00 h by sodium vapour lamps.
Determination of SBPase activity
Frozen leaf discs were ground to a fine powder in liquid nitrogen using a mortar and pestle in 1.4 ml extraction buffer (50 mM HEPES, pH 8.2, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 10% glycerol, 0.1% Triton X-100, 2 mM benzamidine, 2 mM amino caprionic acid, 0.5 mM phenylmethylsulphonylfluoride; and 10 mM dithiothreitol (DTT), transferred to a prechilled 2 ml tube and spun for 1 min at 4 °C. The supernatant was desalted using a NAP-10 column (Pharmacia, Milton Keynes, UK) and equilibrated with desalting buffer (extraction buffer omitting the Triton X-100). Proteins were eluted from the column with 1.5 ml desalting buffer and aliquots stored in liquid nitrogen. To start the reaction, 20 μl of thawed extract was added to 66 μl of assay buffer (50 mM TRIS, pH 8.2, 15 mM MgCl2, 1.5 mM EDTA, 10 mM DTT, 2 mM SBP) and incubated at 25 °C for 5 min. The reaction was stopped by the addition of 50 μl of 1 M perchloric acid and stored on ice. The samples were centrifuged for 5 min and the supernatant assayed for free phosphate. Samples (30 μl) and phosphate standards (0.125–4 nM phosphate) were incubated with for 25 min at room temperature with 300 μl of Biomol Green reagent (Biomol, AK111) and the absorbance at 620 nm was measured (Leegood, 1990).
Fluorescence imaging of seedlings
Images of chlorophyll a fluorescence were obtained as described by Barbargallo et al. (2003) using a CF Imager (Technologica Ltd., Colchester, UK). Seedlings were dark-adapted for 15 min before minimal fluorescence (Fo) was measured using a weak measuring pulse. Then maximal fluorescence (Fm) was measured during an 800 ms exposure with a saturating pulse having a PFD of 4800 μmol m−2 s−1. Plants were then exposed to an actinic PFD of 300 μmol m−2 s−1 for 15 min and steady-state F′ was continuously monitored while Fm′ (maximum fluorescence in the light) was measured at 5 min intervals by applying saturating light pulses. This was repeated at a photon flux density of 500 μmol m−2 s−1. Using the images captured at F′ and Fm′, images of PSII quantum photosynthetic efficiency
were constructed by the imaging software. Non-photochemical quenching (NPQ) was determined from .
Single cell fluorescence imaging
High-resolution chlorophyll a fluorescence imaging analysis of individual cells was employed, as described previously by Lawson et al. (2002). A specially designed chamber attached to a gas exchange system (CIRAS 1, PP Systems, Hitchin, UK) maintained a constant CO2 concentration of 380 μmol mol−1, relative humidity of 60%, and a temperature of 25 °C around the leaf. Chlorophyll a fluorescence was imaged through a 680 nm bandpass filter. All images were taken from the abaxial surface of leaves using a ×40 objective, which provided images of 310×205 μm with a pixel size of 534 nm2. Chloroplasts within guard cell pairs were isolated from images using the ends-in search and editing tools of the FluorImager computer programme (Technologica Ltd., Colchester, UK) as described in Oxborough and Baker (1997), Lawson et al. (2002), and Oxborough (2004).
Effect of light on assimilation rate and stomatal conductance
Gas exchange measurements were made on young plants with nine leaves with a gas exchange system (Li-Cor 6400, Lincoln, Nebraska). For the light response experiments, light was provided by red or blue/red LED light source (Li-Cor 6400-02 and 02B). Leaves were first equilibrated at a PFD of 100 μmol m−2 s−1 for 20–30 min. The PFD was then increased to 1000 μmol m−2 s−1 for 30 min and then returned to 100 μmol m−2 s−1. During the experiment, leaf chamber CO2 and humidity were maintained at 380 μmol mol−1 and 23 mmol mol−1 and leaf temperature at 25 °C. This resulted in a constant leaf to air vapour pressure difference of approximately 1.0 kPa.
Effect of CO2 on assimilation rate and stomatal conductance
Measurements of gas exchange were made on young plants which had eight leaves using the same Li-Cor gas exchange system. Leaves were first equilibrated at a PFD of 1000 μmol m−2 s−1 red light, and a chamber CO2 concentration of 400 μmol mol−1 for 30–50 min. The CO2 concentration was stepwise decreased, followed by stepwise increases to cover a range of CO2 concentrations from 50–1600 μmol mol−1. At each CO2 concentration the leaf was allowed to stabilize to steady-state conditions for >30 min. Throughout the experiment VPD was maintained at 0.85 kPa with a dew point generator (Li-Cor, Lincoln, Nebraska), PFD and temperature were maintained at 1000 μmol m−2 s−1 and at 25 °C, respectively.
Effect of red PFD on gs whilst Ci was maintained constant
The effect of red light on photosynthesis and stomatal conductance with a constant Ci was assessed on several of the plants used for the CO2 response curves using a CIRAS-1 gas exchange system. Leaves were first equilibrated at a PFD of 1000 μmol m−2 s−1 red light (wavelengths >650 nm) provided by using a long pass filter and cold mirror placed in front of a halogen light source. The light was stepwise decreased and the leaf allowed to stabilize to steady-state conditions for about 30 min. Internal CO2 concentration was maintained at about 280 μmol mol−1 by manipulating the external CO2 concentration. Temperature and humidity were maintained throughout the experiment at 26±2 °C and 23 mmol mol−1, respectively.
Statistical analysis
Data shown in Fig. 4 are means (±se) from three to five replicates. Differences in stomatal conductance after 25 min increased light were analysed by ANOVA in Systat 11 (Systat Software Inc., California USA). The rate of stomatal opening and closing were determined using linear regression analysis (Excel 2002, Microsoft Corporation) of the increasing and decreasing slopes of stomatal responses. Differences were analysed using ANOVA (Systat 11, Systat Software Inc., California USA). Correlation analysis (Fig. 6) for all data points was performed in Excel. Data in Fig. 7 are from three or four replicates and differences between stomatal responses to CO2 were determined using ANOVA with a repeated measures design for the different CO2 concentrations (Systat 11).
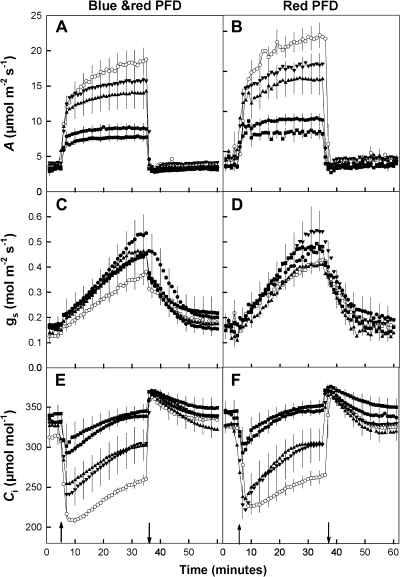
Fig. 4.
Changes in (A, B) CO2 assimilation rate, (C, D) leaf conductance, and (E, F) intercellular CO2 with time after a step change in photon flux density (PFD) from 100 to 1000 μmol m−2 s−1 for WT and transgenic tobacco with SBPase activities of 8.9, 8.3, 7.0, 5.9, and 5.0 μmol m−2 s−1 (open circles n=6, solid inverted triangles n=3, triangles n=4, circles n=4, and squares n=3, respectively). Ambient CO2 and water vapour were maintained at 380 μmol mol−1 and 23 mmol mol−1. Arrows indicate when PFD was increased from 100 to 1000 μmol m−2 s−1 and returned to 100 μmol m−2 s−1. Error bars are the standard error of the mean.
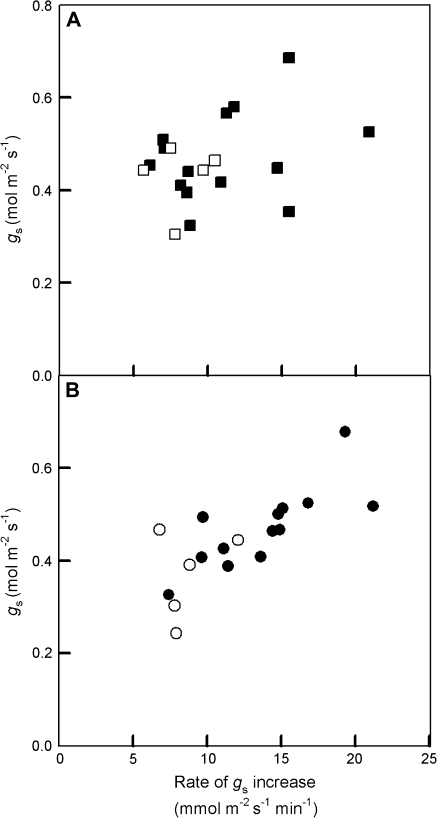
Fig. 6.
Relationship between rate of stomatal opening and final stomatal conductance at the end of 30 min illumination period of 1000 μmol m−2 s−1 photon flux density of (A) mixed blue/red (squares) or (B) red (circles) light. Open symbols represent WT plants and closed symbols antisense SBPase transgenic tobacco. Water vapour was maintained at 23 mmol mol−1 and leaf temperature at 25 °C providing a leaf–air vapour pressure difference of 1.0 kPa.
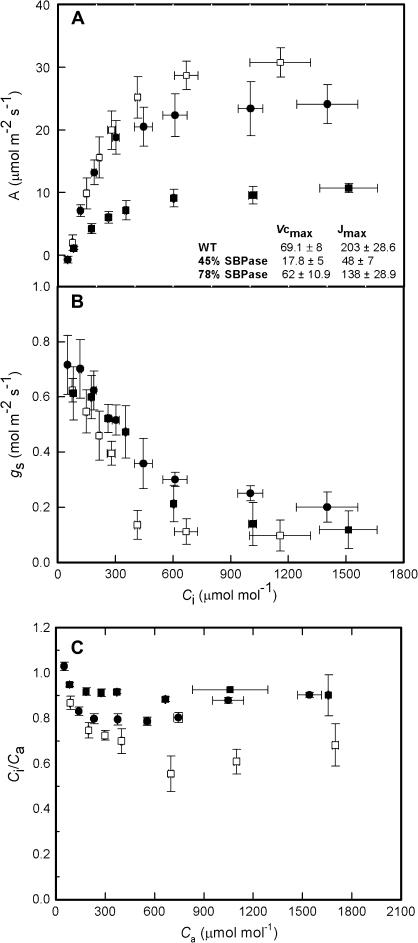
Fig. 7.
(A) Net CO2 assimilation rate and (B) stomatal conductance as a function of internal CO2 concentration (Ci). Values were allowed to stabilize for at least 30 min at each CO2 concentration. (C) The relationship between ambient CO2 concentration Ca and Ci/Ca ratio. WT plants (n=6) are shown by open squares, with antisense SBPase transgenic plants represented by closed squares (78% WT activity, n=4) and closed circles (45% WT activity, n=4). Error bars are the standard error of the mean.
Results
Relationship between photosynthesis and SBPase activity
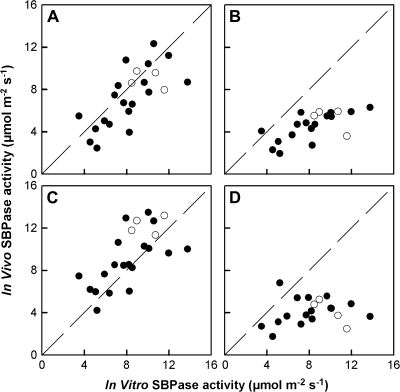
Leaf net CO2 assimilation rates (A) measured in different light and CO2 conditions in 2% O2 were used to estimate in vivo SBPase activity [calculated as (assimilation rate+dark respiration rate)/3], in WT and antisense plants (Fig. 1). There was a strong positive correlation between in vivo and in vitro SBPase activity in high light and moderate or high CO2 (Fig. 1A, C) but not when light or CO2 fluxes were low (Fig. 1B, D). This illustrates that under conditions of high photosynthetic rates SBPase activity has a high control coefficient on mesophyll carbon assimilation (Fig. 1A, C).
Fig. 1.
Relationship between in vitro and estimated in vivo SBPase activity at (A) 1000 μmol m−2 s−1 photon flux density (PFD) and 380 μmol mol−1 CO2 concentration, (B) 300 μmol m−2 s−1 PFD and 380 μmol mol−1 CO2 concentration, (C) 1000 μmol m−2 s−1PFD and 1500 μmol mol−1 CO2 concentration, and (D) 1000 μmol m−2 s−1 PFD and 155 μmol mol−1 CO2 concentration. Estimated in vivo SBPase activity was calculated as (assimilation rate+dark respiration rate)/3. Solid circles represent transgenic antisense plants and open circles WT.
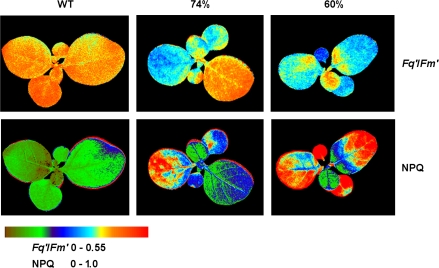
Whole plant chlorophyll a fluorescence imaging was used to determine the influence of SBPase activity on photosynthetic electron transport, and it also provided a rapid, non-invasive screening tool to select antisense plants for further study (Barbagallo et al., 2003). Chlorophyll fluorescence imaging of seedlings showed a positive correlation between SBPase activity and (quantum efficiency of PSII electron transport; data not shown). The images in Fig. 2 show that, in wild-type seedlings, the leaves had the same uniform photosynthetic characteristics while the antisense SBPase plants showed reduced with decreased amounts of SBPase activity and increased non-photochemical quenching as estimated by NPQ (). The older leaves tended to have greater reduction in and increased NPQ compared with the younger leaves. In antisense plants with SBPase activities reduced to 74% and 60% of WT plants, values averaged across all leaves were 0.36 and 0.33, respectively, compared with WT values of 0.41 (reductions by 12% and 20%, respectively). NPQ was higher in the antisense plants, with values of 0.56 and 0.78 for plants with 74% and 60% of WT SBPase activity and 0.26 for WT plants (Fig. 2).
Fig. 2.
Chlorophyll a fluorescence images of and NPQ for WT and SBPase antisense tobacco seedlings at a photon flux density of 300 μmol m−2 s−1. Images are shown for SBPase antisense plants with 74% and 60% WT SBPase activity.
Chlorophyll a fluorescence imaging of guard and mesophyll cells
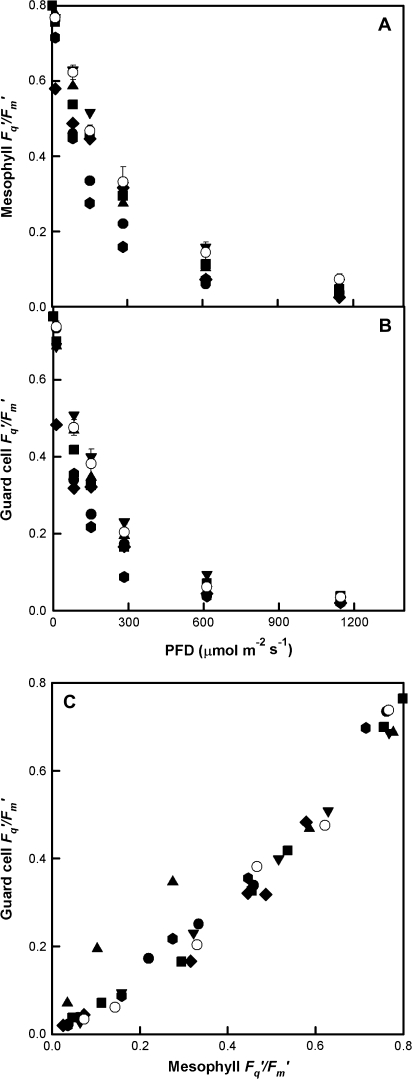
High-resolution chlorophyll a fluorescence imaging with a microscope was used to determine the effect of reductions in SBPase activity on mesophyll and guard cell quantum efficiency of PSII photochemistry. The response of to increasing PFD in fully expanded leaves showed that photosynthetic capacity was reduced by approximately 20% in both guard and mesophyll cells in the antisense SBPase plants with the lowest levels of SBPase activity when compared to WT plants (Fig. 3A, B). Although guard cell was between 5–25% lower than mesophyll , similar decreases in of both guard and mesophyll cells with decreasing SBPase activity were observed (Fig. 3C).
Fig. 3.
Response of to photon flux density in (A) mesophyll cells, (B) guard cells of WT (open symbols) and anti-SBPase (closed symbols) tobacco leaves. Measurements were made on the underside of leaves at 25 °C and ambient CO2 concentration of 380 μmol mol−1. Data for WT are the means of six replicate leaves ±SE. (C) Relationship between of guard cells and adjacent mesophyll cells for measurements shown in (A) and (B).
Red and mixed blue/red light effects on stomatal conductance
To assess the impact of reduced photosynthetic efficiency of electron transport on light-induced stomatal opening, leaves were subjected to a step-increase in PFD and the effect on net CO2 assimilation rate (A) and stomatal conductance (gs) was measured using gas exchange. In WT plants, A increased rapidly from about 4 μmol m−2 s−1 to 12 and 15 μmol m−2 s−1 immediately after a step-increase in either mixed blue/red or pure red PFD, then increased more slowly to a maximum of c. 19 μmol m−2 s−1 in mixed and 22 μmol m−2 s−1 in red PFD (Fig. 4A, B). A similar two-phase response was seen with the transgenic plants but in this case the maximum attained was clearly dependent on SBPase activity. When the PFD was returned to 100 μmol m−2 s−1, CO2 assimilation rate in both WT and transgenic plants returned to the original value.
Stomatal conductance (gs) in both WT and transgenic plants increased at with a step increase in illumination (both mixed blue/red and red alone), from values around 0.13 mol m−2 s−1 to nearly 0.4 mol m−2 s−1 for WT plants. For the antisense SBPase plants gs rose from slightly higher values at low PFD to between 0.45–0.55 mol m−2 s−1 at high PFD. When plants were illuminated with red light alone the rate of stomatal opening was greater in the transgenic plants compared with the WT controls (P <0.05, df 16). By contrast, no statistically significant difference in the rate of opening was observed under mixed blue/red irradiance. However, in both red and blue/red the conductance after 30 min in the light was greater in the transgenic plants (P <0.05). When the PFD was returned to 100 μmol m−2 s−1, gs in both WT and antisense SBPase plants decreased to close to the original values (Fig. 4C, D). The response time of gs to decreasing PFD under both pure red and blue/red illumination was greater in transgenic plants compared with the WT controls (P >0.05). Rates of stomatal opening and closure were closely correlated under pure red (r=0.72, df=16, P <0.001) and blue/red illumination (r=0.68, df=16, P <0.001) (data not shown).
At a PFD of 100 μmol m−2 s−1, Ci values for all plants were around 330 μmol mol−1, irrespective of the illumination wavebands. Ci decreased rapidly when PFD was increased to 1000 μmol m−2 s−1, with the extent of the drop dependent on the amount of SBPase activity. In WT plants, with SBPase activity of 8.9 μmol m−2 s−1, Ci decreased to a minimum of 215 μmol mol−1 and in antisense plants it decreased to 241, 254, 293, and 301 μmol mol−1 with SBPase activities of 8.3, 7.0, 5.9, and 5.0 μmol m−2 s−1, respectively, under mixed blue/red illumination and 222, 238, 293, and 304 μmol mol−1 under red light. During the rest of the high light period, Ci increased in all plants as stomatal conductance rose, but the final Ci reached at the end of the 30 min was different depending on SBPase activity (Fig. 4E, F), but independent of the wavebands used.
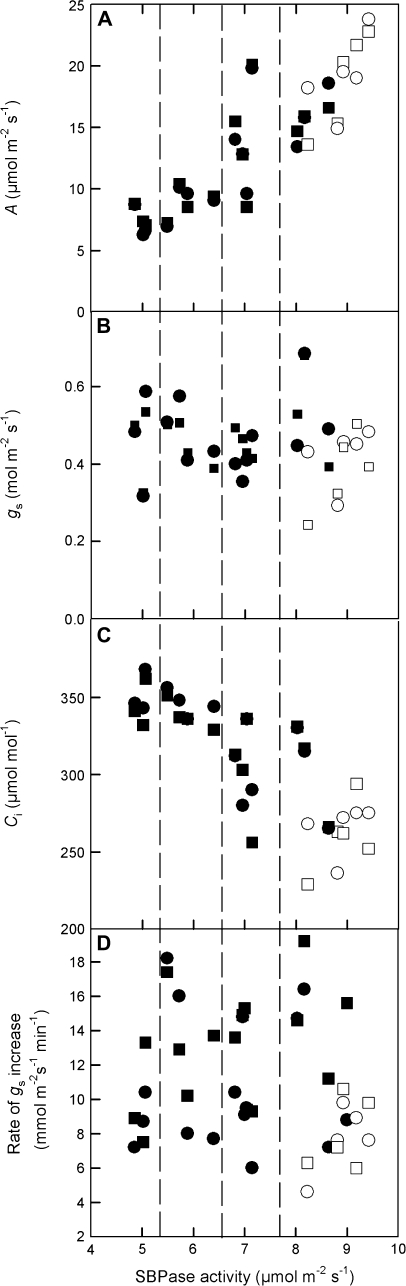
Correlations between SBPase activity and photosynthetic gas exchange parameters
The steady-state values of A, gs, and Ci at the end of the high light period and the initial rate of stomatal opening (calculated as the slope of gs increase between 5 min and 21 min) were plotted against SBPase activity (Fig. 5). Although assimilation rate showed a pronounced decrease with reductions in SBPase activity (Fig. 5A), there was no clear relationship between gs and SBPase activity (Fig. 5B), and therefore Ci increased with lower SBPase activity (Fig. 5C). Similarly, there was no clear relationship between the rate of stomatal opening and SBPase activity (Fig. 5D), although antisense plants in many cases had higher gs than WT and a more rapid rate of stomatal opening which was more pronounced in plants subjected to red light alone. In general, there was a tendency for greater stomatal opening rates to result in higher steady-state conductances (Fig. 6), however the relationship between opening rates and final conductance were only statistically correlated (r=0.78, df=12, P <0.001) in the plants subjected to red light alone (Fig. 6B), but not in plants subject to mixed blue/red illumination (r=0.30, df=12) (Fig. 6A).
Fig. 5.
Relationships between SBPase activity and (A) net CO2 assimilation rate, (B) stomatal conductance, (C) internal CO2 concentration, and (D) the rate of stomatal opening in WT (open symbols) and antisense SBPase transgenic tobacco (closed symbols). Measurements were taken at the end of the 30 min illumination period of 1000 μmol m−2 s−1 photon flux density of red (circles) or red and blue/red (squares) light as shown in Fig. 4. Water vapour was maintained at 23 mmol mol−1 and leaf temperature of 25 °C providing a leaf–air vapour pressure difference of 1.0 kPa. Dashed lines represent the SBPase grouping of Fig. 4.
Effect of CO2 concentration on stomatal and photosynthetic behaviour
The light response data in Fig. 4 showed that stomatal opening in the transgenic plants was similar to or even greater than that in WT plants despite the higher Ci. This suggests that either the stomata in the SBPase plants were less sensitive to Ci or that light was overriding this response. To test this hypothesis, gs was determined at a constant PFD (either red or mixed blue/red) under a range of concentrations of Ci. To allow time for stomata to respond, the external CO2 concentration was increased in steps, with a minimum of 30 min between each increment (Fig. 7). Assimilation rate increased with increasing Ci in a characteristic A/Ci response curve. Maximum assimilation rates were dependent on SBPase activity (Fig. 7A) and was significantly higher in WT plants compared with antisense SBPase plants with 78% SBPase activity (Tukey, P <0.05, df 10) and with those exhibiting 45% SBPase activity (Tukey, P <0.001, df 10). Carboxylation capacity was significantly lower in the 45% SBPase activity plants compared with WT and transgenic plants with 78% SBPase activity (See inset Table in Fig. 7A; Tukey, P <0.001). Potential electron transport rate calculated as Jmax from the A/Ci curves also showed a significant decrease between WT and plants with 78% (Tukey, P <0.05) and 45% WT SBPase activity (Tukey, P <0.05). Changes in Ci revealed a small but significant difference between the conductances in the WT and transgenic plants (repeated measures ANOVA, P <0.001). At Ci values of >600 μmol mol−1, gs did not change in either the WT or antisense plants (Fig. 7B). When Ci was decreased to <600 μmol mol−1, gs increased significantly, reaching a value of between 0.6–0.7 mol m−2 s−1 at the lowest Ci in both the WT and transgenic plants. At Ci concentrations between 300 and 600 μmol mol−1, the transgenic plants tended to have higher gs than the WT plants and the highest conductances values were observed in the antisense plants with 78% SBPase activity. The Ci/Ca ratio was maintained between 0.6–0.7 for the WT plants over a range of Ca concentration from 150 to 1800 μmol mol−1 (Fig. 7C). The transgenic plants with 78% SBPase activity maintained a slightly higher Ci/Ca of between 0.8–0.9 over the same range of Ca concentrations, whilst plants with only 45% SBPase activity maintained an even higher ratio of between 0.9 and 1.0. The same results were found when these measurements were conducted under mixed blue/red PFD (data not shown).
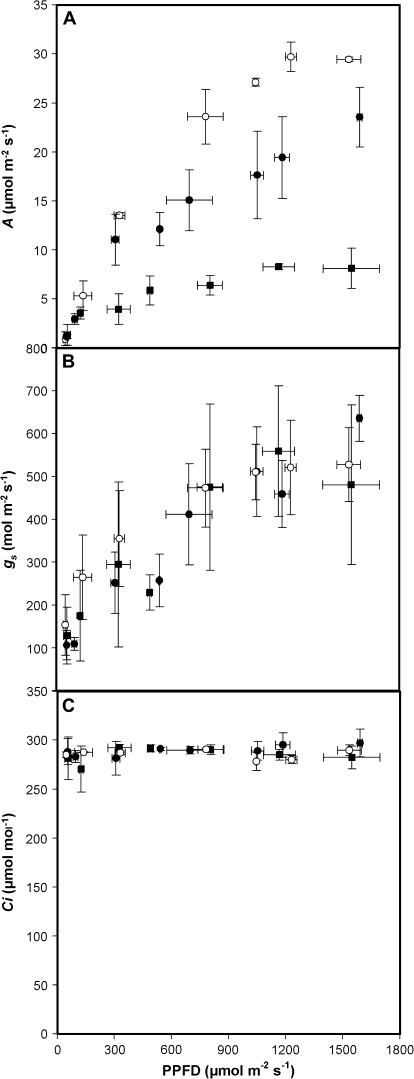
Effect of red light intensity on stomata at a constant Ci
The red light response of stomata is considered to be associated with guard cell photosynthesis, although recent reports have suggested the link with photosynthesis is only due to photosynthetic mesophyll consumption of CO2 and stomatal response to lowering internal CO2 concentration (see Introduction). To eliminate possible effects of changes in Ci in the light response, the effects of increasing red light and PFD on A and gs whilst maintaining Ci at approximately 280 μmol mol−1 (Fig. 8) were examined. Stomatal conductance showed a large (4–6-fold) increase with increasing red PFD in both WT and transgenic plants (Fig. 7B) even though Ci was maintained constant throughout the experiment (Fig. 8C). Although assimilation rates were much lower in the antisense plants (Fig. 8A), the stomatal responses to PFD were indistinguishable from that of the WT plants. Therefore any obligatory role for changing Ci in the observed light responses can be discounted.
Fig. 8.
(A) Net CO2 assimilation rate and (B) stomatal conductance as a function of an increase in red photon flux density whilst maintaining (C) internal CO2 concentration (Ci) at c. 300 μmol mol−1. Values were allowed to stabilize for at least 30 min at each light level, whilst external CO2 concentration was altered to maintain Ci. WT plants (n=4) are shown by open circles, with antisense SBPase transgenic plants represented by closed circles (78% WT activity, n=4) and closed squares (45% WT activity, n=4). Error bars are the standard error of the mean.
Discussion
Using antisense tobacco plants with a range of SBPase activities, it has been demonstrated that reductions in SBPase activity reduced leaf net CO2 assimilation rate substantially (Figs 1, 4–7), and that this reduction in photosynthesis was greatest in high light intensity, particularly at high CO2 concentrations (Figs 1, 3, 7). This is because the reduced SBPase activity affects the regeneration capacity of the Calvin cycle (Fig. 7) and confirms the importance of SBPase in the Calvin cycle (Raines, 2003). For whole seedlings (Fig. 2), decreased SBPase activity reduced the quantum efficiency of PSII electron transport. The imbalance between the capacity for RuBP regeneration and CO2 fixation in the antisense plants led to a reduced consumption of photosynthetic electron transport products (ATP and NADPH) resulting in decreased photosynthetic efficiency and increased NPQ (Fig. 2; see also von Caemmerer et al., 2004). In the antisense plants the photosynthetic efficiency of guard cells was reduced to a similar extent to that in the adjacent mesophyll (Fig. 3), suggesting the same SBPase-caused changes in Calvin cycle activity occurred in the guard cells. This novel observation provides further support for the view that Calvin cycle activity in guard cells is a major sink for ATP and NADPH produced through photosynthetic electron transport (Cardon and Berry, 1992; Lawson et al., 2002, 2003; von Caemmerer et al., 2004).
Despite reductions in both guard and mesophyll cell photosynthetic electron transport and the consequent higher Ci, the stomatal opening and closing responses to either mixed blue/red or red light in the antisense SBPase plants followed a similar pattern to those in WT plants (Fig. 4). These date provide further support for the suggestion from previous antisense studies that photosynthetic CO2 fixation in guard cells is not essential for light-induced stomatal opening in tobacco (von Caemmerer et al., 2004; Barioli et al., 2008). However, some subtle but notable differences were observed in both the rate and extent of stomatal opening in the antisense SBPase plants compared to WT. The gs reached following a large step increase in either mixed blue/red or red light was higher in the antisense SBPase plants than in WT plants. Furthermore illumination with red light alone resulted in a more rapid rate of stomatal opening in the SBPase plants (Fig. 5B, D). The rate of opening under red illumination was strongly correlated with the final conductance, whilst under mixed blue/red illumination duel mechanisms probably masked this relationship (Fig. 6). The fact that no difference in opening rate was observed under mixed blue/red is possibly due to an over-riding specific blue light response (Talbott and Zeiger, 1993), which does not depend on either guard or mesophyll cell photosynthesis (see Introduction). Red-light-induced stomatal opening responses have been shown to be dependent on photosynthetic electron transport (Tominaga et al., 2001). Therefore the 20% decrease in electron transport rate, estimated from , in both the guard cells and the mesophyll of the antisense SBPase plants (Figs 2, 3) might be expected to reduce the light-induced stomatal opening (Fig. 8B). However, inhibition of electron transport using DCMU prevents the development of a proton gradient across the thylakoid membrane, thus reducing ATP availability for cation uptake (Tominaga et al., 2001). By contrast, in the antisense SBPase plants ATP levels may be increased, despite the reduced photosynthetic electron transport, because of the reduced ATP consumption by the Calvin cycle (Figs 4, 5). Baroli et al. (2008) reported that red-light-induced stomatal opening was unaffected in transgenic plants with reduced electron transport and reduced ATP.
Previous experiments have indicated that changes in Ci act as a signal modulating stomatal aperture, with Ci usually maintained around 230–250 μmol mol−1 (Mott, 1988). However, the internal CO2 concentration (Ci) in the SBPase antisense plants was significantly higher than in WT plants, but the stomatal conductance was greater (Figs 4, 5). This result was unexpected as Ci concentrations of >250 μmol mol−1 in the antisense SBPase plants would have been expected to reduce gs (Thomas et al., 1991, in tobacco; Wong et al., 1978; Morison, 1987; Mott, 1990). Despite the well-documented correlation found between Ci and gs it is difficult to explain the results obtained from studies using antisense SBPase and Rubisco plants based on this relationship.
Although it has long been suggested that the opening of stomata in response to red light involves guard cell photosynthesis, Roelfsema et al. (2002) suggested that the PAR light response in intact leaves was due to CO2 depletion by the mesophyll. Our data do not support this proposal as in both wild-type and antisense plants stomata opened in response to increasing light when Ci was kept constant (Fig. 8B), as has also been shown in previous work (Morison and Jarvis, 1983; Messinger et al., 2006). An alternative explanation of these results is that guard cells respond to CO2 concentrations nearer Ca rather than the estimated bulk mesophyll Ci (von Caemmerer et al., 2004). In support of this suggestion, many of the small differences between the stomatal CO2 response curves in WT and antisense plants in Fig. 7B are removed if the abscissa is Ca not Ci (data not shown).
Recently it has been proposed that stomatal responses to light and Ci are dependent on the balance between the photosynthetic carbon reduction reactions and electron transport capacity (Messinger et al., 2006). In this study, a group of antisense plants was identified with small reductions in SBPase activity (20–35% activity) in which the regenerative capacity was reduced without any effects on the carboxylation efficiency. In this group of transgenic plants the closing response to increasing Ci was reduced compared with WT plants. Reduction of Calvin cycle activity, whether through reduced substrate availability (Ci) or antisense technology, could result in increased chloroplastic ATP levels (Farquhar et al., 1980; Farquhar and Wong, 1984; Messinger et al., 2006). Increases in ATP could provide either a sensory mechanism (Buckley et al., 2003) or be directly used for proton pumping at the plasma membrane, which is known to be activated under red light and would increase stomatal aperture (Tominaga et al., 2001). Several years ago, Zhu et al. (1998) proposed that zeaxanthin was the sensory mechanisms for Ci, demonstrating a strong positive correlation between stomatal aperture and guard cell zeaxanthin concentration. This could account for the differences observed; decreased Calvin cycle activity and reduced ATP utilization would result in an increase in the H+ electrochemical gradient across the thylakoid membrane, which in turn would induce zeaxanthin formation as part of plants non-photochemical quenching mechanism (Niyogi et al., 2005). Such an increase in guard cell zeaxanthin concentration would, according to Zhu et al. (1998), result in greater stomatal apertures.
Leaf and guard cell apoplastic sucrose concentration have also been proposed as a mechanism linking photosynthesis and transpiration rate with stomatal movements (Outlaw and De Vlieghere-He, 2001; Kang et al., 2007). These data presented here would fit with the observation that lower photosynthetic rates in apoplastic phloem loaders, such as tobacco, would result in a reduced apoplastic sucrose concentration and consequently greater stomatal aperture (Kang et al., 2007).
Conclusion
Our data demonstrate that both guard and mesophyll photosynthetic electron transport are reduced to a similar degree in antisense plants with reduced levels of SBPase activity. It has been shown that reducing Calvin cycle regenerative capacity led to reductions in guard cell photosynthetic efficiency of electron transport. The fact that pore opening is still entirely functional and that apertures are as great as WT plants, strengthen suggestions from previous antisense studies that photosynthetic CO2 fixation in guard cells is not specifically necessary for the primary light-induced stomatal opening response in tobacco (von Caemmerer et al., 2004; Barioli et al., 2008). However, reductions in SBPase activity resulted in more rapid stomatal opening under red light illumination and a higher steady-state stomatal conductance, suggesting a role for mesophyll or guard cell photosynthesis in the fine-tuning of stomatal responses to light and CO2.
Acknowledgments
Financial support for this research was provided by the Department of Biological Sciences, University of Essex (TL and SL). We acknowledge Susanne von Caemmerer (Australian National University, Canberra) for her suggestions during this study.
References
- Baroli I, Price D, Badger MR, von Caemmerer S. The contribution of photosynthesis to the red light response of stomatal conductance. Plant Physiology. 2008;146:737–747. doi: 10.1104/pp.107.110924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbagallo RP, Oxborough K, Pallett KE, Baker NR. Rapid, noninvasive screening for perturbations of metabolism and plant growth using chlorophyll fluorescence imaging. Plant Physiology. 2003;132:485–493. doi: 10.1104/pp.102.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley TN, Mott KA, Farquhar GD. A hydromechanical and biochemical model of stomatal conductance. Plant, Cell and Environment. 2003;26:1767–1785. [Google Scholar]
- Cardon ZG, Berry J. Effects of O2 and CO2 concentration on the steady-state fluorescence yield of single guard-cell pairs in intact leaf-disks of Tradescantia albiflora: evidence for Rubisco-mediated CO2 fixation and photorespiration in guard cells. Plant Physiology. 1992;99:1238–1244. doi: 10.1104/pp.99.3.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, von Caemmerer S, Berry JA. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta. 1980;149:78–90. doi: 10.1007/BF00386231. [DOI] [PubMed] [Google Scholar]
- Farquhar GD, Wong SC. An empirical model of stomatal conductance. Australian Journal of Plant Physiology. 1984;11:191–210. [Google Scholar]
- Frechilla S, Talbott LD, Zeiger E. The CO2 response of Vicia faba guard cells acclimates to growth environment. Journal of Experimental Botany. 2002;53:545–550. doi: 10.1093/jexbot/53.368.545. [DOI] [PubMed] [Google Scholar]
- Frechilla S, Talbott LD, Zeiger E. The blue light-specific response of Vicia faba stomata acclimates to growth environment. Plant and Cell Physiology. 2004;45:1709–1714. doi: 10.1093/pcp/pch197. [DOI] [PubMed] [Google Scholar]
- Harrison EP, Olcer H, Lloyd JC, Long SP, Raines CA. Small decreases in SBPase cause a linear decline in the apparent RuBP regeneration rate, but do not affect Rubisco carboxylation capacity. Journal of Experimental Botany. 2001;52:1779–1784. doi: 10.1093/jexbot/52.362.1779. [DOI] [PubMed] [Google Scholar]
- Harrison EP, Willingham NM, Lloyd JC, Raines CA. Reduced sedoheptulose-1,7-bisphosphatase levels in transgenic tobacco lead to decreased photosynthetic capacity and altered carbohydrate accumulation. Planta. 1998;20:27–36. [Google Scholar]
- Hudson GS, Evans JR, von Caemmerer S, Arvidsson YBC, Andrews TJ. Reduction of ribulose-1,5-bisphosphate carboxylase/oxygenase content by antisense RNA reduces photosynthesis in transgenic tobacco plants. Plant Physiology. 1992;98:294–302. doi: 10.1104/pp.98.1.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Outlaw WH, Jr, Anderson PC, Fiore GB. Guard cell apoplastic sucrose concentration: a link between leaf photosynthesis and stomatal aperture size in apoplastic phloem loader Vicia faba L. Plant. Cell and Environment. 2007;30:551–558. doi: 10.1111/j.1365-3040.2007.01635.x. [DOI] [PubMed] [Google Scholar]
- Kuiper PJC. Dependence upon wavelength of stomatal movement in epidermal tissue of Senecio odoris. Plant Physiology. 1964;39:952–955. doi: 10.1104/pp.39.6.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR. Responses of photosynthetic electron transport in stomatal guard cells and mesophyll cells in intact leaves to light, CO2, and humidity. Plant Physiology. 2002;128:52–62. [PMC free article] [PubMed] [Google Scholar]
- Lawson T, Oxborough K, Morison JIL, Baker NR. The response of guard cell photosynthesis to CO2, O2, light and water stress in a range of species are similar. Journal of Experimental Botany. 2003;54:1734–1752. doi: 10.1093/jxb/erg186. [DOI] [PubMed] [Google Scholar]
- Leegood RC. Enzymes of the Calvin cycle. Methods in Plant Biochemistry. 1990;3:15–37. [Google Scholar]
- Lodge RJ, Dijkstra P, Drake BG, Morison JIL. Stomatal acclimation to increased CO2 concentration in a Florida scrub oak species Quercus myrtifolia Willd. Plant, Cell and Environment. 2001;24:77–88. [Google Scholar]
- Messinger SM, Buckley TN, Mott KA. Evidence for involvement of photosynthetic processes in the stomatal response to CO2. Plant Physiology. 2006;140:771–778. doi: 10.1104/pp.105.073676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morison JIL. Intercellular CO2 concentration and stomatal response to CO2. In: Zeiger E, Farquhar GD, Cowan IR, editors. Stomatal function. Stanford: Stanford University Press; 1987. pp. 229–251. [Google Scholar]
- Morison JIL, Jarvis PG. Direct and indirect effects of light on stomata. II. In Commelina communis L. Plant. Cell and Environment. 1983;6:103–109. [Google Scholar]
- Mott KA. Do stomata respond to CO2 concentrations other than intercellular? Plant Physiology. 1988;86:200–203. doi: 10.1104/pp.86.1.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA. Sensing of atmospheric CO2 by plants. Plant, Cell and Environment. 1990;13:731–737. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiology Plantarum. 1962;15:473–497. [Google Scholar]
- Muschak M, Willmitzer L, Fisahn J. Gas-exchange analysis of chloroplastic fructose-1,6-bisphosphatase antisense potatoes at different air humidities and at elevated CO2. Planta. 1999;209:104–111. doi: 10.1007/s004250050611. [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Li X-P, Rosenberg V, Jung H-S. Is PsbS the site of non-photochemical quenching in photosynthesis? Journal of Experimental Botany. 2005;56:375–382. doi: 10.1093/jxb/eri056. [DOI] [PubMed] [Google Scholar]
- Olsen RL, Pratt RB, Gump P, Kemper A, Tallman G. Red light activates a chloroplast-dependent ion uptake mechanism for stomatal opening under reduced CO2 concentrations in Vicia spp. New Phytologist. 2002;153:497–508. doi: 10.1046/j.0028-646X.2001.00337.x. [DOI] [PubMed] [Google Scholar]
- Outlaw WH. Integration of cellular and physiological functions of guard cells. Critical Reviews in Plant Science. 2003;22:503–529. [Google Scholar]
- Outlaw WH, Jr, De Vleighere-He X. Transpiration rate: an important factor in controlling sucrose content of the guard cell apoplast of broad bean. Plant Physiology. 2001;126:1716–1724. doi: 10.1104/pp.126.4.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxborough K. Imaging of chlorophyll a fluorescence: theoretical and practical aspects of an emerging technique for the monitoring of photosynthetic performance. Journal of Experimental Botany. 2004;55:1195–1205. doi: 10.1093/jxb/erh145. [DOI] [PubMed] [Google Scholar]
- Oxborough K, Baker NR. Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components: calculation of qP and without measuring . Photosynthesis Research. 1997;54:135–142. [Google Scholar]
- Quick WP, Schurr U, Fichtner K, Schulze E-D, Rodermel SR, Bogorad L, Stitt M. The impact of decreased Rubisco on photosynthesis, growth, allocation and storage in tobacco plants which have been transformed with antisense rbcS. The Plant Journal. 1991;1:51–58. [Google Scholar]
- Raines CA. The Calvin cycle revisited. Photosynthesis Research. 2003;75:1–10. doi: 10.1023/A:1022421515027. [DOI] [PubMed] [Google Scholar]
- Raschke K. Simultaneous requirement of carbon dioxide and abscisic acid for stomatal closing in Xanthium strumarium L. Planta. 1975;125:243–259. doi: 10.1007/BF00385601. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hanstein S, Fell HH, Hedrich R. CO2 provides an intermediate link in the red light response of guard cells. The Plant Journal. 2002;32:65–75. doi: 10.1046/j.1365-313x.2002.01403.x. [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R. In the light of stomatal opening: new insights into ‘the Watergate’. New Phytologist. 2005;167:665–691. doi: 10.1111/j.1469-8137.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- Sharkey TD, Raschke K. Separation and measurement of direct and indirect effects of light on stomata. Plant Physiology. 1981;68:33–40. doi: 10.1104/pp.68.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M, Schulze D. Does Rubisco control the rate of photosynthesis and plant growth: an exercise in molecular ecophysiology. Plant, Cell and Environment. 1994;17:465–487. [Google Scholar]
- Talbott LD, Srivastava A, Zeiger E. Stomata from growth-chamber grown Vicia faba have an enhanced sensitivity to CO2. Plant, Cell and Environment. 1996;19:1188–1194. doi: 10.1111/j.1365-3040.1996.tb00434.x. [DOI] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. Sugar and organic acid accumulation in guard cells of Vicia faba in response to red and blue light. Plant Physiology. 1993;102:1163–1169. doi: 10.1104/pp.102.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbott LD, Zeiger E. The role of sucrose in guard cell osmoregulation. Journal of Experimental Botany. 1998;9:329–337. [Google Scholar]
- Thomas C, Davis SD, Tallman G. Responses of senescing and non-senescing leaves of Nicotiana glauca to changes in intercellular concentrations of carbon dioxide. Plant, Cell and Environment. 1991;14:971–978. [Google Scholar]
- Tominaga M, Kinoshita T, Shimazaki K. Guard-cell chloroplasts provide ATP required for H+ pumping in the plasma membrane and stomatal opening. Plant and Cell Physiology. 2001;42:795–802. doi: 10.1093/pcp/pce101. [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS. Guard cell metabolism and CO2 sensing. New Phytologist. 2005;165:665–682. doi: 10.1111/j.1469-8137.2004.01276.x. [DOI] [PubMed] [Google Scholar]
- von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA. Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. Journal of Experimental Botany. 2004;55:1157–1166. doi: 10.1093/jxb/erh128. [DOI] [PubMed] [Google Scholar]
- Willmer C, Fricker M. Stomata. 2nd edn. London: Chapman and Hall; 1996. [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD. Leaf conductance in relation to assimilation in Eucalyptus pauciflora Sieb. ex Spreng. Influence of irradiance and partial pressure of carbon dioxide. Plant Physiology. 1978;78:821–825. doi: 10.1104/pp.62.4.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WH, Assmann SM. Photosynthesis by guard-cell chloroplasts of Vicia faba L.: effects of factors associated with stomatal movement. Plant and Cell Physiology. 1993;34:1015–1022. [Google Scholar]
- Zeiger E, Talbott LD, Frechilla S, Srivastava A, Zhu JX. The guard cell chloroplast: a perspective for the twenty-first century. New Phytologist. 2002;153:415–424. doi: 10.1046/j.0028-646X.2001.NPH328.doc.x. [DOI] [PubMed] [Google Scholar]
- Zhu J, Talbott LD, Jin X, Zeiger E. The stomatal response to CO2 is linked to changes in guard cell zeaxanthin. Plant, Cell and Environment. 1998;21:813–820. [Google Scholar]