Abstract
Top–down control analysis (TDCA) is a useful tool for quantifying constraints on metabolic pathways that might be overcome by biotechnological approaches. Previous studies on lipid accumulation in oilseed rape have suggested that diacylglycerol acyltransferase (DGAT), which catalyses the final step in seed oil biosynthesis, might be an effective target for enhancing seed oil content. Here, increased seed oil content, increased DGAT activity, and reduced substrate:product ratio are demonstrated, as well as reduced flux control by complex lipid assembly, as determined by TDCA in Brassica napus (canola) lines which overexpress the gene encoding type-1 DGAT. Lines overexpressing DGAT1 also exhibited considerably enhanced seed oil content under drought conditions. These results support the use of TDCA in guiding the rational selection of molecular targets for oilseed modification. The most effective lines had a seed oil increase of 14%. Moreover, overexpression of DGAT1 under drought conditions reduced this environmental penalty on seed oil content.
Keywords: Brassica napus, control analysis, diacylglycerol acyltransferase, oil content, oilseed modification
Introduction
Oil crops have enormous economic importance both as a source of dietary lipids and for renewable industrial feedstocks. Of these crops, oilseed rape (Brassica napus and B. rapa) is the most important in temperate climates, such as northern Europe and Canada, and contributes about 12% to total world oil and fat production (Gunstone et al., 2007). Increasing concerns about sustainable agricultural output, coupled with a desire to use plants as sources of raw materials for industrial products, such as biodiesel, have led to recent initiatives to manipulate the quality and quantity of seed oil (Murphy, 2005). Breeding offers one option, but more direct intervention through genetic manipulation has been limited in many cases by the lack of knowledge about the regulation of lipid metabolism (Ohlrogge and Jaworski, 1997). To that end, attempts have been made using biochemical experiments to pinpoint particular enzymatic steps that are candidates for manipulation. A more comprehensive assessment of regulation can be carried out by metabolic control analysis (Fell, 1992) and one approach of this technique has been applied through top–down control analysis (TDCA) (Brand, 1996) to two important oil crops (Ramli et al., 2002b). Such experiments allow one to identify which parts of a metabolic pathway are more amenable to alteration and, in more detail, which steps exert significant control and may be worth manipulating genetically.
In developing seeds of oleaginous crops, diacylglycerol acyltransferase (DGAT) catalyses the acyl-coenzyme A (acyl-CoA)-dependent acylation of sn-1,2-diacylglycerol (DAG) to generate triacylglycerol (TAG) (Weselake, 2005). Previous experiments with developing seeds of canola (Brassica napus) have suggested that DGAT activity may have a substantial effect on carbon flow into seed oil (Perry and Harwood, 1993; Perry et al., 1999). This has been further supported by the observation that an Arabidopsis thaliana mutant with decreased seed oil content also had decreased DGAT activity (Katavic et al., 1995) and this TAG1 mutant was shown to have a mutation in the gene encoding type-1 DGAT (Zou et al., 1999). Furthermore, the seed oil content of Arabidopsis increased when the cDNA encoding type-1 DGAT from the same species was overexpressed during seed development (Jako et al., 2001). More recently, type-2 DGAT has also been shown to have a substantial influence on the content and composition of some plant seed oils containing unusual fatty acids (Kroon et al., 2006; Cahoon et al., 2007) and DGAT is a key determinant for oil content and composition in maize (Zheng et al., 2008).
In addition to the above, control analysis experiments with B. napus suggested that, in contrast to other crops (Ramli et al., 2002b), there was more control exerted during lipid assembly than during fatty acid synthesis. Moreover, overexpression of a sn-glycerol-3-phosphate dehydrogenase was recently shown to increase seed oil content greatly, which further illustrates the importance of glycerolipid assembly in determining seed oil content in B. napus (Vigeolas et al., 2007). This makes the manipulation of DGAT a more attractive proposition for increasing seed oil content in B. napus compared with other crops. The current study demonstrates, using the example of increased oil content in Brassica napus lines overexpressing DGAT, that metabolic control analysis can effectively identify constraints on biosynthetic pathways. Moreover, such control analysis can provide rational insights into metabolism and, in this way, is helpful for attempts at genetic manipulation.
Materials and methods
DGAT overexpression construct and transformation
cDNA encoding Brassica napus DGAT1 (BnDGAT1) was previously isolated from microspore-derived cell suspension cultures of B. napus L. cv. Jet Neuf (Nykiforuk et al., 2002) and was introduced into B. napus L. cv. Westar. BnDGAT1 cDNA was introduced into a modified binary vector pKYLX71 (Schardl et al., 1987), in which the 35S constitutive promoter was replaced by seed-specific napin (D1) or cruciferin (D2) promoters, respectively. Brassica napus L. cv. Westar was transformed with cDNA encoding DGAT1 that was mobilized into Agrobacterium tumefaciens strain GV3101 by triparental mating using pRK2013 as helper plasmid, essentially according to the protocol of Moloney et al. (1989) with minor modifications. Methods and data verifying transgene integration and expression are provided in Supplementary data available at JXB online.
An Arabidopsis DGAT1 (AtDGAT1) was also cloned (Zou et al., 1999) and the B. napus cultivar Quantum was transformed by the cotyledonary petiole method (Moloney et al., 1989); transgenic lines were screened, selected, and characterized as described previously for Arabidopsis (Jako et al., 2001).
Radiolabelling of embryos and lipid analysis for TDCA
Brassica napus L. cv. Westar (untransformed or expressing BnDGAT1) pods were harvested and opened, and the embryos dissected out quickly and placed in cold 0.1 M potassium phosphate buffer (pH 7.0) containing 0.1 M sorbitol before use. Seeds were selected for uniformity of mass and morphological appearance. The dissected embryos were pooled and incubations carried out with three to six replicates, each containing 10 embryos for seeds 21–24 days after flowering (DAF) and five embryos for seeds 27–35 DAF. Incubations were in 1 ml 0.1 M sorbitol, 0.1 M potassium phosphate buffer (pH 7.0) containing 1 μCi of [1-14C]acetate (2.11 GBq mmol−1) or 1 μCi [U-14C]glycerol (5.51 GBq mmol−1) with gentle shaking at 20 °C for 6 h, which was within the linear labelling period. The addition of radiolabelled precursors was kept to a minimum to avoid changing their substrate pools significantly. At the end of the incubations, samples were washed three times in unlabelled medium and then heated with 1.25 ml isopropanol at 70 °C for 30 min to inactivate endogenous lipase activity.
For single-manipulation TDCA, embryos from pods 27 DAF were used. These were pre-incubated with 2 mM oleic acid dissolved in 1 mM tetramethylammonium hydroxide in 0.1 M sorbitol for 2 h with gentle shaking at 20 °C. After rinsing the embryos with 0.1 M sorbitol–0.1 M potassium phosphate (pH 7.0), they were incubated with radioactivity as above. Control samples (no oleate treatment) were pre-incubated for 2 h with 1 mM tetramethylammonium hydroxide in 0.1 M sorbitol. The standard incubation time for TDCA experiments was 6 h at 20 °C. Such conditions have been used successfully before for TDCA with plant preparations (Ramli et al., 2002b) although it is accepted that these conditions are not physiological. Nevertheless, lipid labelling was linear throughout the 6 h, showing that any disturbance to lipid metabolism during the incubation was minimal.
Following this incubation, lipids were extracted from embryos at 4 °C using a two-phase method to give quantitative extraction even for highly polar components (Garbus et al., 1963) as modified for plant tissues (Smith et al., 1982). Aliquots were taken of the upper aqueous and lower chloroform phases for radioactivity counting. The lower (lipid) phase was separated by thin layer chromatography (TLC) as described by Ramli et al. (2002a). In addition, 2-D TLC used, as first solvent, chloroform/methanol/water (65:25:4, by vol.) and, for the second dimension, chloroform/acetone/methanol/water/acetic acid (50:20:10:10:5, by vol.). Individual bands were routinely revealed with UV light after spraying with 0.2% (w/v) 8-anilino-1-naphthalenesulphonic acid in anhydrous methanol. Further identification of individual lipid classes was by comparison to authentic markers and spraying with colour reagents (Kates, 1986). Separation of fatty acid methyl esters (FAMEs) and quantification of radioactivity were as previously described (Ramli et al., 2002a) except that a Unicam ProGC gas chromatogram was used. If the amount of radioactivity in FAME samples was insufficient for radio-GLC, then silver nitrate TLC was utilized. For the latter, the method of Christie (2003) was used including the procedure to remove silver ions before radioactive counting.
Acyl-CoA and acyl-acyl carrier proteins (acyl-ACPs) were extracted from the aqueous phase obtained from the total lipid extraction by rapid reverse-phase partition chromatography through a Waters Sep-Pak C18 cartridge as described by Stymne and Glad (1981). A full discussion of the method and control experiments is given in Ramli et al. (2002a). Control values for acyl-CoA concentrations for 27 DAF embryos were 7.1±2.2 μM.
sn-Glycerol 3-phosphate was quantified by two enzymic methods (Lang, 1984; Ramli et al., 2002a). The pool size did not change significantly following addition of oleate in the TDCA experiment (data not shown). This result meant that changes in radiolabelling from [U-14C]glycerol were not due to alterations in the glycerol 3-phosphate pool.
In addition to the standard Kennedy pathway using DGAT, two alternative methods for the synthesis of TAG have been reported in plants. Phospholipid:diacylglycerol acyltransferase and diacylglycerol:diacylglycerol acyltransferase activities were assessed by using microsomal fractions and [1-14C]dioleoylphosphatidylcholine and [14C]dioleoylglycerol substrates, respectively (Stahl et al., 2004).
Radioactivity was determined using a LKB Wallac 1209 Rackbeta liquid-scintillation counter (Wallac Oy, Turku, Finland). All samples were evaporated to dryness under a stream of nitrogen in polyethylene disposable vials. The samples were counted in 10 ml of Optifluor scintillant (Canberra Packard, Pangbourne, Berks., UK). Quench correction was made automatically by the external-standard channels-ratio method.
TDCA
To carry out TDCA of lipid biosynthesis it is necessary to divide conceptually the whole pathway into two blocks of reactions which are connected by an intermediate pool. In this case, Block A contains all the reactions of de novo fatty acid synthesis (which take place in the plastid). The product of this synthesis, acyl-CoA, accumulates in the cytosol, and was the chosen intermediate. Within Block A were grouped all of the enzymes which would be used in the metabolism of [1-14C]acetate to fatty acids and this includes acetyl-CoA synthase, acetyl-CoA carboxylase, the individual reactions of Type II fatty acid synthase, stearoyl-ACP synthase, acyl-ACP thioesterases, and acyl-CoA synthases.
Block B contains all the enzymes which form complex lipids from the cytosolic acyl-CoA pool. These reactions take place in the endoplasmic reticulum and include not only the Kennedy pathway for TAG assembly but may include a contribution from additional reactions such as phospholipid:diacylglycerol acyltransferase or diaclyglycerol:diacylglycerol acyltransferase (Stahl et al., 2004) and other acyl exchange reactions (Bates et al., 2007).
For TDCA, changes in the steady-state levels of the acyl-CoA pool were made by the addition of exogenous oleate and these were monitored by following the incorporation of radiolabel into lipids. A full discussion of these methods and details of the specific equations used to calculate group flux control coefficients are given in Ramli et al. (2002b).
Microsome preparation and DGAT activity assay
Microsomes were prepared from frozen developing seeds (27±1 DAF) of B. napus cv. Westar as previously described (Sorensen et al., 2005) with minor modifications. DGAT assays were performed in triplicate according to the method of Little et al. (1994), with final concentrations of 0.2 M HEPES-NaOH, pH 7.4, 3 mM MgCl2, 330 μM sn-1,2-diolein in 0.2% (w/v) Tween 20, and 15 μM [1-14C]oleoyl-CoA in the reaction mixture. Radiolabelled oleoyl-CoA was synthesized according to the method of Taylor et al. (1990). Protein content was determined using the Bio-Rad protein microassay (Bio-Rad Laboratories, Hercules, CA, USA) based on the Bradford procedure (Bradford, 1976) using bovine serum albumin as a standard.
DAG/TAG analysis of developing seeds
Lipid extraction was performed using a hexane:isopropanol method adapted from Hara and Radin (1978). TAG and DAG were isolated from 50 μl of total lipid by TLC (hexane:diethyl ether:acetic acid (80:20:1, by vol.) and scraped into vials for methylation. Total lipid, DAG, and TAG fractions were methylated with 1.2 ml sodium methoxide (2% in methanol, w/v) for 30 min at room temperature. Following the addition of 1 ml of dd H2O, the FAMEs were extracted with hexane (2×2 ml), evaporated to dryness, and resuspended in 0.5 ml hexane for GC analysis. FAME analysis was carried out as previously published (Sorensen et al., 2005). Peaks were identified by comparison of retention times to FAME standards and quantified, based on peak area relative to the internal standards (15:0 or 17:0).
Lipid analysis of mature seeds
Total lipid of mature seed was analysed by low resolution-nuclear magnetic resonance (LR-NMR) and by gravimetric analysis. Approximately 5 g mature seeds were added to flat-bottomed 16×150 mm test tubes (to a fill height of 4 cm) and the total lipid content was measured in a Minispec LR-NMR instrument (Bruker Optics Canada, Milton, ON, Canada). The instrument was calibrated with mature B. napus seed of known oil content obtained from the Grain Research Laboratory of the Canadian Grain Commission (Winnipeg, MB, Canada). Lipid analysis for field-grown B. napus cv. Quantum transformed with AtDGAT1 was performed as described previously (Taylor et al., 2001).
Field trial sites/design
BnDGAT1:
T2 seeds from 26 transformants carrying the BnDGAT1 overexpression construct, 2 null vector controls and one untransformed Westar control were planted in triplicate in a complete randomized block design at two field locations (near Vegreville and Edmonton, AB, Canada) in the summer of 2005. Developing seeds were harvested from the Edmonton trial site at 27 DAF and mature seed was harvested from both locations and analysed as above.
AtDGAT1:
Homozygous T4 B. napus cv. Quantum lines overexpressing AtDGAT1 were grown in confined field trials near Saskatoon, SK, Canada in the summer of 2003. Total annual precipitation at Saskatoon during 2003 was 233 mm, compared with the average annual precipitation of 356±97 (standard deviation) mm between 1993 and 2006 (National Climate Archive, Environment Canada).
Results and discussion
Metabolic control analysis
Single manipulation TDCA was performed on developing seeds of Brassica napus cv. Westar expressing BnDGATI. The principles of these experiments and their application to oil fruit tissues have been published (Ramli et al., 2002b). The lipid biosynthetic pathway was conceptually divided into Block A (fatty acid biosynthesis) and Block B (lipid assembly) with the acyl-CoA pool being the chosen intermediate. The flux through the pathway was changed by the addition of oleate (the major fatty acid in canola oil). This inhibited fatty acid synthesis but stimulated lipid assembly, which was measured independently by using [U-14C]glycerol precursor.
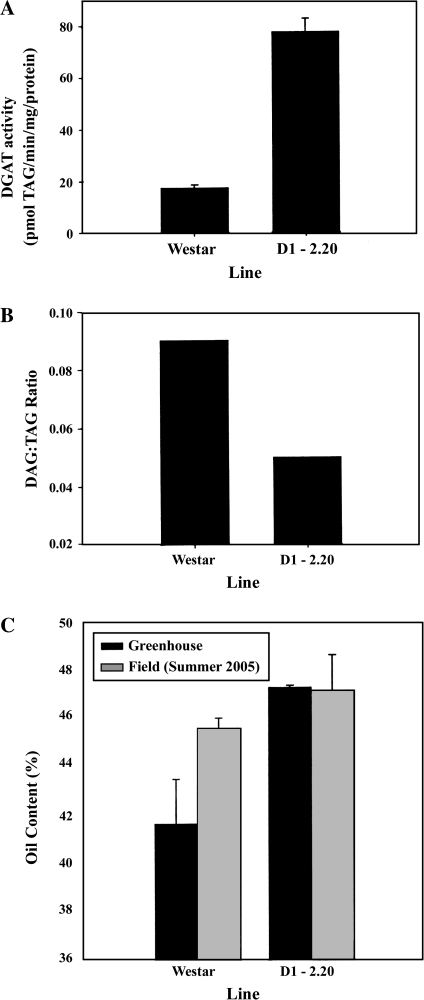
In addition, since the biochemical work described in the Introduction had suggested that DGAT could be constraining carbon flux to lipid accumulation in oilseed rape, it was of interest to see whether TDCA supported that, overall, the reactions in Block B exerted significant flux control. This was important because the ability to affect metabolic pathways through genetic manipulation is clearly dependent on the flux control exerted by those individual steps changed. In Table 1, the results of a number of independent experiments are shown for the B. napus transformant D1-2.20 which contained overexpressed DGATI (see Materials and methods). Exogenous oleate caused a decrease in Block A flux but an increase in that for Block B (Table 1). This was anticipated since the increased availability of fatty acid (oleate) would feedback to reduce de novo fatty acid synthesis through its activation to acyl-CoA, the chosen intermediate of the TDCA. The increase in total lipid labelling from [U-14C]glycerol indicates that the supply of fatty acids can exert a significant effect on total lipid assembly in keeping with observations for other oil crops (Bao and Ohlrogge, 1999). These data, together with those for control cv. Westar, were used to derive Block flux control coefficients (Table 2) using the principles and equations described previously (Ramli et al., 2002b). In contrast to other crops such as olive and oil palm (Ramli et al., 2002b), oilseed rape contained the majority of the control of flux within the Block B reactions. The flux control exerted by Block B decreased from 69% in non-transformed B. napus to 51% in transformant D1-2.20. This observation is entirely consistent with DGAT exerting significant flux control, which can be reduced if the activity of the enzyme during seed development is increased. Indeed, microsomal DGAT activity in T3 developing seeds of the transformed line was ∼4-fold greater than untransformed Westar (Fig. 1A), while the DAG:TAG ratio in developing T2 seeds (27 DAF) was reduced from 0.09 to 0.05 (Fig. 1B). This is consistent with a more efficient conversion of DAG to TAG in the transformed line, which also showed a significant increase in seed oil content at maturity, under both greenhouse (T2 generation, Fig. 1C) and field conditions (T3 generation, Fig. 1C). The seed oil content in transformant D1-2.20 was the same (∼47%) under both greenhouse and field conditions, while non-transformed Westar demonstrated higher seed oil content in the field than in the greenhouse (Fig. 1C). The higher DGAT activity and its beneficial effect of increasing seed oil content of transformant D1-2.20 was further supported by an increase in the abundance of BnDGAT1 transcript in the transformant (Fig. S1 in Supplementary data available at JXB online).
Table 1.
Effect of exogenous oleate on the incorporation of radioactivity from [1-14C]acetate or [U-14C]glycerol into lipids in oilseed rape (transgenic D1-2.20)
| Experiment | [1-14C]acetate | [U-14C]glycerol | |
| 1 | Control | 11665±1028 (n=4) | 13408±1158 (n=3) |
| + Oleate | 6728±661 (n=4) | 17733±1353 (n=3) | |
| 2 | Control | 3372±659 (n=5) | 4034±172 (n=5) |
| + Oleate | 1692±127 (n=5) | 6499±595 (n=5) | |
| 3 | Control | 19793±1116 (n=4) | 6509±609 (n=4) |
| + Oleate | 12687±1585 (n=4) | 8512±384 (n=4) |
Data expressed as d.p.m./mg fresh wt. embryos and means ±SD (n=x) for separate experiments. Exogenous oleate was added at 2 mM.
Table 2.
Up-regulation of DGAT 1 changes significantly the flux control exerted over lipid biosynthesis
| Group flux control coefficients |
|||
| Experiment | *CBlkAJTL | *CBlkBJTL | |
| Transgenic D1-2.20 | 1 | 0.44 | 0.56 |
| 2 | 0.56 | 0.44 | |
| 3 | 0.47 | 0.53 | |
| (mean ±SD) | 1–3 | 0.49±0.05 | 0.51±0.05 |
| Control tissue (mean ±SD) | 4–8 | 0.31±0.02 | 0.69±0.02 |
For equations used to calculate Block flux control coefficients, see Ramli et al. (2002b).
Fig. 1.
Seed properties of B. napus cv. Westar transformed with BnDGAT1 cDNA. Westar=non-transformed control; D1-2.20=transformed line. (A) Microsomal DGAT activity during seed development [27±1 days after flowering (DAF), greenhouse conditions]. Error bars indicate standard deviation (n=3). (B) DAG:TAG ratio during seed development (27±1 DAF, greenhouse conditions). (C) Seed oil content in mature seeds grown under greenhouse conditions or field conditions (Alberta, summer 2005; error bars indicate standard deviation, n=6; P <0.05 by Student's t-test for D1-2.20 compared with Westar).
Thus, the previous evidence from biochemical experiments (Perry and Harwood, 1993; Perry et al., 1999) that DGAT may have a substantial effect on carbon flow into oilseed rape lipid accumulation has been validated by transgenic manipulation. It has been shown that increased activity of DGAT in transgenic D1-2.20 (Fig. 1A) is accompanied by a reduction in the DAG:TAG ratio (Fig. 1B) and an increased TAG content in the mature seeds (Fig. 1C). These changes in lipid biochemistry were paralleled by a noticeable reduction in the control exerted by the lipid assembly block of reactions (Block B). The control exerted by Block B decreased from 69% in cv. Westar to 51% in its transgenic line D1-2.20 (Table 2). Therefore, TDCA has supported the biochemical observations and quantified the degree of control under the experimental conditions.
Field studies under drought conditions
A field study was also conducted with B. napus L. cv. Quantum overexpressing a cDNA encoding Arabidopsis DGAT1 grown during a season when drought conditions were experienced. Non-transformed B. napus (cv. Quantum) exhibited severely reduced seed oil content (30%), compared with normal values for cv. Quantum (38%; DC Taylor, unpublished data) while the transformed lines consistently showed a higher seed oil content than non-transformed B. napus (Table 3). For the transgenic lines of cv. Quantum expressing the AtDGAT1, the yield penalty of drought conditions was reduced with almost half of the proportional loss being restored in some lines. These data demonstrate the potential utility of DGAT transgenics when grown under drought conditions.
Table 3.
Transgene copy number and seed oil content at maturity of B. napus L. cv. Quantum and several transgenic lines transformed with AtDGAT1 grown in Saskatchewan under drought conditions in the summer of 2003
| Line | Oil content (%)a | DGAT transgene copy number |
| Nt-Conb | 30.10±0.38 | – |
| 4a-2 | 31.73±0.76 | 1 |
| 7a-1 | 32.43±0.29 | 1 |
| 13a-2 | 32.28±0.61 | 1 |
| 15b-1 | 33.82±0.92 | 2–3 |
| 16b-3 | 34.20±0.73 | 1 |
| 17a-3 | 32.70±1.00 | 1 |
| 18a-4 | 32.55±0.76 | 1 |
| 21a-2 | 32.10±1.24 | 1 |
| 23a-1 | 32.59±0.76 | 1 |
| 43–1 | 33.28±0.97 | 2 |
±SE, n=4–5.
Non-transformed control.
It is well documented that seed oil content in canola is drastically reduced by severe summer weather conditions (high temperature and/or drought) during flowering (Mailer and Cornish, 1987; Nielsen, 1997; Tesfarmarium, 2004) and the present data suggest that overexpression of DGAT1 reduces this decrease in oil content. It should be noted that in the field trial (and also in greenhouse experiments) no difference in the average mass of seed per plant was found. In other field trials (data not shown) there was a negative correlation (P <0.001) between oil and protein, suggesting that seed oil increases, at least partly, at the expense of protein content.
The increased oil content for transgenics under drought conditions, together with the fact that the increase in oil content under optimal field conditions was less than the increase observed in the greenhouse, suggests there may be environmental influences on the regulation of DGAT activity and its control over glycerolipid assembly. We speculate that overexpression of DGAT1 is most effective at enhancing oil accumulation under conditions where the plant is otherwise unlikely to reach its full physiological potential for oil content due to environmental stress. Indeed, it has been demonstrated in vitro that maize embryos exposed to abscisic acid and/or high osmoticum maintain elevated levels of DGAT activity (Pacheco-Moises et al., 1997), while Arabidopsis mutants deficient in DGAT displayed heightened sensitivity to ABA and osmotic stress (Lu and Hills, 2002). Given the role of abscisic acid in mediating abiotic stress responses, these studies support the observation that DGAT overexpression reduces the penalty on oil content under suboptimal growth conditions.
Conclusions
TDCA can serve as an effective way of studying the regulation and control of metabolism. It provides quantitative information which can then be used to confirm biochemical experiments as well as to inform rational genetic manipulation. Of course, as in all scientific experiments (including growth trials), the data are applicable to the precise conditions used and this should be used as a caveat when trying to apply the results to other conditions and systems. Indeed, as we have already pointed out, there are marked differences in the control exerted by Block A and Block B reactions for lipid biosynthesis in different oil crops.
In the present study, the change in flux control following overexpression of DGAT1 in oilseed rape has been quantified. Such transgenic lines show increases in seed oil content of up to 14% as well as increased resistance to the reduction of oil content caused by drought. The use of TDCA in combination with biochemistry to define strategies to enhance the seed oil content in canola can, potentially, be of great benefit in expanding the food and non-food applications of this crop.
Supplementary data
The following supplementary data are available at JXB online.
Supplementary methods.
Fig. S1. Northern blot analysis of BnDGAT1 transcript in developing seeds of B. napus L. cv Westar.
Fig. S2. Southern blot illustrating insertion of the BnDGAT1 transgene in Brassica napus L.
Supplementary Material
Acknowledgments
This paper is dedicated to D. Philip Raney, who passed away during its preparation. This work was supported by the Alberta Agricultural Research Institute, Alberta Crop Industry Development Fund, BBRSC, UK (Grant 72/P16420), Canada Research Chairs Program, Canada Foundation for Innovation, Natural Sciences and Engineering Research Council of Canada, Saskatchewan Agriculture Development Fund, and the University of Alberta.
References
- Bao X, Ohlrogge J. Supply of fatty acid is one limiting factor in the accumulation of triacylglycerol in developing embryos. Plant Physiology. 1999;120:1057–1062. doi: 10.1104/pp.120.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PD, Ohlrogge JB, Pollard M. Incorporation of newly synthezised fatty acids into cytosolic glycerolipids in pea leaves occurs via acyl editing. Journal of Biological Chemistry. 2007;282:31206–31216. doi: 10.1074/jbc.M705447200. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brand MD. Top down metabolic control analysis. Journal of Theoretical Biology. 1996;182:351–360. doi: 10.1006/jtbi.1996.0174. [DOI] [PubMed] [Google Scholar]
- Cahoon EB, Shockey JM, Dietrich CR, Gidda SK, Mullen RT, Dyer JM. Engineering oilseeds for sustainable production of industrial and nutritional feedstocks: solving bottlenecks in fatty acid flux. Current Opinion in Plant Biology. 2007;10:236–244. doi: 10.1016/j.pbi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Christie WW. Lipid analysis. 3rd edn. Bridgewater, UK: Oily Press; 2003. [Google Scholar]
- Fell DA. Metabolic control analysis: a survey of its theoretical and experimental development. Biochemical Journal. 1992;286:313–330. doi: 10.1042/bj2860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbus J, DeLuca HF, Loomans ME, Strong FM. The rapid incorporation of phosphate into mitochondrial lipids. Journal of Biological Chemistry. 1963;238:59–63. [PubMed] [Google Scholar]
- Gunstone FD, Harwood JL, Dijkstra AJ, editors. The lipid handbook. 3rd edn. Boca Raton, FL: Taylor and Francis; 2007. [Google Scholar]
- Hara A, Radin NS. Lipid extraction of tissues with a low-toxicity solvent. Analytical Biochemistry. 1978;90:420–426. doi: 10.1016/0003-2697(78)90046-5. [DOI] [PubMed] [Google Scholar]
- Jako C, Kumar A, Wei Y, Zou J, Barton DL, Giblin EM, Covello PS, Taylor DC. Seed-specific over-expression of an Arabidopsis cDNA encoding a diacylglycerol acyltransferase enhances seed oil content and seed weight. Plant Physiology. 2001;126:861–874. doi: 10.1104/pp.126.2.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou J, Mackenzie SL, Covello PS, Kunst L. Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiology. 1995;108:399–409. doi: 10.1104/pp.108.1.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates M. Techniques in lipidology. Amsterdam: Elsevier; 1986. [Google Scholar]
- Kroon JT, Wei W, Simon WJ, Slabas AR. Identification and functional expression of a type-2 acyl-CoA:diacylglycerol acyltransferase (DGAT2) in developing castor bean seeds which has high homology to the major triglyceride biosynthetic enzyme of fungi and animals. Phytochemistry. 2006;67:2541–2549. doi: 10.1016/j.phytochem.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Lang G. Glycerol 3-phosphate. In: Bergmeyer J, Grassl M, editors. Methods of enzymatic analysis. 3rd edn. Weinheim: Verlag Chemie; 1984. pp. 525–530. [Google Scholar]
- Little D, Weselake R, Pomeroy K, Furukawa-Stoffer T, Bagu J. Solubilization and characterization of diacylglycerol acyltransferase from microspore-derived cultures of oilseed rape. Biochemical Journal. 1994;304:951–958. doi: 10.1042/bj3040951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Hills J. Arabidopsis mutants deficient in diacylglycerol acyltransferase display increased sensitivity to abscisic acid, sugars, and osmotic stress during germination and seedling development. Plant Physiology. 2002;12:1352–1358. doi: 10.1104/pp.006122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailer RJ, Cornish PS. Effects of water stress on glucosinolate and oil concentrations in the seeds of rape (Brassica napus L.) and turnip rape (Brassica rapa L. var silvestris [Lam.] Briggs) Australian Journal of Experimental Agriculture. 1987;27:707–711. [Google Scholar]
- Moloney M, Walker JM, Sharma KK. High efficiency transformation of Brassica napus using Agrobacterium vectors. Plant Cell Reports. 1989;8:238–242. doi: 10.1007/BF00778542. [DOI] [PubMed] [Google Scholar]
- Murphy DJ, editor. Plant lipids: biology, utilization and manipulation. Oxford: Blackwell Publishing; 2005. [Google Scholar]
- Neilsen D. Water use and yield of canola under dryland conditions in the Central Great Plains. Journal of Production Agriculture. 1997;10:307–313. [Google Scholar]
- Nykiforuk CL, Furukawa-Stoffer TL, Huff PW, Sarna M, Laroche A, Moloney MM, Weselake RJ. Characterization of cDNAs encoding diacylglycerol acyltransferase from cultures of Brassica napus and sucrose-mediated induction of enzyme biosynthesis. Biochimica et Biophysica Acta. 2002;1580:95–109. doi: 10.1016/s1388-1981(01)00200-1. [DOI] [PubMed] [Google Scholar]
- Ohlrogge JB, Jaworski JG. Regulation of fatty acid synthesis. Annual Review of Plant Physiology and Plant Molecular Biology. 1997;48:109–136. doi: 10.1146/annurev.arplant.48.1.109. [DOI] [PubMed] [Google Scholar]
- Pacheco-Moises F, Valencia-Turcotte L, Altuzar-Martineze M, Rodriguez-Sotres R. Regulation of acyltransferase activity in immature maize embryos by abscisic acid and the osmotic environment. Plant Physiology. 1997;114:1095–1101. doi: 10.1104/pp.114.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry HJ, Bligny R, Gout E, Harwood JL. Changes in Kennedy pathway intermediates associated with increased triacylglycerol synthesis in oil-seed rape. Phytochemistry. 1999;52:799–804. [Google Scholar]
- Perry HJ, Harwood JL. Use of [2-3H]glycerol precursor in radiolabelling studies of acyl lipids in developing seeds of Brassica napus. Phytochemistry. 1993;34:69–73. [Google Scholar]
- Ramli US, Baker DS, Quant PA, Harwood JL. Control mechanisms operating for lipid biosynthesis differ in oil-palm (Elaeis guineensis Jacq.) and olive (Olea europaea L.) callus cultures. Biochemical Journal. 2002a;364:385–391. doi: 10.1042/BJ20010202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramli US, Baker DS, Quant PA, Harwood JL. Control analysis of lipid biosynthesis in tissue cultures from oil crops shows that flux control is shared between fatty acid synthesis and lipid assembly. Biochemical Journal. 2002b;364:393–401. doi: 10.1042/BJ20010203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardl CL, Byrd AD, Benzion G, Altschuler MA, Hildebrand DF, Hunt AG. Design and construction of a versatile system for the expression of foreign genes in plants. Gene. 1987;61:1–11. doi: 10.1016/0378-1119(87)90359-3. [DOI] [PubMed] [Google Scholar]
- Smith KL, Douce R, Harwood JL. Phospholipid metabolism in the brown alga, Fucus serratus. Phytochemistry. 1982;21:569–573. [Google Scholar]
- Sorensen BM, Furukawa-Stoffer TL, Marshall KS, Page EK, Mir Z, Forster RJ, Weselake RJ. Storage lipid accumulation and acyltransferase action in developing flaxseed. Lipids. 2005;40:1043–1049. doi: 10.1007/s11745-005-1467-0. [DOI] [PubMed] [Google Scholar]
- Stahl U, Carlsson AS, Lenman M, Dahlqvist A, Huang B, Banas W, Banas A, Styme S. Cloning and functional characterization of a phospholipid: diacylglycerol acyltransferase from Arabidopsis. Plant Physiology. 2004;135:1324–1335. doi: 10.1104/pp.104.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Styme S, Glad G. Acyl exchange between oleoyl-CoA and phosphatidylcholine in microsomes of developing soya bean and its role in fatty acid desaturation. Lipids. 1981;16:298–305. [Google Scholar]
- Taylor DC, Katavic V, Zou J-T, et al. Field-testing of transgenic rapeseed cv. Hero transformed with a yeast sn-2 acyltransferase results in increased oil content, erucic acid content and seed yield. Molecular Breeding. 2001;8:317–322. [Google Scholar]
- Taylor DC, Weber N, Hogge LR, Underhill EW. A simple enzymatic method for the preparation of radiolabelled erucoyl-CoA and other long-chain fatty acid-CoAs and their characterization by mass spectrometry. Analytical Biochemistry. 1990;184:311–316. doi: 10.1016/0003-2697(90)90686-4. [DOI] [PubMed] [Google Scholar]
- Tesfarmarium EH. Modelling the soil water balance of canola (Brassica napus L. Hoyola 60) Pretoria, South Africa: University of Pretoria; 2004. [Google Scholar]
- Vigeolas H, Waldeck P, Zank T, Geigenberger P. Increasing seed oil content in oil-seed rape (Brassica napus L.) by over-expression of a yeast glycerol-3-phosphate dehydrogenase under the control of a seed-specific promoter. Plant Biotechnology Journal. 2007;5:431–441. doi: 10.1111/j.1467-7652.2007.00252.x. [DOI] [PubMed] [Google Scholar]
- Weselake RJ. Storage lipids. In: Murphy DJ, editor. Plant lipids: biology, utilization and manipulation. Oxford: Blackwell Publishing; 2005. pp. 162–225. [Google Scholar]
- Zheng P, Allen WB, Roesler K, et al. A phenylalanine in DGAT is a key determinant of oil content and composition in maize. Nature Genetics. 2008;40:367–373. doi: 10.1038/ng.85. [DOI] [PubMed] [Google Scholar]
- Zou J, Wei Y, Jako C, Kumar A, Selvaraj G, Taylor DC. The Arabidopsis thaliana TAG1 mutant has a mutation in a diacylglycerol acyltransferase gene. The Plant Journal. 1999;19:645–653. doi: 10.1046/j.1365-313x.1999.00555.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.