Abstract
Phospholipase A2 (PLA2) catalyses the hydrolysis of phospholipids into lysophospholipids and free fatty acids. Physiological studies have indicated that PLA2 is involved in stomatal movement. However, genetic evidence of a role of PLA2 in guard cell signalling has not yet been reported. To identify PLA2 gene(s) that is (are) involved in light-induced stomatal opening, stomatal movement was examined in Arabidopsis thaliana plants in which the expression of PLA2 isoforms was reduced or knocked-out. Light-induced stomatal opening in PLA2α knockout plants did not differ from wild-type plants. Plants in which PLA2β was silenced by RNA interference exhibited delayed light-induced stomatal opening, and this phenotype was reversed by exogenous lysophospholipids, which are products of PLA2. Stomatal opening in transgenic plants that over-expressed PLA2β was faster than wild-type plants. The expression of PLA2β was localized to the endoplasmic reticulum of guard cells, and increased in response to light in the mature leaf. Aristolochic acid, which inhibits light-induced stomatal opening, inhibited the activity of purified PLA2β. Collectively, these results provide evidence that PLA2β is involved in light-induced stomatal opening in Arabidopsis.
Keywords: Guard cell, light signal transduction, phospholipase A2, stomata
Introduction
Light-induced stomatal opening is critical for the uptake of CO2 during photosynthesis. Guard cells perceive light, trigger elaborate signalling pathways, and thereby regulate the stomatal aperture. Light is sensed by photoreceptors in guard cells, and induces the activation of a plasma membrane H+-ATPase through a mechanism that involves phosphorylation of the H+ ATPase C-terminus (Kinoshita and Shimazaki, 1999). C-terminal phosphorylation allows binding of 14-3-3 and activation of the proton pump (Emi et al., 2001; Kinoshita and Shimazaki, 2002). However, signalling components that are involved in the activation of H+-ATPase in response to light are still poorly identified.
It has been shown that exogenous lysophospholipids (LPLs) and free fatty acids (FFAs), which are products of PLA2 hydrolysis, activate the proton pump (Palmgren et al., 1988). Polyunsaturated fatty acids enhance light-induced stomatal opening, potentiate inward K+ channel currents, and inhibit outward K+ channel currents in guard cells (Lee et al., 1994). Thus, it has been speculated that a certain type of PLA2 may also be involved in the activation of the proton pump. However, the identity of PLA2 isoform(s) involved in guard cell signalling has not been reported. In the Arabidopsis genome, there are two groups of PLA2 genes: those that encode the low molecular weight PLA2 isoforms, and those that encode the patatin-like PLAs, which have combined PLA1 and PLA2 activities. The calcium-dependent cytosolic form of PLA2 that has been implicated in signal transduction in animal cells has not been identified in plants (Ryu, 2004). Previous studies have demonstrated that inhibitors of the low molecular weight PLA2 isoforms, such as 4-bromophenacyl bromide, aristolochic acid, and manoalide, inhibit light-induced stomatal opening (Suh et al., 1998). Four low molecular weight PLA2 isoforms have been identified in the Arabidopsis genome sequence database and shown to encode functional PLA2 enzymes (Bahn et al., 2003; Lee et al., 2005). Arabidopsis plants that are deficient in the expression of PLA2, or have reduced expression levels, may represent potentially valuable tools for identifying the role of PLA2 in guard cell signalling.
PLA2 catalyses the hydrolysis of phospholipids to produce LPLs and FFAs (including polyunsaturated fatty acids). PLA2 has been reported to be important in diverse signal transduction pathways in animal cells. Fatty acids released by PLA2, such as arachidonic acid, function as second messengers (Berk and Stump, 1999; Gijon and Leslie, 1999) and as precursors of eicosanoids, which are potent mediators of inflammation and signal transduction (Austin and Funk, 1999; Bingham III and Austen, 1999; Devillier et al., 1999). The other products of PLA2-mediated hydrolysis, LPLs, function as biological mediators to induce the activation of cellular signalling pathways, and are precursors of platelet-activating factor (Moolenaar et al., 1997). PLA2 has been shown to function in signal transduction and many other cellular processes in plant cells as well (reviewed in Munnik et al., 1998; Wang, 2001, 2004; Scherer, 2002; Ryu, 2004). The best known example of PLA2 function in plants is in the shoot gravitropic response and cell elongation (Lee et al., 2003). Plant PLA2 has also been suggested to be important for auxin-induced cell growth (Yi et al., 1996; Scherer, 2002).
To identify the PLA2 gene(s) that is (are) involved in the regulation of stomatal opening, the stomatal movements were examined of plants that had reduced expression levels of low molecular weight PLA2 genes based on our previous studies using PLA2 inhibitors (Suh et al., 1998). Genetic evidence is presented here for a role of PLA2β in the transduction of light signals that regulate stomatal opening in Arabidopsis.
Materials and methods
Plant material and chemicals
Plants (Arabidopsis thaliana Col-0) were grown for 3–4 weeks in a greenhouse at 22±2 °C with a light/dark cycle of 16/8 h. Lysophosphatidylethanolamine (LPE) and lysophosphatidylcholine (LPC) derived from soybean were purchased from Avanti Lipids Ltd., reconstituted in chloroform, dried under nitrogen gas, and then sonicated in incubation buffer (10 mM KCl and 30 mM MES-KOH, pH 6.1) immediately before use. Plants that over-expressed PLA2β or a gene fusion construct of the PLA2β promoter and glucuronidase (GUS) (PLA2β promoter::GUS) have been described in Lee et al. (2003). The generation of PLA2β RNAi-silenced plants was previously described by Lee et al. (2003). Seeds of PLA2α knockout plants, which contained a T-DNA insertion into chromosome II, 42 bp upstream of the start codon of the PLA2α gene, were obtained from TAIR (Salk_099415; At2G06925).
Assay of stomatal opening
Intact leaves of Arabidopsis were floated on a solution containing 10 mM KCl and 30 mM MES-KOH (pH 6.1) with or without LPLs. Leaf samples were incubated in the dark beginning 0.5 h prior to the photoperiod. To determine whether supplementation with LPL complemented the defect in light-induced stomatal opening in PLA2β RNAi-silenced plants, leaves were floated on incubation buffer containing LPE or LPC (50 mg l−1) and a non-ionic surfactant (0.01%) silwet (L-77), then irradiated with white light at a dose of 220 μmol m−2 s−1. Control leaves were floated on incubation buffer containing a similar concentration of the silwet but lacking LPLs, and irradiated with the same dose of white light. The abaxial epidermal layers of the leaves were peeled at 1 h intervals, and observed using bright-field microscopy (Axioskop 2, Carl Zeiss, Jena, Germany). Images were captured using a CCD camera (Axio Cam, Carl Zeiss, Jena, Germany). Aperture size was measured from the photographs using the Interactive Measurement software package AxioVision 3.0.6 (Carl Zeiss, Germany).
RNA analysis
4-week-old plants were exposed to white light (200–250 μmol m−2 s−1), then collected at the indicated time points, frozen in liquid nitrogen, and stored at –80 °C. Total RNA was extracted from the frozen tissue using an RNA isolation kit (Invitrogen). First-strand cDNAs were synthesized from 4 μg of total RNA using random primers and the ThermoScript reverse transcriptase from ThermoScript RT-PCR system (Invitrogen), according to the manufacturer's instructions. PCR amplification was carried out using 3 μl of the cDNA reaction mixture, and the following primers: 5′-GCGGCTCCGATCATACTTT-3′ and 5′-GGTTGCTTCTTCTGGCTGAA-3′ for PLA2α; or 5′-TCGCACTTCATTGATGCG-3′ and 5′-TCATAGCTCTGTTTTCATATCATTACCT-3′ for PLA2β.
Histochemical glucuronidase (GUS) staining
GUS activity was assayed in leaves and epidermis. To observe guard cells in the absence of mesophyll cells, epidermal strips were peeled off the abaxial side of the leaf. Leaves or epidermal strips were incubated in X-Gluc solution containing 50 mM sodium phosphate (pH 7.2), 0.5 mM potassium ferri- and ferro-cyanide, 2 mM X-gluc, and 0.05% Triton X-100 overnight at 37 °C. Chlorophyll was removed by sequential incubation in 50%, 70%, and 100% ethanol, for several hours each. After rehydration, samples were observed by microscopy.
PLA2 inhibitor assay
Low molecular weight PLA2β was expressed and purified using the expression vector pET-40b(+) and BL21(DE3)pLys cells (Novagen) (Lee et al., 2005). The radiolabelled substrate 1-palmitoyl-2-[14C]palmitoyl-PC was purchased from Amersham Pharmacia Biotech. To prepare the substrate solution, radiolabelled PC (1.0 μCi, 108 mCi mmol−1) was mixed with 2 μmol of unlabelled PC in chloroform, dried under a stream of N2 gas and emulsified in an appropriate volume of 1× reaction buffer by gentle sonication. Preincubation of PLA2β was initiated by the addition of inhibitor, dissolved in 0.4 N NaOH, to a final concentration of 20 μM, or NaOH alone (for the mock control samples) in a total reaction volume of 50 μl of 100 mM TRIS pH 8.0. Samples were incubated at 40 °C for 40 min, then aliquots (5 μl) were removed from the preincubation mixture and diluted into normal reaction mixture, and PLA2β enzymatic activity was determined. Reaction mixtures contained 5 μl (∼1 μg) of PLA2β in 100 μl of 100 mM TRIS-HCl (pH 8.0), emulsified PC and 1-palmitoyl-2-[14C]palmitoyl-PC, and 10 mM CaCl2. The reaction products were extracted and separated on TLC plates, as previously described by Lee et al. (2003). Bee venom low molecular weight PLA2 was analysed in parallel to determine the position of 14C-palmitic acid.
Subcellular localization of PLA2β
The TargetP program (http://www.cbs.dtu.dk/services/TargetP) predicted that the cleavage site of the signal peptide of PLA2β is located between Ser-28 and Glu-29. The putative signal peptide of PLA2β was fused in-frame to the N-terminus (XbaI-BamH1 site) of green fluorescent protein (GFP), using the transient expression vector p326mGFP-3G (a kind gift from Dr I Hwang, POSTECH). The full-length sequence of PLA2β minus the putative signal peptide, was then fused to the C-terminus (SmaI-XhoI site) of GFP to create the expression construct sp-GFP-PLA2β. sp-GFP-PLA2β was introduced into guard cells of Vicia faba by biolistic bombardment. After incubation for 16 h in the dark, the subcellular distribution of GFP was examined by fluorescence microscopy (Axioskop 2, Carl Zeiss, Jena, Germany). BiP-RFP was used as a marker protein for endoplasmic reticulum (ER) localization.
Results
Light-induced stomatal opening is induced by PLA2β
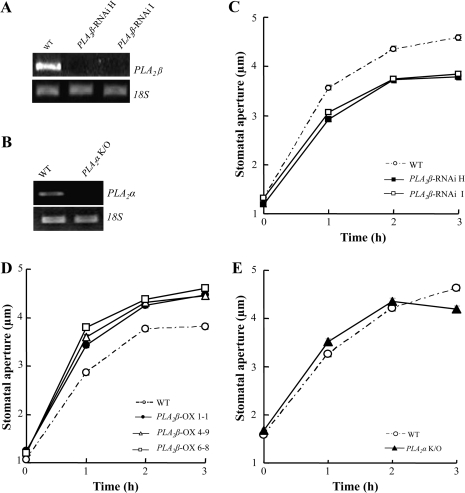
Among the four low molecular weight PLA2 genes in Arabidopsis, PLA2α and PLA2β are expressed throughout plant tissues, while PLA2γ and PLA2δ are expressed almost exclusively in the floral tissues (Lee et al., 2005). To determine whether PLA2α and PLA2β are involved in stomatal opening, several genetically modified plant lines were analysed: two RNAi lines (PLA2β-RNAi H and I), in which PLA2β mRNA levels were reduced by RNAi silencing (Fig. 1A); PLA2α knockout plants in which PLA2α mRNA was undetectable (Fig. 1B); and three plant lines over-expressing PLA2β (PLA2β OX 1-1, 4-9, and 6-8), in which the levels of PLA2β transcripts were increased, and PLA2β activity was approximately 1.5–1.6-fold higher compared to wild-type plants (Lee et al., 2003). The RNAi lines showed similar growth to that of the wild type under normal growth and experimental conditions with a short photoperiod, although, under long-day conditions, the RNAi lines grew more slowly than the wild type at the early developmental stages. This is probably because PLA2 is also involved in cell elongation (Lee et al., 2003).
Fig. 1.
Light-induced stomatal opening in PLA2β-silenced, PLA2β over-expressing, and PLA2α knockout plants. (A) The levels of PLA2β mRNA in wild-type and PLA2β RNAi-silenced plants, determined by RT-PCR. (B) The levels of PLA2α mRNA in wild-type and PLA2α knockout plants, determined by RT-PCR. (C–E) Light-induced stomatal opening in PLA2β RNAi-silenced (C), PLA2β over-expressing (D), and PLA2α knockout (E) Arabidopsis plants. Leaves were floated on a solution containing 10 mM KCl and 30 mM MES-KOH (pH 6.1), then illuminated with white light (200–250 μmol m−2 s−1) for 3 h. Values represent the means ±SE of n=140–228 for (C), 138–203 for (D) and 230–320 for (E) from three independent experiments.
Light-induced stomatal opening was slower in the two independent PLA2β RNAi-silenced plant lines, and the apertures were smaller compared to wild-type plants (Fig. 1C). For the light-induced stomatal opening assay, a light intensity of 200–250 μmol m−2 s−1 was used to illuminate the leaves, as described in Jeon et al. (2008). Under this light intensity, guard cells remained healthy and stomatal movements were normal. Stomatal movements were also tested under a reduced light intensity of 90 μmol m−2s−1, and a similar difference in stomatal movements between the wild type and RNAi mutant plants was observed (see Supplementary Fig. S1 at JXB online). In the three independent PLA2β-over-expressing lines, light-induced stomatal opening was faster and larger than wild-type plants (Fig. 1D). To test whether PLA2β is also involved in the ABA-inhibition of stomatal opening, stomatal opening movements were analysed in wild-type and mutant plants treated with ABA. ABA inhibited light-induced stomatal opening to similar extents in all genotypes, including the wild type and the PLA2β RNAi-silenced and PLA2β-over-expressing lines (see Supplementary Fig. S2 at JXB online). These results suggested that PLA2β is involved in the light-signal transduction that induces stomatal opening, and that this involvement is independent of ABA signalling. In PLA2α knockout plants, the time-course and size of light-induced stomatal opening were similar to wild-type plants (Fig. 1E), suggesting that PLA2α is not likely to be involved in light-induced stomatal regulation.
Effect of LPL on stomatal aperture in wild-type and PLA2β RNAi-silenced plants
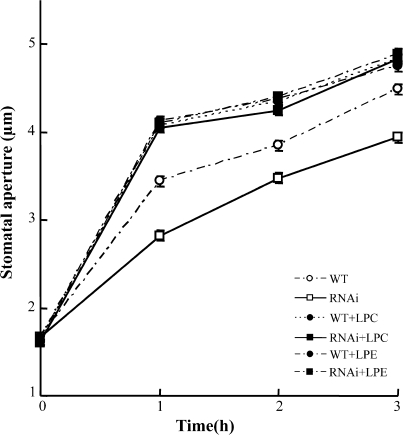
To determine whether the reduction in stomatal opening in PLA2β RNAi-silenced plants was due to reduced PLA2β activity, it was examined whether exogenous supplementation with LPLs, which are products of PLA2β hydrolysis, restore the stomatal opening. When the leaves of PLA2β RNAi-silenced plants were treated with either LPC or LPE, the difference in stomatal opening between the wild-type and PLA2β RNAi-silenced plants was eliminated, and both wild-type and RNAi-silenced plants showed similarly elevated stomatal openings in response to light (Fig. 2). Interestingly, the levels of stomatal opening restored by the PLA2 products exceeded the opening level of the wild type, suggesting that the PLA2 products are rate-limiting for maximum stomatal opening in wild-type plants under the current experimental conditions.
Fig. 2.
Effect of LPLs on stomatal opening in wild-type (WT) and PLA2β RNAi-silenced plants. Stomatal opening was measured initially before exposure to light, and then again at 1 h intervals after exposure to light in media supplemented with or without the indicated LPLs. Guard cells were treated with 50 μg ml−1 lysophosphatidylcholine (LPC) or lysophosphatidylethanolamine (LPE). Values represent the means ±SE of three independent experiments. n=140–200.
Light-induced expression of PLA2β
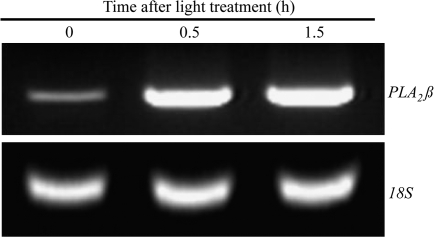
Next it was examined whether the expression of PLA2β was responsive to light. The level of PLA2β mRNA was very low under dark conditions, and increased upon white light irradiation for 0.5 h (Fig. 3). The elevated level of PLA2β mRNA was maintained upon exposure to light for up to 1.5 h (Fig. 3). There was a similar induction of PLA2β gene expression when etiolated seedlings were exposed to blue light (20 μmol m−2 s−1) (data not shown), which suggested that PLA2β is involved in blue-light induced signal transduction.
Fig. 3.
Light-induced expression of PLA2β. Four week-old wild-type plants were exposed to white light (200–250 μmol m−2 s−1) for 0.5 h and 1.5 h. Leaves were harvested and total RNA was extracted, and then analysed by RT-PCR. Amplification of 18S rRNA served as an internal standard.
Inhibition of PLA2β by aristolochic acid, an inhibitor of low molecular weight PLA2
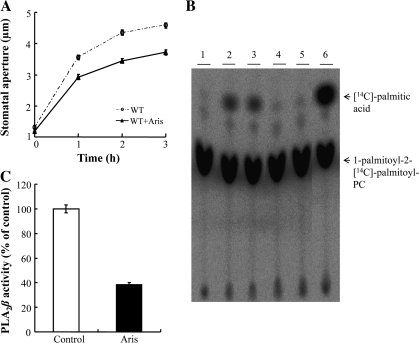
Aristolochic acid (Aris) has previously been shown to inhibit light-induced stomatal opening in Commelina communis (Suh et al., 1998). It was also tested whether or not Aris inhibits stomatal opening in Arabidopsis. The stomata of Aris-treated Arabidopsis guard cells opened more slowly than the stomata of untreated cells in response to light (Fig. 4A). To determine whether the activity of PLA2β is affected by this inhibitor, the effect of Aris on the activity of purified PLA2β was examined using a radiolabelled substrate, 1-palmitoyl-2-[14C]palmitoyl-PC. In control samples, the radioactive substrate was hydrolysed into the corresponding FFA, and readily detectable by thin-layer chromatography (TLC) (Fig. 4B, lanes 2 and 3). The amount of radiolabelled FFA decreased (Fig. 4B, lanes 4 and 5) to 38±1.8% (average ±SE, Fig. 4C) when PLA2β was preincubated with Aris, which indicated that PLA2β activity was strongly inhibited. These results suggested that the inhibitory effect of Aris on light-induced stomatal opening is due to its ability to inhibit PLA2β.
Fig. 4.
The effect of aristolochic acid (Aris), a low molecular weight PLA2 inhibitor, on the activity of purified PLA2β. (A) Light-induced stomatal opening of wild-type Arabidopsis in the presence or absence of 20 μM Aris. (B) Thin layer chromatography (TLC) analysis of the hydrolytic activity of recombinant PLA2β. Purified PLA2β was incubated in 100 mM TRIS–HCl (pH 8.0) at 40 °C in the presence or absence of 20 μM Aris for 40 min, and then PLA2β hydrolytic activity was assayed in the presence of 1-palmitoyl-2-[14C]palmitoyl-PC. The acyl-hydrolysis activity of the recombinant protein was greatly inhibited by Aris, compared to the solvent controls. Lane 1, substrate only; lanes 2–3, solvent controls (40 μM NaOH); lanes 4–5, 20 μM Aris dissolved in NaOH; lane 6, bee venom low molecular weight secretory PLA2 was used instead of purified PLA2β to identify the position of 14C-palmitic acid in the TLC plate. (C) The results from TLC were quantified using a phosphoimager (n=4).
Expression of PLA2β in guard cells
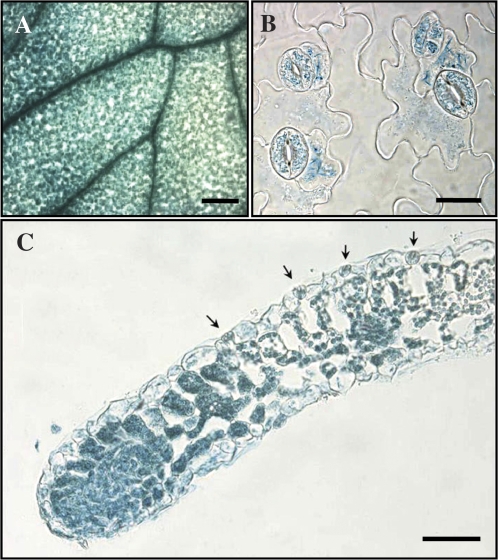
It has been shown that PLA2β is expressed in young seedlings in all tissues, in the flowers of mature plants, and in the vascular tissues of both young and old plants (Lee et al., 2003). To determine the sites of expression of PLA2β in more detail, the pattern of expression of a PLA2β promoter::GUS fusion construct in transformed plants was analysed. Three independent transgenic plant lines exhibited similar patterns of expression of GUS. GUS activity was the highest in vascular tissues (Fig. 5A), and was also found in all other cell types of the leaf, including mesophyll, epidermal, and guard cells. The expression of PLA2β in guard cells was confirmed in epidermal strips that were free of mesophyll cell background (Fig. 5B), and in leaf cross-sections (Fig. 5C).
Fig. 5.
GUS staining of A. thaliana leaves transformed with a PLA2β promoter::GUS fusion construct. (A–C) GUS expression pattern in the intact leaf, bar = 200 μm (A), peeled epidermis, bar = 10 μm (B), and leaf cross-sections, bar = 20 μm (C). Guard cells are indicated by arrows (C).
Subcellular localization of PLA2β
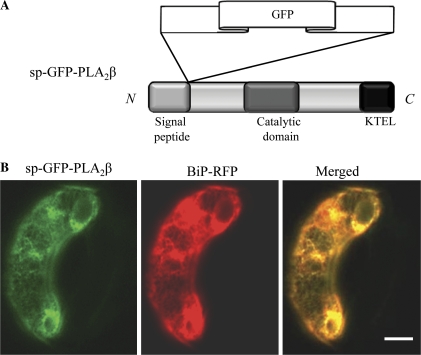
Similar to animal cells, low molecular weight PLA2 isoforms in plants contain a putative N-terminal signal peptide (sp) that directs protein secretion. To determine whether PLA2β was indeed secreted from plant cells, sp-GFP-PLA2β was generated, an expression construct in which a GFP-PLA2β fusion protein was fused to the C-terminal of predicted signal peptide of PLA2β, such that the GFP moiety would not interfere with the functions of either the putative N-terminal signal peptides or the putative C-terminal ER retention signal (Fig. 6A). The expression construct was introduced into guard cells of Vicia faba by biolistic bombardment. Exogenously expressed PLA2β localized to the ER, and the fluorescence signals corresponding to GFP-PLA2β co-localized extensively with the ER marker protein, BiP-RFP (Jin et al., 2001; Fig. 6B).
Fig. 6.
Co-localization of GFP-PLA2β and an ER marker protein in Vicia faba guard cells. (A) A schematic representation of the domain structure of sp-GFP-PLA2β. (B) Fluorescent images of intact V. faba guard cells transformed with sp-GFP-PLA2β (left), and BiP-RFP (middle), and the superimposed images of GFP-PLA2ββ and BiP-RFP (right). Bar=10 μm.
Discussion
In this paper, we identified PLA2β as an enzyme that can provide LPLs and FFAs during the light signal transduction pathway in guard cells, which leads to stomatal opening. The phenotypes of PLA2β mutant plants support this conclusion. Plant lines in which PLA2β expression was reduced by RNAi silencing exhibited delayed stomatal opening; plants that over-expressed PLA2β exhibited accelerated stomatal opening in response to light; and the phenotype of RNAi-silenced plants was reversed by exogenous LPLs, which are the products of PLA2 hydrolysis. In addition, light exposure induced the expression of PLA2β in the leaf, and an inhibitor of light-induced stomatal opening, Aris, inhibited the activity of purified PLA2β. Therefore, it is proposed that PLA2β contributes to the light-induced opening of stomata, and represents a new signal mediator in the pathways that regulate light-induced stomatal movement.
To obtain further supporting evidence, a biochemical assay of PLA2 activity in irradiated Arabidopsis guard cell protoplasts and intact leaves was also performed, but it was not possible reliably to detect changes in total PLA2 activity in response to light. It is speculated that this is because the level of expression of PLA2β in guard cells is low. It is also possible that PLA2 is more responsive to handling and wounding (Narvaez-Vasquez et al., 1999) than to light. In addition, the possibility cannot be excluded that other PLA isoforms also modulate stomatal movement. Characterization of the stomatal phenotypes of plants that carry mutations in the patatin-like PLAs will provide additional information on the mechanism of regulation of stomatal movement in plant cells by PLA2.
PLA-mediated hydrolysis generates second messengers, i.e. fatty acids and LPLs, that can enhance stomatal opening. FFAs have been shown to stimulate H+ pump activity (Palmgren et al., 1988). It has also been shown that polyunsaturated fatty acids increase inward K+ channel currents and inhibit outward K+ channel currents in guard cells (Lee et al., 1994), which can facilitate K+ uptake into guard cells along the electrochemical gradient established by the H+ pump. In the current study, it is shown that LPLs also enhance stomatal opening (Fig. 2). The mechanism by which LPLs enhance stomatal opening probably involves activation of the H+ pump (Palmgren et al., 1988). The H+ pump drives stomatal opening in response to both blue and red light (Assmann et al., 1985; Serrano et al., 1988). Since white light, comprising both blue and red light, was used, PLA2β could be a downstream target for both blue and red light signal transduction. The identity of the photoreceptor responsible for PLA2β activation remains to be determined. LPLs have been shown to be involved in auxin-induced cell elongation (Yi et al., 1996; reviewed in Scherer, 2002), which also requires activation of the H+ pump. LPL-mediated activation of the H+ pump may involve phosphorylation of the H+ pump by a protein kinase that is activated by LPLs (Scherer et al., 1993). Thus, upon activation by a signal receptor, PLA can potentially generate two potent second messengers that can mediate increases in cell volume, a process that is necessary for stomatal opening and cell elongation.
PLA2β is expressed not only in guard cells but also in all other leaf cell types, including palisade parenchyma, spongy parenchyma, and epidermal cells. PLA2β may have various functions in different plant cell types. Other PLA2 genes have also been shown to function in signal transduction as well as in many other cellular processes in plant cells (reviewed in Munnik et al., 1998; Wang, 2001, 2004; Scherer, 2002; Ryu, 2004).
One of the interesting findings of this study was that GFP-PLA2β localized to the ER. PLA2β contains a KTEL sequence in its C-terminus, which may be responsible for ER retention of the protein, as it is similar to the canonical ER retention signal KDEL (Fig. 6). Previously it was shown that a C-terminal GFP fusion protein of PLA2β is secreted into the cell wall/extracellular space when expressed in onion epidermal cells (Lee et al., 2003). It is possible that the fusion of GFP to the C-terminus of PLA2β obstructed its C-terminal KTEL domain.
Since PLA2β is localized in the ER of guard cells, what is the possible mechanism of activation of the H+-ATPase at the plasma membrane by PLA2β? Previously it was reported that LPC, generated by PLA2 at the plasma membrane, transduces elicitor-induced signals to activate a tonoplast H+/Na+ antiporter (Viehweger et al., 2002). This result suggests that LPC molecules are highly mobile in intact cells. The following model of activation of H+-ATPase in guard cells is proposed: PLA2β is activated by light, and LPLs formed in the ER by PLA2β-mediated hydrolysis move to the plasma membrane, where they activate H+-ATPase, thereby facilitating stomatal opening. Thus the daily breakdown of phospholipids by PLA2β for stomatal opening may not occur in the plasma membrane but rather in the ER where there are relatively plentiful substrates for PLA2β, the endoplasmic membrane phospholipids. Alternatively, a low level of PLA2β may be localized to the plasma membrane area and function there, as observed previously for the 22-kDa auxin-binding protein that has a KDEL motif. Although it is found mainly in the ER, it is also present at a low level at the plasma membrane where it performs auxin-related functions (Jones and Herman, 1993).
In summary, it has been demonstrated that PLA2 functions as a light signal mediator in guard cells, and in concert with a variety of other signalling molecules and pathways, participates in the regulation of the plant response to light.
Supplementary data
Supplementary data are available at JXB online.
Fig. S1. Stomatal opening of PLA2β-silenced and wild-type plants induced by 90 μmol m−2 s−1 white light.
Fig. S2. Effect of ABA on light-induced stomatal opening in wild-type and PLA2β mutant plants.
Supplementary Material
Acknowledgments
This work was supported by a grant awarded to YL from the Crop Functional Genomics Center of Korea (Grant number CG1-1-23), and to SBR from the Plant Diversity Research Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology of the Korea (PF06305-01 and in part KRF-C00368).
References
- Assmann SM, Simoncini L, Schroeder JI. Blue light activates electrogenic ion pumping in guard cell protoplasts of Vicia faba. Nature. 1985;318:285–287. [Google Scholar]
- Austin SC, Funk CD. Insight into prostaglandin, leukotriene, and other eicosanoid functions using mice with targeted gene disruptions. Prostaglandins and other Lipid Mediators. 1999;58:231–252. doi: 10.1016/s0090-6980(99)00041-6. [DOI] [PubMed] [Google Scholar]
- Bahn SC, Lee HY, Kim HJ, Ryu SB, Shin JS. Characterization of Arabidopsis secretory phospholipase A2-cDNA and its enzymatic properties. FEBS Letters. 2003;553:113–118. doi: 10.1016/s0014-5793(03)00982-7. [DOI] [PubMed] [Google Scholar]
- Berk PD, Stump DD. Mechanisms of cellular uptake of long chain free fatty acids. Molecular and Cellular Biochemistry. 1999;192:17–31. [PubMed] [Google Scholar]
- Bingham CO, III, Austen KF. Phospholipase A2 enzymes in eicosanoid generation. Proceedings of the Association of American Physicians. 1999;111:516–524. doi: 10.1046/j.1525-1381.1999.99321.x. [DOI] [PubMed] [Google Scholar]
- Devillier P, Baccard N, Advenier C. Leukotrienes, leukotriene receptor antagonists and leukotriene synthesis inhibitors in asthma. Pharmacology Research. 1999;40:3–13. doi: 10.1006/phrs.1998.0458. [DOI] [PubMed] [Google Scholar]
- Emi T, Kinoshita T, Shimazaki K. Specific binding of vf14-3-3a isoform to the plasma membrane H+-ATPase in response to blue light and fusicoccin in guard cells of broad bean. Plant Physiology. 2001;125:1115–1125. doi: 10.1104/pp.125.2.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gijon MA, Leslie CC. Regulation of arachidonic acid release and cytosolic phospholipase A2 activation. Journal of Leukocyte Biology. 1999;65:330–336. doi: 10.1002/jlb.65.3.330. [DOI] [PubMed] [Google Scholar]
- Jin JB, Kim YA, Kim SJ, Lee SH, Kim DH, Cheong G, Hwang I. A new dynamin-like protein, ADL6, is involved in trafficking from the trans-Golgi network to the central vacuole in Arabidopsis. The Plant Cell. 2001;13:1511–1525. doi: 10.1105/TPC.000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon BW, Hwang JU, Hwang Y, et al. The Arabidopsis small G protein ROP2 is activated by light in guard cells and inhibits light-induced stomatal opening. The Plant Cell. 2008;20:75–87. doi: 10.1105/tpc.107.054544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Herman EM. KDEL-containing auxin-binding protein is secreted to the plasma membrane and cell wall. Plant Physiology. 1993;101:595–606. doi: 10.1104/pp.101.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. The EMBO Journal. 1999;18:5548–5558. doi: 10.1093/emboj/18.20.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K. Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant and Cell Physiology. 2002;43:1359–1365. doi: 10.1093/pcp/pcf167. [DOI] [PubMed] [Google Scholar]
- Lee HY, Bahn SC, Kang YM, Lee KH, Kim HJ, Noh EK, Palta JP, Shin JS, Ryu SB. Secretory low molecular weight phospholipase A2 plays important roles in cell elongation and shoot gravitropism in Arabidopsis. The Plant Cell. 2003;15:1900–2002. doi: 10.1105/tpc.014423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HY, Bahn SC, Shin JS, Hwang I, Back K, Doelling JH, Ryu SB. Multiple forms of secretory phospholipase A2 in plants. Progress in Lipid Research. 2005;44:52–67. doi: 10.1016/j.plipres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Lee Y, Lee HJ, Crain RC, Lee A, Korn SJ. Polyunsaturated fatty acids modulate stomatal aperture and two distinct K+ channel currents in guard cells. Cell Signaling. 1994;6:181–186. doi: 10.1016/0898-6568(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Moolenaar WH, Kranenburg O, Postma FR, Zondag GC. Lysophosphatidic acid: G protein signaling and cellular responses. Current Opinion in Cell Biology. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- Munnik T, Irvine RF, Musgrave A. Phospholipid signalling in plants. Biochimica et Biophysica Acta. 1998;1389:222–272. doi: 10.1016/s0005-2760(97)00158-6. [DOI] [PubMed] [Google Scholar]
- Narvaez-Vasquez J, Florin-Christensen J, Ryan CA. Positional specificity of a phospholipase A activity induced by wounding, systemin, and oligosaccharide elicitors in tomato leaves. The Plant Cell. 1999;11:2249–2260. doi: 10.1105/tpc.11.11.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG, Sommarin M, Ulvskov P, Jorgensen PL. Modulation of plasma membrane H+-ATPase from oat roots by lysophosphatidyl-choline, free fatty acids and phospholipase A2. Physiologia Plantarum. 1988;74:11–19. [Google Scholar]
- Ryu SB. Phospholipid-derived signaling mediated by phospholipase A in plants. Trends in Plant Science. 2004;9:229–235. doi: 10.1016/j.tplants.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Scherer GFE. Secondary messengers and phospholipase A2 in auxin signal transduction. Plant Molecular Biology. 2002;49:357–372. [PubMed] [Google Scholar]
- Scherer GFE, Hecker D, Muller J. Ca++ ions lysophospholipids activate phosphorylation of different proteins in plasma membrane and tonoplast. Journal of Plant Physiology. 1993;142:425–431. [Google Scholar]
- Serrano EE, Zeiger E, Hagiwara S. Red light stimulates an electrogenic proton pump in Vicia guard cell protoplasts. Proceedings of the National Academy of Sciences, USA. 1988;85:436–440. doi: 10.1073/pnas.85.2.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh S, Park J, Lee Y. Possible involvement of phospholipase A2 in light signal transduction of guard cells of Commelina communis. Physiologia Plantarum. 1998;104:306–310. [Google Scholar]
- Viehweger K, Dordschbal B, Roos W. Elicitor-activated phospholipase A2 generates lysophosphatidylcholines that mobilize the vacuolar H+-pool for pH signaling via the activation of Na+-dependent proton fluxes. The Plant Cell. 2002;14:1509–1525. doi: 10.1105/tpc.002329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X. Plant phospholipases. Annual Review of Plant Physiology and Plant Molecular Biology. 2001;52:211–231. doi: 10.1146/annurev.arplant.52.1.211. [DOI] [PubMed] [Google Scholar]
- Wang X. Lipid signaling. Current Opinion in Plant Biology. 2004;7:329–336. doi: 10.1016/j.pbi.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Yi H, Park D, Lee Y. In vivo evidence for the involvement of phospholipase A and protein kinase in the signal transduction pathway for auxin-induced corn coleoptiles elongation. Physiologia Plantarum. 1996;96:359–368. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.