Abstract
The antioxidant lipoic acid (LA) treats and prevents the animal model of multiple sclerosis (MS), experimental autoimmune encephalomyelitis (EAE). In an effort to understand the therapeutic potential of LA in MS, we sought to define the cellular mechanisms that mediate the effects of LA on human natural killer (NK) cells, which are important in innate immunity as the first line of defense against invading pathogens and tumor cells. We discovered that LA stimulates cAMP production in NK cells in a dose-dependent manner. Studies using pharmacological inhibitors and receptor transfection experiments indicate that LA stimulates cAMP production via activation of the EP2 and EP4 prostanoid receptors and adenylyl cyclase. In addition, LA suppressed interleukin (IL)-12/IL-18 induced IFNγ secretion and cytotoxicity in NK cells. These novel findings suggest that LA may inhibit NK cell function via the cAMP signaling pathway.
Keywords: Multiple sclerosis, thioctic acid, Natural Killer cells, cAMP, IFNγ
1. Introduction
Multiple sclerosis (MS) is a chronic, inflammatory disorder of the central nervous system (CNS) that is characterized by immune cell infiltration and destruction of oligodendrocytes, the myelin sheath and axons. Though early studies implicate CD4+ myelin-reactive T cells as the cells responsible for initiating the inflammatory response, the actual cellular mediators of tissue injury is becoming more complex. Several reports have recently focused on the potential role of NK cells in MS (Morandi et al., 2008).
NK cells are large granular cells that express CD16 and/or CD56, but not CD3. They are important components of the innate immune response to invading pathogens, including bacteria, and viruses and tumor cells (French and Yokoyama, 2003; Korbel et al., 2004). How NK cells contribute to the pathogenesis of MS is still being studied. There is evidence that NK cells can both exacerbate and protect against experimental autoimmune encephalomyelitis (EAE) and may also be pathogenic or protective in MS ((Morandi et al., 2008). For instance, NK cells are implicated in mediating cytotoxicity toward oligodendrocytes as a mechanism for tissue injury in MS (Antel et al., 1998). Shi et al. reported an IFNγ dependent and disease promoting effect of NK cells in a mouse model of EAE (Shi et al., 2000). Conversely, a study by Aranami and colleagues (Aranami et al., 2006) revealed that NK cells differentially express the CD11c marker, which coincides with disease course of MS. The investigators suggested that CD11chigh expressing NK cells are associated with more unstable and severe condition of MS whereas those expressing CD11clow were associated with remission. Similarly, Saraste et al. (Saraste et al., 2007) demonstrated expansion of CD56Bright NK cells in the peripheral blood of MS patients receiving interferon beta therapy, suggesting that these NK cell subpopulations may be involved in alleviating MS. Thus, the dual role of NK cells may be due to the subset of NK cells present, and the phenotype that is expressed. It is, therefore, possible that treatment strategies that promote less pathogenic NK cells could help ameliorate MS.
Antioxidants are naturally occurring compounds that are being investigated for treatment of many diseases, including cancer, diabetes, cardiovascular and neurodegenerative diseases. Recent studies identified the antioxidant lipoic acid (LA) as a potential agent to treat MS (Marracci et al., 2002; Marracci et al., 2004; Morini et al., 2004). LA is a ring-containing fatty acid that functions as a free radical scavenger, and as a cofactor for many mitochondrial dehydrogenase enzymes. Marracci et al. (Marracci et al., 2002) showed that LA suppresses EAE in SJL mice when daily administration commenced prior to disease onset. Importantly, mice receiving LA beginning at disease onset demonstrated a significant reduction in the severity of clinical and histological EAE. A study by two independent groups also demonstrated that LA prevented the development of EAE in both the mouse and rat model of MS (Morini et al., 2004; Schreibelt et al., 2006). Furthermore, LA inhibits T cell migration into the CNS (Marracci et al., 2002) and inhibits ICAM-1 and VCAM-1 (proteins involved in T cell adhesion) expression by CNS endothelial cells (Chaudhary et al., 2006). An early pilot trial of oral LA in MS subjects conducted by Yadav et al. (Yadav et al., 2005) demonstrated that oral LA was well tolerated and appeared to reduce serum MMP-9 and sICAM-1 levels. These proteins are associated with inflammatory disease activity and have been targeted for therapy.
Recently, our lab discovered that LA stimulates cyclic AMP (cAMP) production in NK cells (Schillace et al., 2007). cAMP is a small molecule second messenger that is involved in the transduction of signals from the extracellular milieu into the intracellular environment. Activation of the cAMP-dependent signaling pathway modulates many aspects of the immune function. For instance, an increase in intracellular cAMP inhibits IL-2 production and expression of the cognate receptor (Anderson et al., 1997). T cell proliferation and activation is also inhibited (Kuklina and Shirshev, 2000). In NK cells, cAMP inducing agents such as butyrate and prostaglandin E1 (PGE1) suppresses NK cytolytic function (Roder et al., 1980) independent of the target-cell recognition phase. PGE2, another cAMP inducing agent, also inhibited NK cell cytotoxicity, as well as interleukin (IL)-15, IL-12 or IL-18 induced interferon gamma (IFNγ) mRNA expression and protein secretion (Joshi et al., 2001). Collectively, these reports suggest that LA may modulate NK cell activation and function.
This study presents novel evidence that LA stimulates cAMP production in NK cells via activation of G-protein coupled receptors (GPCR) and adenylyl cyclase, and that LA modulates NK cell activation and function. In addition, LA suppressed interleukin (IL)-12/IL-18 induced interferon gamma (IFN)γ synthesis and cytotoxicity in NK cells. These findings lay the foundation for studying LA mediated modulation of NK cells in an effort to understand how LA can be used as an effective new treatment strategy for MS.
2. Materials and Methods
2.1. Materials
RPMI, high glucose DMEM, Lipofectamine 2000 and all other tissue culture reagents were purchased from Invitrogen (Carlsbad, CA). The Dynal and EasySep NK cell negative purification kits were purchased from Invitrogen and Stem Cell Technologies Inc. (Vancouver, Bristish Columbia), respectively. The cAMP kits were purchased from BioAssay Designs (Ann Arbor, MI). Lipoic acid, PGE2, AH6809, AH23848 and 2′5′-dideoxyadenosine inhibitors were obtained from Sigma (St. Louis, MO). Rolipram was purchased from Biomol (Plymouth Meeting, PA). The BCA protein assay kit was obtained from Pierce Biotechnology (Rockford, IL). HEK 293 EBNA cell line was purchased from ATCC (Manassas, VA). Recombinant human IL-12, IL-18, IFNγ, monoclonal anti-human IFNγ and biotinylated anti-human IFNγ antibodies were obtained from R&D systems (Minneapolis, MN).
2.2. Cell culture
Human peripheral blood mononuclear cells (PBMCs) were obtained from source leukocytes (buffy coat) from the Red Cross in Portland, OR (approval #VACARR). Enriched leukocytes were subjected to ficoll purification (Amersham) and centrifugation at 1400 rpm with the brake set off for 30 minutes to remove contaminating red blood cells and platelets. The interface was collected, washed with RPMI 1640 and centrifuged at 1300 rpm for 10 minutes. The supernatant was decanted and the cells were subjected to two more wash steps. Cells were resuspended in freezing medium (RPMI + 25% FCS + 12% DMSO) and stored in liquid nitrogen for future use.
HEK 293 EBNA cells were maintained in a humidified 5% CO2 atmosphere chamber at 37ºC in high glucose DMEM supplemented with 10% fetal bovine serum (FBS). Media was changed every three days.
2.3. NK cell purification
Previously frozen PBMCs were thawed and NK cells purified using the Dynal or EasySep negative purification kits following the manufacture’s protocol.
2.4. Cyclic AMP assay
For dose response, 1–2 × 105 NK cells in 500 μl RPMI were treated with 0, 10, 25, 50, 75 and 100 μg/ml LA or 0.001. 0.01, 0.1, 1.0, 10. 100 μM for 1 minute. Samples were centrifuged at 1300 rpm for 1 minute and supernatants were then decanted. Cells were lysed with the addition of 400 μl 0.1M HCl and boiling for 10 minutes. Samples were centrifuged at 1300 rpm and 100 μl was used for cAMP assays following the manufacture’s protocol. The absorbance was measured at 405 nm using a colorimetric 96-well plate reader. Results in pmol/ml were then divided by the protein concentration to obtain pmol of cAMP per milligram of protein.
For inhibitor studies, 1 × 105 NK cells were pre-incubated with 50 μM AH6809, 50 μM AH23848 or 2′5′-dideoxyadenosine (0.1, 0.25 or 0.5 mM), which is referred to as 2′5′ddAdo, for 30 minutes and then stimulated with LA or prostaglandin E2 (PGE2) positive control for 1 minute. The drug concentration and pre-incubation times were conditions used successfully by other investigators (Chen et al., 2006; Sanchez and Moreno, 2002; Schillace et al., 2007; Walker and Rotondo, 2004). Samples were centrifuged and prepared as described above. AH6809 inhibits prostaglandin D receptors as well as the PGE2 receptors 1 (EP1) and EP2. The EP1 receptor modulates calcium signaling and the EP2 receptor stimulates cAMP production. AH23848 is an antagonist of the EP4 receptor. 2′5′ddAdo is an inhibitor of AC.
2.5. Bicinchoninic acid (BCA) assay
Depending on the experiment, varying volumes of supernatants (20–60 μl) that were leftover from cAMP assays were used to determine total protein concentrations using the BCA assay kit (Pierce, Rockford, IL) following the manufacturer’s protocol. Absorbance readings were measured at 562 nm. Bovine serum albumin (BSA) standards were prepared in 0.1 M HCl at concentrations ranging from 0–1 mg/ml. Protein concentrations for unknown samples were extrapolated from the standard curve using Softmax Pro software (Molecular Devices, Sunnyvale, CA).
2.6. Receptor transfection
HEK293 EBNA cells were seeded into a 12-well plate at 2 × 105 cells per well and transfected 24 hours later using Lipofectamine 2000. Briefly, for each well to be transfected, 5 μg pcDNA3 EP2 or pcDNA3 EP4 receptor DNA were combined with 50 μl Opti-mem and incubated at room temperature (RT) for 5 minutes (DNA mixture). Simultaneously, 1.2 μl lipofectamine and 50 μl Opti-mem were mixed and incubated for 5 minutes at RT (lipofectamine mixture). The DNA and lipofectamine mixtures were combined, incubated at RT for 30 minutes and added to each well in a dropwise fashion. Forty-eight hours post-transfection, cells were pre-incubated with 100 μM IBMX with or without 50 uM each AH6809 or AH23848 for 30 minutes and stimulated with LA or PGE2 for 1 hour, as reported by Schillace et al. (Schillace et al., 2007). IBMX is a phosphodiesterase inhibitor which is used to prevent degradation of cAMP. The media was aspirated, cells were scraped in 400 μl 0.1 M HCl and boiled for 10 minutes. Samples were vortexed and centrifuged at 1300 rpm for 1 minute. The supernatants were used for cAMP assays as described above.
2.7. Quantitation of INFγ by ELISA
Supernatants obtained from cells treated with a mixture containing 10 ng/ml IL-12 and IL-18 for 24 hours in the absence or presence of 25 or 50μg/ml LA or 1 mM rolipram were used for detection of secreted IFNγ by ELISA. A 96-well plate was coated with 100 μl αIFNγ capture antibody (MAB2852) at 4 μg/ml and incubated for 2 hours at 37°C. Wells were washed 5X with TTBS, blocked with 3% BSA/TTBS (200 μl) for 1 hour at RT and washed again. Recombinant human IFNγ standards (285-IF100) or treated sample supernatants were added to each well (100 μl) and incubated at RT for 2 hours. Wells were washed with TTBS 5X and incubated with 100 μl biotinylated αIFNγ (200 ng/ml) (BAF285) for 2 hours at RT. After washing, wells were incubated with 100 μl streptavidin-HRP for 20 minutes at RT in the dark. Wells were washed with TTBS and incubated with 100 μl TMB substrate (52-00-01) for 20 minutes at RT in the dark. The reaction was stopped with the addition of 50 μl 1M H2SO4 and absorbance was measured at 450 nm.
2.8. Cytotoxicity Assay
NK cell cytotoxicity towards K-562 target cells was measured using the lactate dehydrogenase (LDH) release assay from Promega (Madison, WI) according to the manufacturer’s protocol. Briefly, using a 6:1 effector cell to target cell ratio, 1.2 × 105 NK cells were plated in the presence of 1 mM rolipram (15 minutes) or 25 or 50 μg/ml LA (5 minutes) in a round bottom 96-well plate. K-562 cells (2 × 104) were added to the wells and the plate was then incubated at 37C for 4 hours. Cells were pelleted with centrifugation at 250 × g for 4 minutes. The supernatant (50 μl) was transferred to a new 96- well plate and 50 μl reconstituted Substrate Mix was then added. The plate was incubated at RT for 30 minutes in the dark. The reaction was stopped with the addition of 50 μl Stop Solution and the absorbance was measured at 490 nm.
2.9. Statistical Analysis
The data were analyzed by one way ANOVA analysis and Student’s t-test and were considered statistically significant at a p value of <0.05.
3. Results
3.1. Stimulation of cAMP production
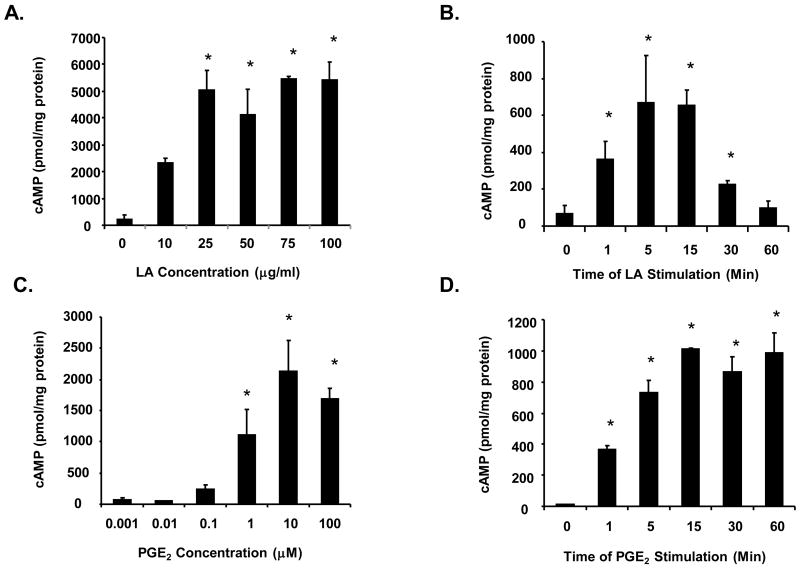
PGE2 induced increases in cAMP levels have been shown to regulate various NK cell functions. To determine if NK cell functions are regulated by LA mediated increases in cAMP, we first confirmed and expanded our original finding that LA increases cAMP levels in NK cells (Schillace, et al., 2007). In figure 1A we present a representative data from one experiment illustrating a concentration dependent increase in cAMP levels induced by increasing concentrations of LA (N=4 different donors in duplicate). Purified human NK cells were stimulated with 0, 10, 25, 50, 75 and 100 μg/ml LA for 1 minute, and the synthesis of cAMP was assayed as described in the materials and methods. Results indicate that 10 μg/ml LA was sufficient to stimulate cAMP production where the average fold increase in cAMP level was 14 fold. However, due to the variability between donors, the increase was not statistically significant (p value =0.06). The concentration that achieved maximal stimulation of cAMP production varied between 25–100 μg/ml depending on the donor with maximal cAMP levels between 400–6000 pmol/mg protein. The average of the fold-change increase in cAMP compared to unstimulated control demonstrate that 10, 25, 50, 75 and 100 μg/ml LA induced 14, 19, 20, 25 and 21 fold induction in cAMP production, respectively (data not shown). In order to elicit maximal cAMP production in all donors used, many of the subsequent studies were conducted using 100 μg/ml. A timecourse using 100 μg/ml LA demonstrates that cAMP production is transient over 60 minutes at which time cAMP levels were reduced to nearly basal amounts (Fig. 1B). The observed tmax was 5 minutes. Similarly, PGE2 also stimulated cAMP production in a concentration dependent fashion. However, cAMP levels were sustained over 60 minutes (Figures 1C and 1D). These data demonstrate that both LA and PGE2 stimulate cAMP production in NK cells.
Figure 1. Lipoic acid and PGE2 stimulates cAMP in a concentration-dependent manner.
(A) Purified human NK cells (1–2 × 105) were stimulated with 0, 10, 25, 50, 75 and 100 μg/ml LA for 1 minute and centrifuged. The pellets were resuspended in 0.1 M HCl and lysed with boiling for 10 minutes. The supernatants were used in cAMP assays. N = 4 donors in duplicate, * indicates statistically significant compared to unstimulated control, p < 0.05. (B) Purified human NK cells were stimulated with 100 μg/ml LA for 0, 1, 5, 15, 30 and 60 minutes (B), 0.001, 0.01, 0.1, 1, 10 and 100 μM PGE2 for 1 min (C), or 10 μM PGE2 for 0, 1, 5, 15, 30 and 60 minutes (D). Samples were processed as described in A. N = 3 donors in duplicate for B–D, * indicates statistically significant compared to unstimulated control, p < 0.05.
3.2. The prostaglandin EP2 and EP4 receptors mediate cAMP production
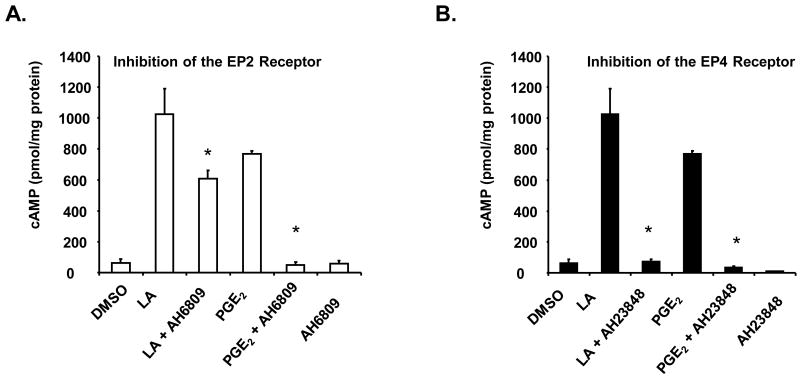
Induction of intracellular cAMP levels is dependent on the activation of G-protein coupled receptors (GPCRs). Some of the most studied GPCRs are the prostaglandin receptors designated subtypes EP1, EP2, EP3 and EP4. To determine if the EP2 and EP4 receptors mediate LA stimulated cAMP production in NK cells, we pre-treated the cells with pharmacological inhibitors (AH6809 and AH23848, 50 μM each) against the EP2 or EP4 receptors for 30 minutes (Matlhagela and Taub, 2006; Sanchez and Moreno, 2002; Walker and Rotondo, 2004). AH6809 has higher affinity for PGD receptors, but will also inhibit EP1 and EP2 receptors. However, the EP1 receptor mediates Ca2+ production and signaling, not cAMP. AH23848 is an antagonist of the EP4 receptor. After pre-incubation with the inhibitors, cells were stimulated LA and cAMP levels were assayed. We first used 100 μg/ml LA, but we did not observe an inhibitory effect with either AH6809 or AH23848. It is possible that 100 μg/ml LA stimulated saturated cAMP levels, which then limited our ability to detect any differences with or without the drugs. Thus, we used 10 μg/ml LA to stimulate cAMP production for this study. Prostaglandin E2 (PGE2) at 1μM was used as a positive control since the EP2 and EP4 receptors are activated upon binding with PGE2 (Sugimoto and Narumiya, 2007; Wang and Klein, 2007). This concentration better mimics physiological conditions and was used successfully by Walker and Rotondo (Walker and Rotondo, 2004). As illustrated in figure 2, pre-incubation with AH6809 prior to stimulation with LA resulted in a significant inhibition of cAMP production by approximately 40–60 percent depending on the donors used. Preincubation with AH23848 yielded 66–94 percent inhibition. Similarly, blocking the EP2 and EP4 receptors resulted in approximately 49–93 percent and 35–95 percent reduction, respectively, in cAMP production upon stimulation with the PGE2 control. These novel data indicate that LA stimulates cAMP production in NK cells via the EP2 and EP4 receptors.
Figure 2. LA stimulates cAMP production via the EP2 and EP4 receptors.
NK cells (1 × 105) were pre-incubated with 50 μM AH6809 (A) or AH23848 (B) for 30 minutes in RPMI and then stimulated with 10 μg/ml LA or 1 μM PGE2 for 1 minute. Media was removed and cells were lysed with 0.1 M HCl and boiling for 10 minutes. cAMP assays were determined by ELISA as described in materials and method. Data are graphical representatives of 3 donors in duplicate. * indicates statistically significant compared to stimulated controls, p< 0.05.
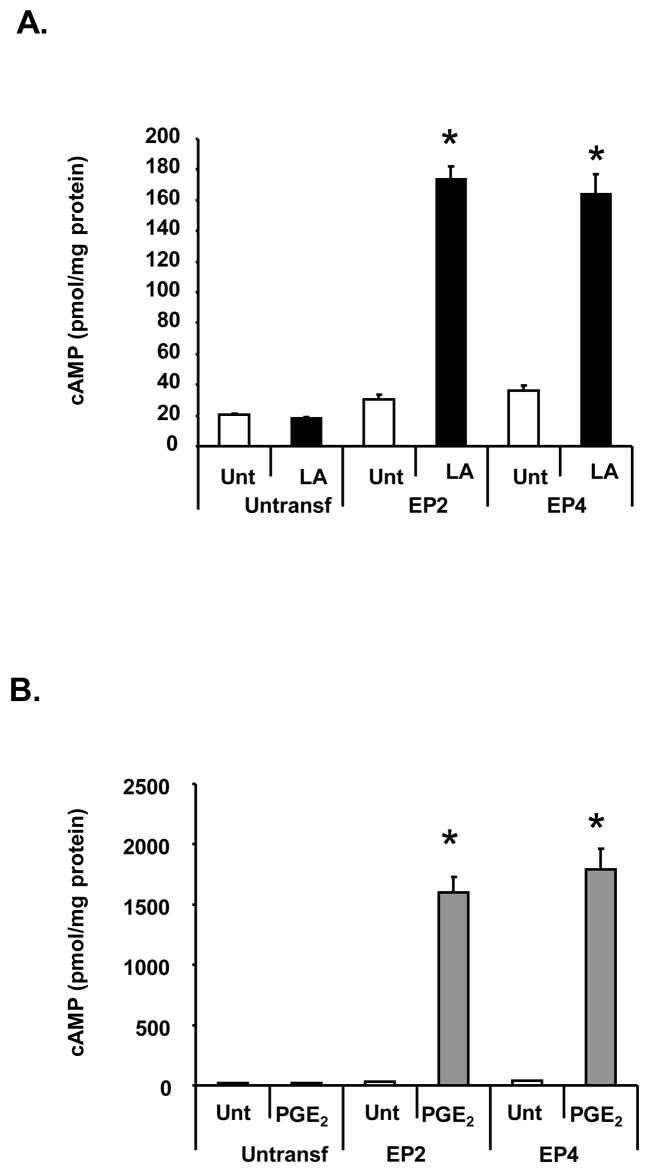
To confirm our observations, HEK 293 EBNA cells were transfected with either the EP2 or EP4 receptor DNA. Forty-eight hours post-transfection, cells were stimulated with 100 μg/ml LA or 1 nM PGE2 in the presence of the phosphodiesterase inhibitor (IBMX) and cAMP levels were determined. Since the levels of the EP2 and EP4 receptors are increased in this system, we reduced the PGE2 concentration to 1 nM to generate cAMP levels within the standard curve range. Neither LA nor PGE2 stimulated cAMP production in non-transfected cells (Fig. 3). LA significantly induced cAMP production in cells transfected with either the EP2 or EP4 receptors by 4 to 5 fold (Fig. 3A). The PGE2 positive control also stimulated cAMP production (Fig. 3B). Notably, higher cAMP values were generated compared to those obtained from NK cells, which is attributed to increased expression of the EP2 and EP4 receptors on the membrane of the transfected cells.
Figure 3. LA stimulates cAMP production in HEK 293 EBNA cells transfected with the EP2 or EP4 receptors.
HEK 293 EBNA cells were plated on a 12 well plate at 2 × 105 cells per well for 24 hours. Cells were transfected with 5 μg EP2 or EP4 DNA using lipofectamine 2000 and stimulated 48 hours later with 100 μ/ml LA (A) or 1 nM PGE2 (B) in the presence of 100 μM IBMX for 1 hour. Cells were scraped in 0.1 M HCl and boiled for 10 minutes. The supernatant was then used for determining cAMP levels by ELISA. Depicted is a representative of 3 donors in duplicate. * indicates statistically significant compared to untreated, transfected cells, p< 0.05.
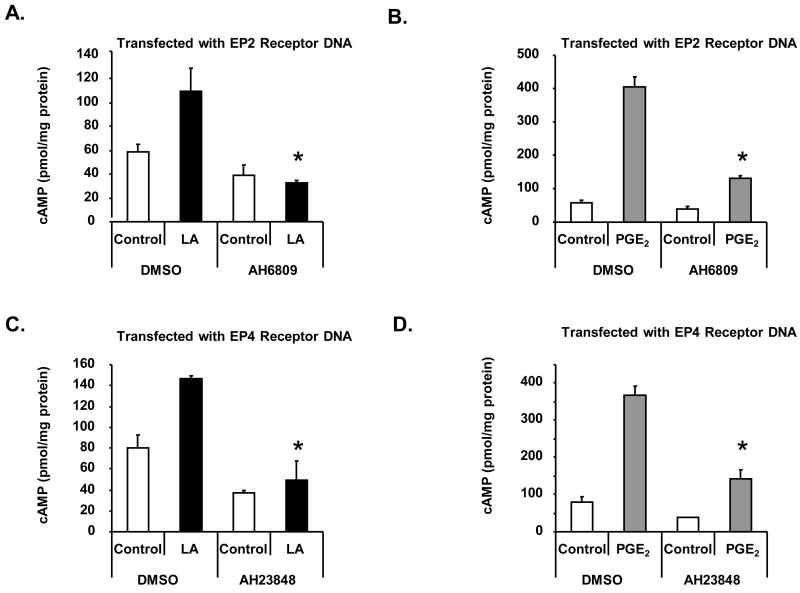
Collectively, these data suggest that LA stimulates cAMP production via the EP2 and EP4 receptors. To conclusively provide evidence to support this notion, we blocked the EP2 and EP4 receptors in transfected HEK 293 EBNA cells with 50μM each AH6809 or AH23848. As illustrated in figures 4A–B, pre-incubation of AH6809 prior to stimulation with LA or PGE2 significantly inhibited cAMP synthesis in cells transfected with the EP2 receptor DNA compared to DMSO vehicle control. Pre-incubation with AH23848 blocked cAMP production in cells transfected with the EP4 receptor DNA. These data, combined with inhibitor studies using NK cells, provide strong evidence that LA stimulation of cAMP production is mediated in part by the EP2 and EP4 prostanoid receptors.
Figure 4. EP2 and EP4 inhibitors block cAMP production in HEK EBNA cells transfected with the EP2 or EP4 receptors.
HEK 293 EBNA cells were plated on a 12 well plate at 2 × 105 cells per well for 24 hours. Cells were transfected using lipofectamine 2000 and pre-incubated with AH6809 (A-B) or AH23848 (C-D) 48 hours later in the presence of 100 μM IBMX. After 30 minutes, cells were then stimulated with 100 μg/ml LA (A,C) or 1 nM PGE2 (B,D) for 1 hour. Cells were scraped in 0.1 M HCl and boiled for 10 minutes. The supernatant was then used for determining cAMP levels by ELISA. Depicted is a representative of 3 donors in duplicate. * indicates statistically significant compared to stimulated controls, p< 0.05.
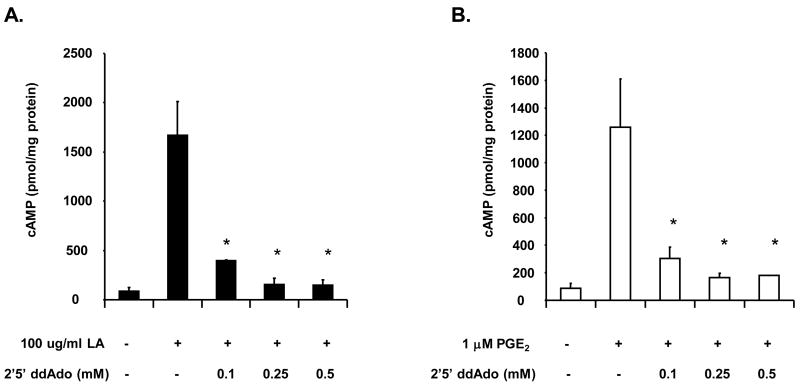
3.3. LA stimulates cAMP production via activation of adenylyl cyclase
cAMP production is regulated by two classes of enzymes called adenylyl cyclases (AC) and phosphodiesterases (PDE). ACs generate cAMP from ATP while PDEs degrade cAMP into ATP and Pi. In order to determine if AC mediates cAMP production upon stimulation with LA, we inhibited AC activation using 2′5′-dideoxyadenosine (ddAdo), a cell permeable inhibitor of AC. NK cells were incubated with varying concentrations of ddAdo for 30 minutes and then stimulated with 100 μg/ml LA or 1 μM PGE2 control. As shown in figure 5, pre-incubation with 0.1 mM ddAdo reduced cAMP levels by 70–75 percent in cells stimulated with LA or PGE2. Pre-incubation with 0.25 and 0.5 mM ddAdo inhibited cAMP synthesis by 85–95 percent. These data provide evidence to support the hypothesis that LA induces cAMP production by activating AC upon engagement of the EP2 and/or EP4 receptors.
Figure 5. The AC inhibitor (2′5′-dideoxyadenosine or ddAdo) blocks cAMP production in human NK cells.
NK cells (1 × 105) were pre-incubated with the indicated concentrations of 2′5′ddAdo for 30 minutes in RPMI and then stimulated with 100 μg/ml LA (A) or 1 μM PGE2 (B) for 1 minute. Media was removed and cells were lysed with 0.1 M HCl and boiling for 10 minutes. cAMP assays were determined by ELISA. Depicted is a representative of 3 donors in duplicate. * indicates statistically significant compared to stimulated controls, p< 0.05.
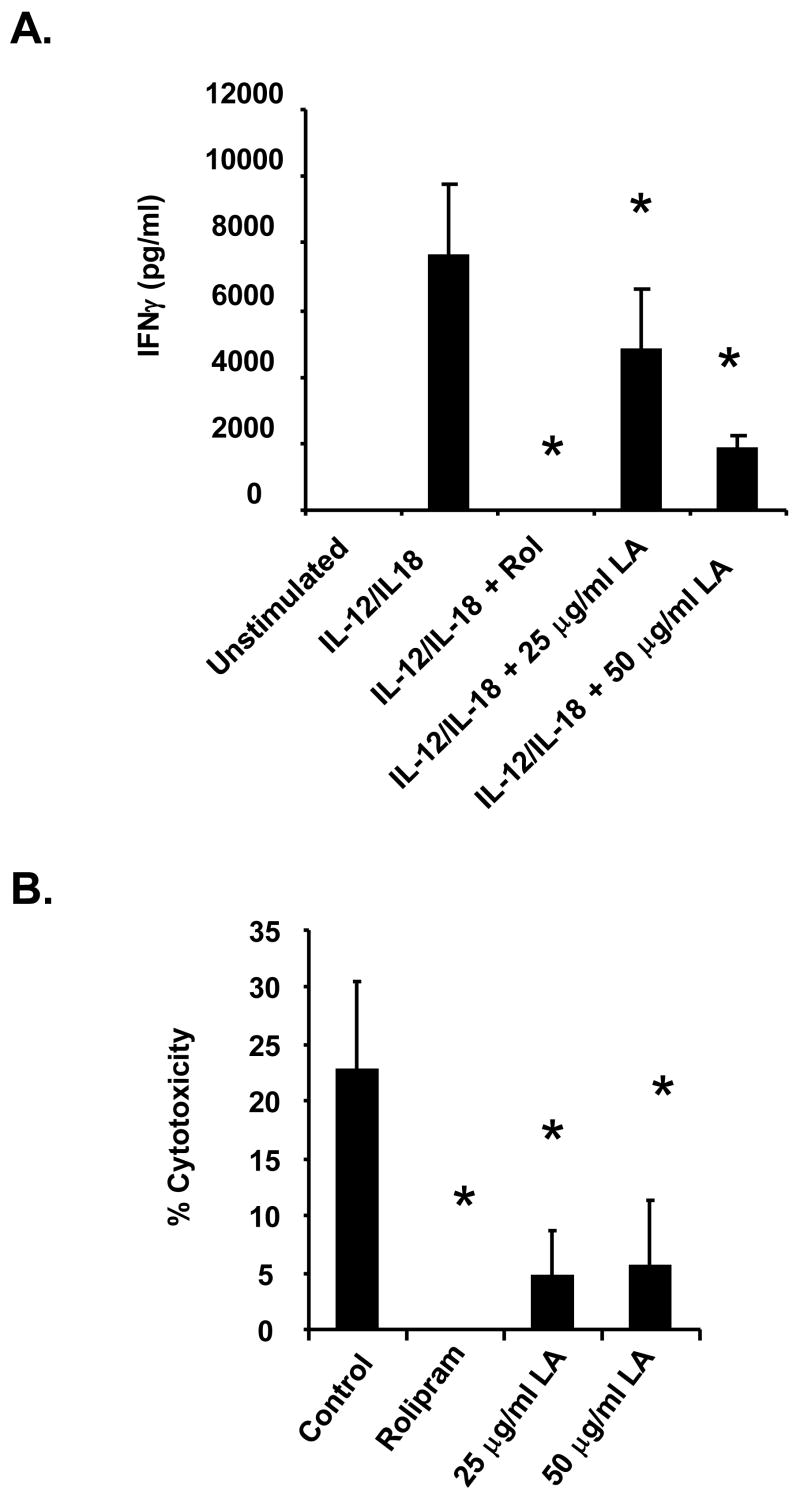
3.4. LA stimulation inhibits interferon gamma (IFNγ) secretion
Numerous reports indicate that cAMP inducing agents such as forskolin and prostaglandin modulate NK cell activation and function (Katz et al., 1982; Whalen and Bankhurst, 1990; Whalen and Crews, 2000). Joshi and colleagues (Joshi et al., 2001) showed that PGE2 suppresses IL-15 mediated IFNγ synthesis and cytotoxicity in human NK cells. Bariagaber and Whalen (Bariagaber and Whalen, 2003), however, showed that decreased AC and cAMP inhibits the cytotoxic function of human NK cells. These studies, clearly illustrate that the role of cAMP in NK cell activation and function is very complex. Thus, we evaluated the effect of LA on human NK cell activation by measuring IFNγ synthesis and secretion, which is a standard method for determining NK cell activation. NK cells were stimulated with a combination of IL-12 and IL-18 for 24 hours. As depicted in Figure 6A, unstimulated cells did not produce any detectable amount of IFNγ. Treatment with IL-12/IL-18 induced IFNγ secretion at a concentration of 8 ng/ml. The NK cells were then pre-incubated with rolipram and LA prior to stimulation with IL-12/IL-18. Rolipram is a PDE 4 inhibitor that increases endogenous cAMP levels and served as a positive control for these experiments. Rolipram suppressed IL-12/IL-18 induced IFNγ secretion to levels that were not detectable. Though not as potent as rolipram, LA also significantly inhibited IFNγ secretion. Pre-incubation with 25 or 50 μg/ml LA resulted in 25 and 75 percent, respectively, suppression compared to IL-12/IL-18 stimulated control. These data indicate that LA inhibits human NK cell activation as determined by measurement of IFNγ synthesis.
Figure 6. LA inhibits IFNγ secretion and NK cell cytotoxicity.
(A) Purified NK cells were plated on a 96-well plate at 1 × 105 cells per well in RPMI. Cells were pre-treated with 1 mM rolipram (30 min) or 25 or 50 μg/ml LA (1 min) then stimulated with a combination of IL-12/IL-18 for 24 hours. Ten percent FBS was added after treatment to ensure cell survival overnight. Supernatants were used to measure IFNγ secretion by ELISA. Depicted is a representative of 3 donors in duplicate. * indicates statistically significant compared to stimulated control, p< 0.05. (B) LDH release. NK cells were pre-incubated with rolipram or LA prior to addition of K562 target cells at a 6:1 effector:target ratio. The supernatant was removed and used for measurement of LDH release as in materials and method. Depicted is a representative of 3 donors in duplicate. * indicates statistically significant compared to untreated control, p< 0.05.
3.5. LA stimulation inhibits NK cell cytotoxicity
The ability of NK cells to lyse K562 target cells was measured using a lactate dehydrogenase (LDH) release assay from Promega. LDH is a stable cytosolic enzyme that is released into the media upon cell lysis. The media is collected and the amount of LDH is measured using a LDH release assay as described previously. In the control, NK cells were incubated with K562 cells at a 6:1 effector:target ratio. Results indicate that the NK cells exhibited ~20–30% cytotoxicity. We then investigated the effect of LA on the cytolytic activity of the NK cells. The cells were pre-incubated with 25, 50 or 100 μg/ml LA for 5 minutes prior to addition of the K562 cells. As illustrated in Figure 6B, LA significantly inhibited NK cell cytotoxicity by 60–75 percent at all concentrations used. Similarly, pre-incubation with rolipram suppressed NK cell cytotoxicity to below detectable levels. These data indicates that LA suppresses NK cell function.
4. Discussion
The role of NK cells in MS is quite controversial. There is evidence that NK cells may contribute to the destruction of the oligodendrocytes and myelin sheath, as well as contribute to other pathogenic features of MS, such as the perpetuation of the inflammatory response (Antel et al., 1998; Morandi et al., 2008; Morse et al., 2001). NK cells interact with the adaptive immune response to facilitate priming of T cells and DCs that modulate both the priming phase and effector phase of an immune response (Morandi et al., 2008). IFN-γ secreting NK cells were also shown to promote the development of murine EAE (Shi et al., 2000), which may be dependent upon the presence of IL-21 (Vollmer et al., 2005). Reports that NK cells are protective in MS are also very compelling (Huang et al., 2006; Lu et al., 2007; Matsumoto et al., 1998; Zhang et al., 1997). Zhang et al. demonstrated that mice depleted of NK cells developed more serious EAE associated with relapse (Zhang et al., 1997). Similar findings were reported in Lewis rats (Matsumoto et al., 1998). As discussed in a recent review by Morandi et al. (2008), the contrasting data in support of NK cells promoting or protecting against EAE could result from differences in manipulation of NK cells or differences in the NK cell subsets. In addition, the localization of NK cells and their interaction with various organ specific factors could trigger differential effector functions in NK cells. Thus, more studies are necessary to elucidate the role of NK cells in MS.
In this study, we discovered that addition of LA to NK cells increases the levels of cAMP, a potent immunosuppressant. The increase in cAMP levels is concentration dependent and is mediated via stimulation of the PGE2 stimulated g-protein coupled receptors EP2 and EP4 and adenylyl cyclase. We also report the novel findings that LA inhibits IFNγ secretion and cytotoxicity in NK cells. Taken together, these results indicate that LA can significantly alter NK cell function, suggesting that LA may ameliorate EAE and MS in part by modulating the activity of these cells. It will be exciting to pursue these studies in addition to investigations on the effect of LA in other immune cell populations to determine its protective properties in EAE.
After surveying 4 human donors, we found that a concentration of 10 μg/ml LA was sufficient to stimulate cAMP production in NK cells in vitro. Because both the basal and cAMP values in response to 10 μg/ml LA varied greatly from 4–251 and 8–2500 pmol/mg protein, respectively, the data were not statistically significant (p=0.06) even though the average fold increase was 14-fold. This concentration is detectable in the serum of healthy human subjects. These individuals received 1200 mg LA per day and had serum LA levels between 2–19 μg/ml after 60–240 minutes after ingestion (Yadav et al., 2005). Carlson et al. reported that 600 mg oral dosing of 12 healthy subjects with sodium salt conjugated R-LA resulted in peak serum LA levels between 10.6–33.8 μg/ml (Carlson et al., 2007). However, it is unknown if higher concentrations can be achieved since LA is rapidly taken up by eurythrocytes and other cell types, and is quickly metabolized. Thus, our data suggest that physiological concentrations of LA is sufficient to stimulate cAMP production.
cAMP is a small molecule second messenger that is involved in the transduction of signals from the extracellular milieu into the intracellular environment, resulting in the subsequent activation of a diverse array of signaling cascades. Consequently, cAMP regulates many biological functions including proliferation, migration, apoptosis and gene expression (Brosens and Gellersen, 2006; Chen et al., 2000). Thus, the cAMP signaling pathway is a therapeutic target for diseases such as cardiovascular disease, rheumatoid arthritis and Alzheimers (McPhee et al., 2005; Osadchii, 2007). Many cAMP inducing agents, such as PGE2 or forskolin, and phosphodiesterase inhibiting compounds, including rolipram, have been used as therapeutics. Since LA also stimulates cAMP production, LA may potentially be useful as a therapeutic strategy for MS and other chronic inflammatory diseases.
The generation of cAMP is initiated by activation of GPCRs. Thus, we sought to identify the receptors that mediate the production of cAMP in NK cells in response to LA stimulation. Pharmacological inhibitors were employed to block access to the prostanoid EP2 and EP4 receptors prior to stimulation with LA. Results show for the first time that pre-incubation with the inhibitors attenuated LA stimulated cAMP production in NK cells, suggesting that the EP2 and EP4 receptors mediate cAMP production. We further confirmed this data by overexpressing the EP2 and EP4 receptors in HEK 293 EBNA cells. Regan and colleagues have demonstrated that untransfected HEK293 EBNA cells do not produce cAMP in response to PGE2. However, after stable transfection with the EP2 or EP4 receptor, incubation with PGE2 results in measurable increases in cAMP levels (Fujino et al., 2002). We initially repeated their experiment by transiently transfecting HEK293 EBNA cells with EP2 or EP4 and incubated with PGE2 or LA 24 hrs post transfection. We found that PGE2 induced increases in cAMP levels, but LA did not (Schillace et al., 2007). For the current study, we repeated this experiment using cells that had been transfected for 24, 48 or 72 hours and found that in agreement with our published data, LA did not stimulate cAMP production 24 hrs post transfection; however, after 48 (Figure 3) or 72 (data not shown) hours the cells produced cAMP in response to both LA and PGE2. Review of the literature and consultation with Regan and colleagues confirm that longer times are needed for full maturation of the receptor signaling complex in the cell. We also showed that blocking the transfected EP2 or EP4 receptors with chemical inhibitors resulted in inhibition of cAMP production after stimulation with LA. This data, combined with initial inhibition studies in NK cells, support the notion that LA, similar to PGE2, induces cAMP production at least in part via activation of the EP2 and EP4 receptors.
It is unclear how PGE2 and LA, two seemingly different structural compounds, can possibly activate the same receptors. However, the binding of multiple, different ligands to the same receptor is not unique. The receptor may have multiple binding sites or different ligands may bind to the same or overlapping sites. In addition, receptor binding is a dynamic process where the binding pocket is defined by the incoming ligand. Nussinov and colleagues (Ma et al., 2002) argue that ligand binding simply reflects the existence of populations of protein conformers, which is the outcome of protein topology, and is modulated by the types of amino acid residues present. They further argue that the size and shape of protein side-chains and the flexibility of these side-chains also determine the geometry of the conformational space that is utilized for protein binding. For example, the Fc fragment of immunoglobulin G binds to its natural ligand, protein A and G, rheumatoid factor and synthetic peptides. The natural proteins are structurally dissimilar to the peptides, yet all bind to the Fc fragment. The binding resulted in slightly altered conformations in each of the complexes (DeLano et al., 2000). Small molecules are also capable of binding the same receptor even though they are structurally different. For example, synthetic molecules including SRT1720 and SRT1460 binds to SIRT1, an NAD+ dependent deacetylase, at the same site as the natural compound Resveratrol (Milne et al., 2007). Lipoic acid and PGE2 have a number of qualities in common from a physiochemical point of view. Both are hydrophobic and can exist in multiple conformers. Thus, it is reasonable that these two compounds could bind to the same receptors. Further studies are necessary to determine the mechanism by which LA binds to the EP2 and EP4 receptors.
We next investigated the functional consequences of LA stimulation in NK cells. Studies by Marracci and colleagues (Marracci et al., 2002) demonstrated inhibition of T cell migration into the spinal cord of EAE mice treated with LA as well as inhibition of human T cell migration in vitro (Marracci et al., 2004). Other investigators showed that LA inhibited endothelial apoptosis and proliferation (Artwohl et al., 2007) and tumor necrosis factor (TNF) α secretion (Zhang et al., 2007). Based on these studies and the fact that the activation state of NK cells influences their function, we examined the effect of LA on NK cell activation. NK cell activation can result in the production of a multitude of cytokines that can modulate immune responses to pathogens and directly affect the target cell as well. Pivotal among these cytokines is interferon (IFN)γ, which is a pleiotropic cytokine capable of influencing both the innate and adaptive immune systems. In addition, IFNγ activates antigen presenting cells (APCs) and promotes Th1 differentiation. In MS, IFNγ is present within the inflamed CNS (Hartung et al., 1992; Merrill, 1992) and can contribute to oligodendrocyte tissue injury (Horwitz et al., 1997). Most importantly, a pilot clinical trial of IFNγ showed that it increased relapse rates in MS (Panitch et al., 1987a; Panitch et al., 1987b).
We discovered that LA suppressed IL-12/IL-18 induced IFNγ secretion in NK cells, suggesting that LA may inhibit NK cell activation. We compared this response to that elicited by rolipram, a phosphodiesterase 4 inhibitor used as an anti-inflammatory drug. Rolipram also suppressed IL-12/IL-18 induced IFNγ secretion from NK cells. This is consistent with studies by other investigators who demonstrated that other cAMP producing compounds also attenuate IFNγ secretion. For instance, Joshi and colleagues (Joshi et al., 2001) demonstrated that PGE2 suppresses IL-15 induced IFNγ production in NK cells at both the transcriptional and secretional levels. Walker and Rotondo (Walker and Rotondo, 2004) also showed that PGE2 inhibited IFNγ synthesis. Since IFNγ contributes to the pathogenesis of MS, the ability of LA to inhibit IFNγ synthesis and NK cell activation provides a novel mechanism that may explain how LA can treat and prevent EAE and, by extension, MS.
Activation of NK cells leads to a dramatic increase in cytolytic activity. Some studies demonstrate a high number of activated NK cells in the peripheral blood of MS patients (Filaci et al., 1999; Munschauer et al., 1995). Activated NK cells, in turn, may contribute to increased killing of CNS oligodendrocytes (Antel et al., 1998). Furthermore, it was demonstrated that the perforin/granzyme system mediates NK cell killing of the oligodendrocytes. Since LA suppressed IFNγ secretion, we postulated that LA may also inhibit NK cell cytotoxicity. Our data showed that pre-incubation with LA inhibited the ability of NK cells to spontaneously lyse K562 target cells. This data, taken together with current literature and our finding that LA inhibits IFNγ secretion, support the idea that LA may ameliorate EAE by regulating NK cell activation and cytotoxicity towards oligodendrocytes. Future studies whereby LA is administered to NK cell depleted mice with EAE will help determine if these cells are mediating the effects of LA.
To summarize, we provide evidence that LA stimulates cAMP production in NK cells via activation of the EP2 and EP4 receptors and adenylyl cyclase, and that LA inhibits IFNγ secretion and NK cell cytotoxicity. These data provide a foundation for future studies, both in vitro and in patients with MS, to elucidate the molecular mechanisms by which LA ameliorates the symptoms of MS.
Acknowledgments
We would like to thank Sarah Fiedler and Casey Miller for helpful critique of the manuscript. This research was supported by the Department of Veterans Affairs Biomedical Laboratory Research & Development Service (D.W.C. and D.N.B.), NIH Grant P50AT00066-01 (D.N.B.), and the Nancy Davis Center Without Walls (D.N.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson KA, Ribar TJ, Illario M, Means AR. Defective survival and activation of thymocytes in transgenic mice expressing a catalytically inactive form of Ca2+/calmodulin-dependent protein kinase IV. Mol Endocrinol. 1997;11:725–737. doi: 10.1210/mend.11.6.0011. [DOI] [PubMed] [Google Scholar]
- Antel JP, McCrea E, Ladiwala U, Qin YF, Becher B. Non-MHC-restricted cell-mediated lysis of human oligodendrocytes in vitro: relation with CD56 expression. J Immunol. 1998;160:1606–1611. [PubMed] [Google Scholar]
- Aranami T, Miyake S, Yamamura T. Differential expression of CD11c by peripheral blood NK cells reflects temporal activity of multiple sclerosis. J Immunol. 2006;177:5659–5667. doi: 10.4049/jimmunol.177.8.5659. [DOI] [PubMed] [Google Scholar]
- Artwohl M, Muth K, Kosulin K, de Martin R, Holzenbein T, Rainer G, Freudenthaler A, Huttary N, Schmetterer L, Waldhausl WK, Baumgartner-Parzer SM. R-(+)-alpha-lipoic acid inhibits endothelial cell apoptosis and proliferation: involvement of Akt and retinoblastoma protein/E2F-1. Am J Physiol Endocrinol Metab. 2007;293:E681–689. doi: 10.1152/ajpendo.00584.2006. [DOI] [PubMed] [Google Scholar]
- Bariagaber AK, Whalen MM. Decreased adenylyl cyclase and cAMP-dependent protein kinase activities inhibit the cytotoxic function of human natural killer cells. Hum Immunol. 2003;64:866–873. doi: 10.1016/s0198-8859(03)00154-x. [DOI] [PubMed] [Google Scholar]
- Brosens JJ, Gellersen B. Death or survival--progesterone-dependent cell fate decisions in the human endometrial stroma. J Mol Endocrinol. 2006;36:389–398. doi: 10.1677/jme.1.02060. [DOI] [PubMed] [Google Scholar]
- Carlson DA, Smith AR, Fischer SJ, Young KL, Packer L. The plasma pharmacokinetics of R-(+)-lipoic acid administered as sodium R-(+)-lipoate to healthy human subjects. Altern Med Rev. 2007;12:343–351. [PubMed] [Google Scholar]
- Chaudhary P, Marracci GH, Bourdette DN. Lipoic acid inhibits expression of ICAM-1 and VCAM-1 by CNS endothelial cells and T cell migration into the spinal cord in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;175:87–96. doi: 10.1016/j.jneuroim.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Chen BC, Liao CC, Hsu MJ, Liao YT, Lin CC, Sheu JR, Lin CH. Peptidoglycan-induced IL-6 production in RAW 264.7 macrophages is mediated by cyclooxygenase-2, PGE2/PGE4 receptors, protein kinase A, I kappa B kinase, and NF-kappa B. J Immunol. 2006;177:681–693. doi: 10.4049/jimmunol.177.1.681. [DOI] [PubMed] [Google Scholar]
- Chen CH, Zhang DH, LaPorte JM, Ray A. Cyclic AMP activates p38 mitogen-activated protein kinase in Th2 cells: phosphorylation of GATA-3 and stimulation of Th2 cytokine gene expression. J Immunol. 2000;165:5597–5605. doi: 10.4049/jimmunol.165.10.5597. [DOI] [PubMed] [Google Scholar]
- DeLano WL, Ultsch MH, de Vos AM, Wells JA. Convergent solutions to binding at a protein-protein interface. Science. 2000;287:1279–1283. doi: 10.1126/science.287.5456.1279. [DOI] [PubMed] [Google Scholar]
- Filaci G, Bacchetta R, Zanetti M. Is there a role for NK cells in the pathogenesis of multiple sclerosis? A case study. Hum Immunol. 1999;60:231–238. doi: 10.1016/s0198-8859(98)00121-9. [DOI] [PubMed] [Google Scholar]
- French AR, Yokoyama WM. Natural killer cells and viral infections. Curr Opin Immunol. 2003;15:45–51. doi: 10.1016/s095279150200002x. [DOI] [PubMed] [Google Scholar]
- Fujino H, West KA, Regan JW. Phosphorylation of glycogen synthase kinase-3 and stimulation of T-cell factor signaling following activation of EP2 and EP4 prostanoid receptors by prostaglandin E2. J Biol Chem. 2002;277:2614–2619. doi: 10.1074/jbc.M109440200. [DOI] [PubMed] [Google Scholar]
- Hartung HP, Jung S, Stoll G, Zielasek J, Schmidt B, Archelos JJ, Toyka KV. Inflammatory mediators in demyelinating disorders of the CNS and PNS. J Neuroimmunol. 1992;40:197–210. doi: 10.1016/0165-5728(92)90134-7. [DOI] [PubMed] [Google Scholar]
- Horwitz MS, Evans CF, McGavern DB, Rodriguez M, Oldstone MB. Primary demyelination in transgenic mice expressing interferon-gamma. Nat Med. 1997;3:1037–1041. doi: 10.1038/nm0997-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Shi FD, Jung S, Pien GC, Wang J, Salazar-Mather TP, He TT, Weaver JT, Ljunggren HG, Biron CA, Littman DR, Ransohoff RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. Faseb J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- Joshi PC, Zhou X, Cuchens M, Jones Q. Prostaglandin E2 suppressed IL-15-mediated human NK cell function through down-regulation of common gamma-chain. J Immunol. 2001;166:885–891. doi: 10.4049/jimmunol.166.2.885. [DOI] [PubMed] [Google Scholar]
- Katz P, Zaytoun AM, Fauci AS. Mechanisms of human cell-mediated cytotoxicity. I. Modulation of natural killer cell activity by cyclic nucleotides. J Immunol. 1982;129:287–296. [PubMed] [Google Scholar]
- Korbel DS, Finney OC, Riley EM. Natural killer cells and innate immunity to protozoan pathogens. Int J Parasitol. 2004;34:1517–1528. doi: 10.1016/j.ijpara.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Kuklina EM, Shirshev SV. Role of cAMP-dependent signal transduction in the control of T lymphocyte activation. Biochemistry (Mosc) 2000;65:629–639. [PubMed] [Google Scholar]
- Lu L, Ikizawa K, Hu D, Werneck MB, Wucherpfennig KW, Cantor H. Regulation of activated CD4+ T cells by NK cells via the Qa-1-NKG2A inhibitory pathway. Immunity. 2007;26:593–604. doi: 10.1016/j.immuni.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B, Shatsky M, Wolfson HJ, Nussinov R. Multiple diverse ligands binding at a single protein site: a matter of pre-existing populations. Protein Sci. 2002;11:184–197. doi: 10.1110/ps.21302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marracci GH, Jones RE, McKeon GP, Bourdette DN. Alpha lipoic acid inhibits T cell migration into the spinal cord and suppresses and treats experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;131:104–114. doi: 10.1016/s0165-5728(02)00269-2. [DOI] [PubMed] [Google Scholar]
- Marracci GH, McKeon GP, Marquardt WE, Winter RW, Riscoe MK, Bourdette DN. Alpha lipoic acid inhibits human T-cell migration: implications for multiple sclerosis. J Neurosci Res. 2004;78:362–370. doi: 10.1002/jnr.20255. [DOI] [PubMed] [Google Scholar]
- Matlhagela K, Taub M. Involvement of EP1 and EP2 receptors in the regulation of the Na, K-ATPase by prostaglandins in MDCK cells. Prostaglandins Other Lipid Mediat. 2006;79:101–113. doi: 10.1016/j.prostaglandins.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto Y, Kohyama K, Aikawa Y, Shin T, Kawazoe Y, Suzuki Y, Tanuma N. Role of natural killer cells and TCR gamma delta T cells in acute autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1681–1688. doi: 10.1002/(SICI)1521-4141(199805)28:05<1681::AID-IMMU1681>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- McPhee I, Gibson LC, Kewney J, Darroch C, Stevens PA, Spinks D, Cooreman A, MacKenzie SJ. Cyclic nucleotide signalling: a molecular approach to drug discovery for Alzheimer’s disease. Biochem Soc Trans. 2005;33:1330–1332. doi: 10.1042/BST0331330. [DOI] [PubMed] [Google Scholar]
- Merrill JE. Proinflammatory and antiinflammatory cytokines in multiple sclerosis and central nervous system acquired immunodeficiency syndrome. J Immunother. 1992;12:167–170. doi: 10.1097/00002371-199210000-00004. [DOI] [PubMed] [Google Scholar]
- Milne JC, Lambert PD, Schenk S, Carney DP, Smith JJ, Gagne DJ, Jin L, Boss O, Perni RB, Vu CB, Bemis JE, Xie R, Disch JS, Ng PY, Nunes JJ, Lynch AV, Yang H, Galonek H, Israelian K, Choy W, Iffland A, Lavu S, Medvedik O, Sinclair DA, Olefsky JM, Jirousek MR, Elliott PJ, Westphal CH. Small molecule activators of SIRT1 as therapeutics for the treatment of type 2 diabetes. Nature. 2007;450:712–716. doi: 10.1038/nature06261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morandi B, Bramanti P, Bonaccorsi I, Montalto E, Oliveri D, Pezzino G, Navarra M, Ferlazzo G. Role of natural killer cells in the pathogenesis and progression of multiple sclerosis. Pharmacol Res. 2008;57:1–5. doi: 10.1016/j.phrs.2007.11.003. [DOI] [PubMed] [Google Scholar]
- Morini M, Roccatagliata L, Dell’Eva R, Pedemonte E, Furlan R, Minghelli S, Giunti D, Pfeffer U, Marchese M, Noonan D, Mancardi G, Albini A, Uccelli A. Alpha-lipoic acid is effective in prevention and treatment of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2004;148:146–153. doi: 10.1016/j.jneuroim.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Morse RH, Seguin R, McCrea EL, Antel JP. NK cell-mediated lysis of autologous human oligodendrocytes. J Neuroimmunol. 2001;116:107–115. doi: 10.1016/s0165-5728(01)00289-2. [DOI] [PubMed] [Google Scholar]
- Munschauer FE, Hartrich LA, Stewart CC, Jacobs L. Circulating natural killer cells but not cytotoxic T lymphocytes are reduced in patients with active relapsing multiple sclerosis and little clinical disability as compared to controls. J Neuroimmunol. 1995;62:177–181. doi: 10.1016/0165-5728(95)00115-9. [DOI] [PubMed] [Google Scholar]
- Osadchii OE. Myocardial phosphodiesterases and regulation of cardiac contractility in health and cardiac disease. Cardiovasc Drugs Ther. 2007;21:171–194. doi: 10.1007/s10557-007-6014-6. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987a;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987b;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Roder JC, Argov S, Klein M, Petersson C, Kiessling R, Andersson K, Hansson M. Target-effector cell interaction in the natural killer cell system. V. Energy requirements, membrane integrity, and the possible involvement of lysosomal enzymes. Immunology. 1980;40:107–116. [PMC free article] [PubMed] [Google Scholar]
- Sanchez T, Moreno JJ. Role of EP(1) and EP(4) PGE(2) subtype receptors in serum-induced 3T6 fibroblast cycle progression and proliferation. Am J Physiol Cell Physiol. 2002;282:C280–288. doi: 10.1152/ajpcell.00128.2001. [DOI] [PubMed] [Google Scholar]
- Saraste M, Irjala H, Airas L. Expansion of CD56Bright natural killer cells in the peripheral blood of multiple sclerosis patients treated with interferon-beta. Neurol Sci. 2007;28:121–126. doi: 10.1007/s10072-007-0803-3. [DOI] [PubMed] [Google Scholar]
- Schillace RV, Pisenti N, Pattamanuch N, Galligan S, Marracci GH, Bourdette DN, Carr DW. Lipoic acid stimulates cAMP production in T lymphocytes and NK cells. Biochem Biophys Res Commun. 2007;354:259–264. doi: 10.1016/j.bbrc.2006.12.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreibelt G, Musters RJ, Reijerkerk A, de Groot LR, van der Pol SM, Hendrikx EM, Dopp ED, Dijkstra CD, Drukarch B, de Vries HE. Lipoic acid affects cellular migration into the central nervous system and stabilizes blood-brain barrier integrity. J Immunol. 2006;177:2630–2637. doi: 10.4049/jimmunol.177.4.2630. [DOI] [PubMed] [Google Scholar]
- Shi FD, Takeda K, Akira S, Sarvetnick N, Ljunggren HG. IL-18 directs autoreactive T cells and promotes autodestruction in the central nervous system via induction of IFN-gamma by NK cells. J Immunol. 2000;165:3099–3104. doi: 10.4049/jimmunol.165.6.3099. [DOI] [PubMed] [Google Scholar]
- Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282:11613–11617. doi: 10.1074/jbc.R600038200. [DOI] [PubMed] [Google Scholar]
- Vollmer TL, Liu R, Price M, Rhodes S, La Cava A, Shi FD. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J Immunol. 2005;174:2696–2701. doi: 10.4049/jimmunol.174.5.2696. [DOI] [PubMed] [Google Scholar]
- Walker W, Rotondo D. Prostaglandin E2 is a potent regulator of interleukin-12-and interleukin-18-induced natural killer cell interferon-gamma synthesis. Immunology. 2004;111:298–305. doi: 10.1111/j.1365-2567.2004.01810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Klein RD. Prostaglandin E2 induces vascular endothelial growth factor secretion in prostate cancer cells through EP2 receptor-mediated cAMP pathway. Mol Carcinog. 2007;46:912–923. doi: 10.1002/mc.20320. [DOI] [PubMed] [Google Scholar]
- Whalen MM, Bankhurst AD. Effects of beta-adrenergic receptor activation, cholera toxin and forskolin on human natural killer cell function. Biochem J. 1990;272:327–331. doi: 10.1042/bj2720327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen MM, Crews JD. Inhibition of a phosphodiesterase III in the lysis-sensitive target-induced elevation of cyclic AMP (cAMP) in human natural killer cells. Biochem Pharmacol. 2000;60:499–506. doi: 10.1016/s0006-2952(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Yadav V, Marracci G, Lovera J, Woodward W, Bogardus K, Marquardt W, Shinto L, Morris C, Bourdette D. Lipoic acid in multiple sclerosis: a pilot study. Mult Scler. 2005;11:159–165. doi: 10.1191/1352458505ms1143oa. [DOI] [PubMed] [Google Scholar]
- Zhang B, Yamamura T, Kondo T, Fujiwara M, Tabira T. Regulation of experimental autoimmune encephalomyelitis by natural killer (NK) cells. J Exp Med. 1997;186:1677–1687. doi: 10.1084/jem.186.10.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WJ, Wei H, Hagen T, Frei B. Alpha-lipoic acid attenuates LPS-induced inflammatory responses by activating the phosphoinositide 3-kinase/Akt signaling pathway. Proc Natl Acad Sci U S A. 2007;104:4077–4082. doi: 10.1073/pnas.0700305104. [DOI] [PMC free article] [PubMed] [Google Scholar]