Abstract
PMCA2, a major calcium pump, is expressed at particularly high levels in Purkinje neurons. Accordingly, PMCA2-null mice exhibit ataxia suggesting cerebellar pathology. It is not yet known how changes in PMCA2 expression or activity affect molecular pathways in Purkinje neurons. We now report that the levels of metabotropic glutamate receptor 1 (mGluR1), which plays essential roles in motor coordination, synaptic plasticity, and associative learning, are reduced in the cerebellum of PMCA2-null mice as compared to wild type littermates. The levels of inositol 1,4,5-triphosphate receptor type 1 (IP3R1), an effector downstream to mGluR1, which mediates intracellular calcium signaling, and the expression of Homer 1b/c and Homer 3, scaffold proteins that couple mGluR1 to IP3R1, are also reduced in somata and dendrites of some Purkinje cell subpopulations. In contrast, no alterations occur in the levels of mGluR1 and its downstream effectors in the hippocampus, indicating that the effects are region specific. The reduction in cerebellar mGluR1, IP3R1 and Homer 3 levels are neither due to a generic decrease in Purkinje proteins nor extensive dendritic loss as immunoreactivity to total and non-phosphorylated neurofilament H (NFH) is increased in Purkinje dendrites and microtubule associated protein 2 (MAP2) staining reveals a dense dendritic network in the molecular layer of the PMCA2-null mouse cerebellum. PMCA2 coimmunoprecipitates with mGluR1, Homer 3 and IP3R1, suggesting that the calcium pump is a constituent of the mGluR1 signaling complex. Our results suggest that the decrease in the expression of mGluR1 and its downstream effectors and perturbations in the mGluR1 signaling complex in the absence of PMCA2 may cumulatively result in aberrant metabotropic glutamate receptor signaling in Purkinje neurons leading to cerebellar deficits in the PMCA2-null mouse.
Introduction
PMCAs are P-type ATPases that play a major role in expelling calcium from cells. Four isoforms, PMCA1-4, encoded by different genes, have been described (Strehler and Zacharias, 2001). PMCA isoform 2 is enriched in brain and heart and is predominantly expressed in neurons (Stahl et al., 1992; Filotea et al., 1997; Stauffer et al., 1995; 1997; Lehotsky et al., 1999; Burette et al., 2003). PMCA2 is particularly abundant in Purkinje neurons of the cerebellum and is found both in cell bodies and dendrites (Stahl et al., 1992; Stauffer et al., 1997). The pivotal role of PMCA2 in the function of the cerebellum is indicated by the phenotype of the PMCA2-null mouse and the deafwaddler 2J (dfw2J), a mouse with a spontaneous mutation in the PMCA2 gene which causes null pump activity (Kozel et al., 1998; Street et al., 1998; Penheiter et al., 2001). Both PMCA2-null and dfw2J mice manifest motor deficits and ataxia, which may be partially attributable to cerebellar pathology. In fact, an increase in the density of Purkinje neurons and a reduction in the thickness of the molecular layer in the cerebellum of PMCA2-null mice have been documented (Kozel et al., 1998). Additional studies further pinpoint the importance of PMCA2 in the function of other CNS regions (Lehotsky et al., 2002). Loss of motor neurons in the spinal cord of PMCA2-null and dfw2J mice has recently been reported (Kurnellas et al., 2005). Accordingly, PMCA2-null and dfw2J mice manifest hindlimb weakness and loss of grip strength in addition to the aforementioned neural deficits. A decrease in PMCA2 levels in the inflamed spinal cord of rodents affected by experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis (MS), has also been documented (Nicot et al., 2003; 2005).
Our earlier studies, which focused on the proteome analysis of the PMCA2-null versus wild type mouse cerebellum, indicated a significant decrease in the levels of IP3R1 (Hu et al., 2006). This finding was of particular interest as IP3R1 is an effector downstream to mGluR1, a glutamate receptor subtype, which plays a pivotal role in important cerebellar functions including motor coordination, synaptic plasticity, associative learning and developmental innervation of Purkinje cells by climbing fibers (Ichise et al., 2000; Kano et al., 1997). Activation of mGluR1 in parallel fiber-Purkinje cell synapses leads to production of IP3, which binds IP3Rs on the endoplasmic reticulum, a key step in the induction of intracellular calcium release and signaling (Knöpfel and Grandes, 2002). Coupling of IP3R1 to mGluR1 is mediated by Homer proteins, which are components of the molecular scaffold at postsynaptic densities of excitatory synapses (Brakeman et al., 1997; Xiao et al., 1998; Tu et al., 1998). This family of small proteins comprises several members including Homer 1a, 1b/c, 2 and 3. Homer 3 is highly expressed in the cerebellum, especially in the dendrites of Purkinje cells and Homer proteins regulate the localization, expression and function of group I mGluRs (Xiao et al., 1998; Sheng, 1997; Thomas, 2002). Co-localization of IP3R with Homer 1b/c and PMCA in Purkinje cells has been reported, although the antibodies used could not differentiate between different PMCA isoforms (Sandona et al., 2003). Our double-labeling studies also indicated co-localization of mGluR1 with PMCA2 in dendrites of Purkinje neurons (Kurnellas et al., 2006). Furthermore, the co-expression of Ania-3/Homer with PMCA2 in soma and dendrites of hippocampal neurons and the interaction of Ania-3/Homer with the b-splice form of all PMCAs via their PDZ binding domain has been documented (Sgambato-Faure et al., 2006).
The present studies were undertaken in order to determine the molecular pathways that are affected in Purkinje neurons of PMCA2-null mice with especial emphasis on the mGluR1 signaling pathway and to define a potential link between PMCA2 and the components of the mGluR1-Homer3-IP3R1 complex in the cerebellum.
RESULTS
Previous studies reported an increase in the density of Purkinje neurons and a small but significant decrease (17%) in the thickness of the molecular layer in the cerebellum of PMCA2-null mice as compared to wild type controls, indicating that lack of PMCA2 induces morphological changes (Kozel et al., 1998). However, the molecular alterations in the cerebellum of the PMCA2-null mouse have not yet been studied. We undertook novel studies in order to gain an initial insight into this issue. Proteomic analysis of the PMCA2-null cerebellum as compared to the wild type littermates provided the first hints on potential pathways which might be affected. These investigations indicated a decrease in IP3R1 (Hu et al., 2006). Additional analysis of the proteome by two dimensional gel electrophoresis followed by mass spectrometry raised the possibility of reductions in Homer 3 levels (unpublished results). As Homer proteins link mGluRs to IP3Rs and mGluR1 is essential for cerebellar function, we further assessed whether levels of mGluR1 and its downstream effectors are reduced in PMCA2-null mouse cerebellum and in which cells and cellular compartments the changes occur.
Levels of mGluR1, Homer proteins and IP3R1 are selectively decreased in the cerebellum of the PMCA2-null mouse
To determine whether there are alterations in the levels of mGluR1 and its downstream effectors, we performed Western blot analysis using individual cerebella obtained from PMCA2-null and wild type littermates. There was a significant reduction in mGluR1 (41.0 ± 12.7 %, p < 0.03) levels in cerebella of knockout mice as compared to wild type littermates (Fig. 1). Furthermore, we corroborated the results obtained by proteomic analysis using isobaric tags for relative and absolute quantitation (iTRAQ) and confirmed the decrease in IP3R1 levels by Western analysis (40 ± 2.4 %, p < 0.0004). As mGluR1 and IP3R1 can be physically linked via Homer 1b/c and Homer 3, which are abundantly expressed in Purkinje neurons, we further analyzed potential changes in the expression of these proteins (Xiao et al., 1998; Knopfel and Grandes, 2002; Brakeman et al., 1997). Antibodies specific for Homer 3 or Homer 1b/c visualized bands at 47 kDa, the expected molecular weight. Levels of Homer 1b/c and Homer 3 were significantly decreased (Fig. 1; 51.9 ± 7.5, p < 0.0004 and 41.8 ± 8.7 %, p < 0.002, respectively).
Fig. 1.
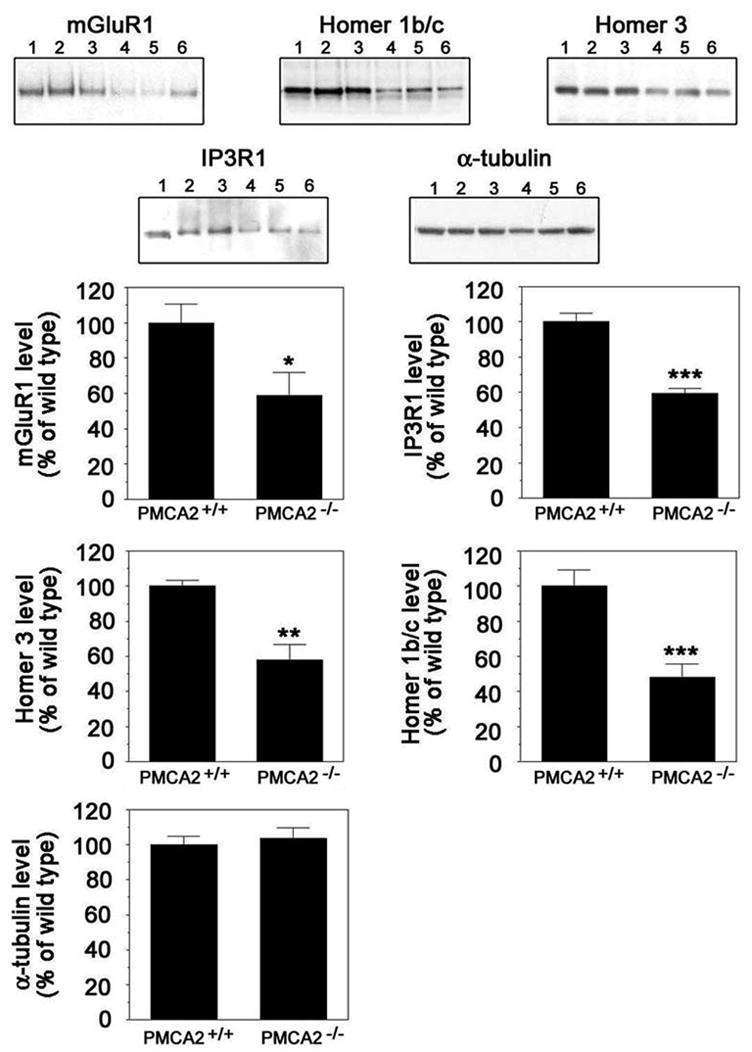
Quantification of mGluR1, IP3R1, Homer 1b/c and Homer 3 levels in the cerebellum of PMCA2+/+ and PMCA2−/− mice by Western blot analysis. The top panel shows representative Western blots. Lanes 1–3 and 4–6 are samples obtained from the cerebellum of three distinct PMCA2+/+ and PMCA2−/− mice, respectively. Antibodies specific for each protein recognized bands of expected molecular weights; mGluR1: ~133 kDa, Homer 1b/c and Homer 3: ~47 kDa, and IP3R1: ~240 kDa. α-Tubulin (~50 kDa), remained unchanged and was used as a control for experimental variations. The bottom panel is the schematic representation of two to three experiments combined (n=6–8). Values in graphs represent mean ± S.E.M. after normalization to α-tubulin. *p<0.03, **p<0.002 and ***p< 0.0004 by Student’s t-test.
To determine whether the changes in the levels of mGluR1, Homer 3 and IP3R1 are selective to the cerebellum, we examined the hippocampus, another brain region which expresses all three proteins as well as PMCA2. We did not find any significant alterations in the levels of mGluR1, Homer 3 and IP3R1 in the hippocampus of PMCA2−/− mice as compared to wild type controls (Fig. 2). These results support the notion that the decrease in the levels of mGluR1 and its downstream effectors selectively occur in the cerebellum.
Fig. 2.
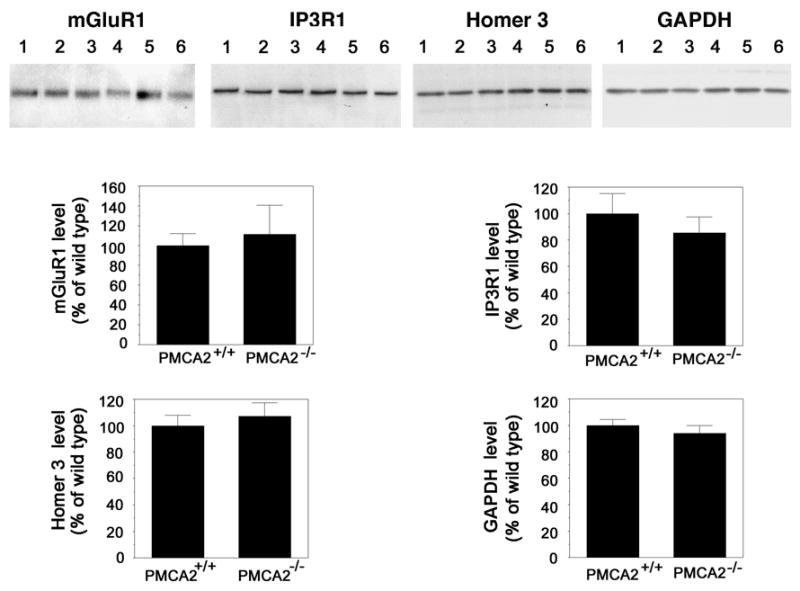
Quantification of mGluR1, IP3R1 and Homer 3 levels in the hippocampus of PMCA2+/+ and PMCA2−/− mice by Western analysis. The top panel shows representative Western blots. Lanes 1–3 and 4–6 are samples obtained from the hippocampus of three distinct PMCA2+/+ and PMCA2−/− mice, respectively. The bottom panel is the schematic representation of the results obtained by Western analysis (n=4–5). As α-tubulin was variable in the hippocampus of the wild type versus knockout mouse, another housekeeping gene, GADPH (~36 kDa), was used as control for experimental variations. Values in graphs represent mean ± S.E.M. after normalization to GAPDH. No significant changes were observed.
The reduction in IP3R1 and Homer 3 levels occur in clusters of Purkinje cells and dendrites
To identify the cell types where changes took place, we performed immunocytochemistry on sections of PMCA2+/+ and PMCA2−/− mouse cerebellum (Figs. 3 and 4). In general, IP3R1 staining was predominantly localized to molecular and Purkinje cell layers (Fig. 3A). The cell bodies of granule neurons did not show staining above background whereas occasional immunopositive fibers could be detected in the granule cell layer and white matter. In wild type mice, most Purkinje somata were IP3R1 positive. Dense and punctate staining was also detectable in the molecular layer (Fig. 3A). Occasionally, some non-punctate staining could be observed along the proximal apical dendrites. In cerebellar sections of the wild type mouse, IP3R1 staining appeared to be evenly distributed throughout the Purkinje cell and molecular layer of the cerebellum irrespective of the different folia. In contrast, in the PMCA2-null mouse a decrease in the staining intensity was observed in clusters of Purkinje cells especially in regions along the cerebellar sulci (Figs. 3B and 4C). In these areas, a reduction in staining intensity was evident both in the molecular layer and in somata of Purkinje neurons (Fig. 3B). On the contrary, the intensity of IP3R1 immunoreactivity did not appear modified in Purkinje neurons and dendrites located in the folial crowns of the PMCA2−/− cerebellum (Figs. 4A and 4B). However, in these regions, the staining pattern in the molecular layer of the PMCA2-null mouse cerebellum appeared different than that observed in the wild type. The immunoreactivity was less punctate and concentrated on apical dendrites and many small dendritic branches.
Fig. 3.
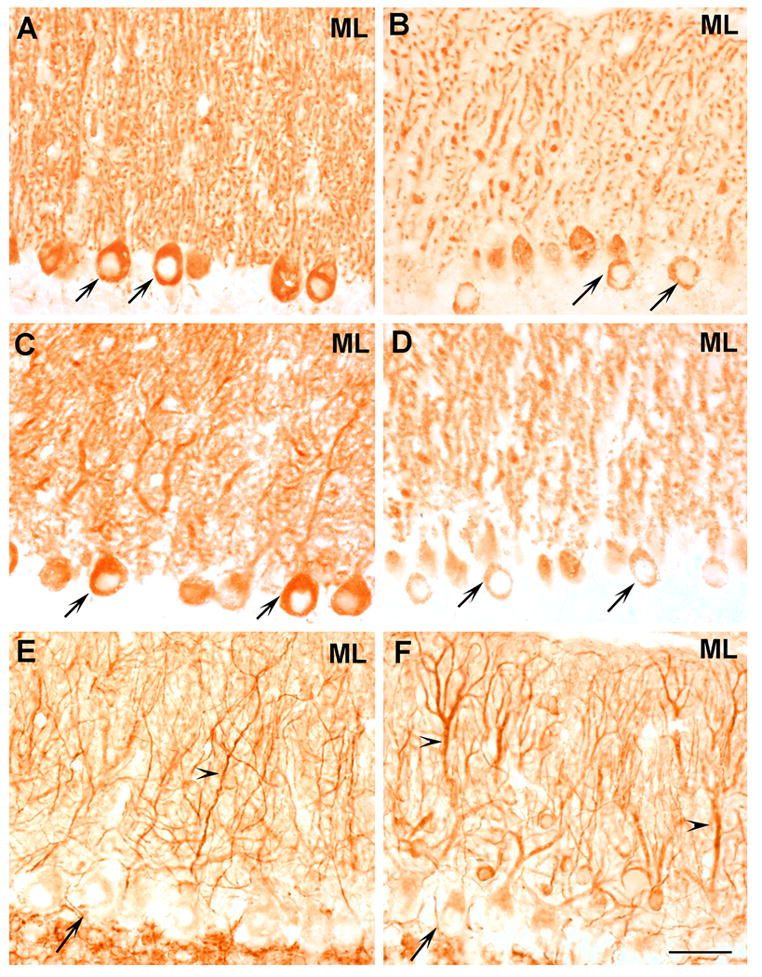
IP3R1, Homer 3 and MAP2 immunoreactivity in sagittal sections of the PMCA2+/+ and PMCA2−/− mouse cerebellum. IP3R1 staining was predominantly localized to the molecular layer (ML) and Purkinje somata (arrows) of the wild type mouse cerebellum (A). No staining was observed in cell bodies of granule neurons. The staining intensity was decreased in Purkinje neuron cell bodies (arrows) and in the ML of the PMCA2−/− mouse (B). Similarly, Homer 3 antibody revealed diffuse immunoreactivity in the ML and strong staining in Purkinje somata (arrows) of the wild type cerebellum (C). The staining was decreased both in cell bodies (arrows) and ML of the PMCA2-null mouse (D). The decrease in immunoreactivity occurred in clusters of Purkinje cells especially along cerebellar sulci (see figure 4). Immunostaining with an anti-MAP2 antibody revealed the extensive dendritic network both in wild type (E) and knockout mouse (F). n=4, Bar represents 100 μm.
Fig. 4.
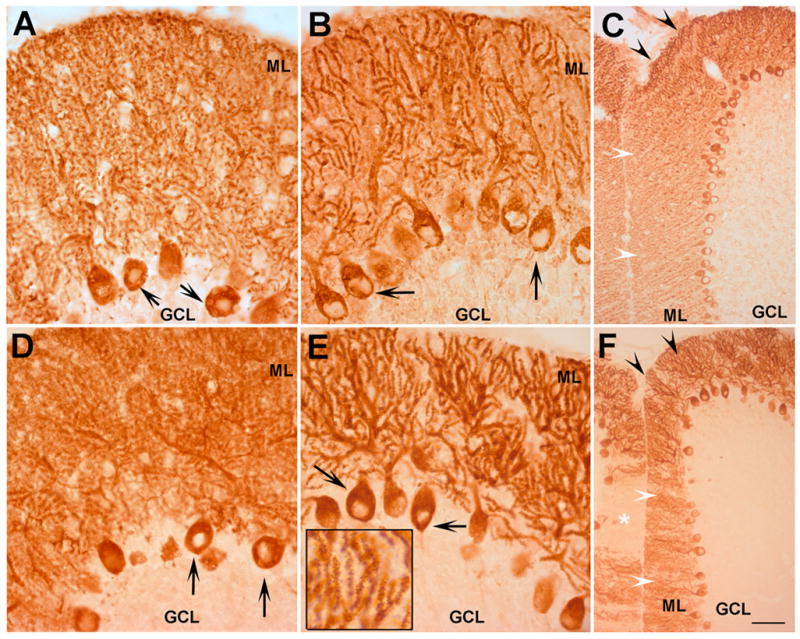
IP3R1 and Homer 3 immunoreactivity in folial crowns of PMCA2+/+ and PMCA2−/− mouse cerebellum. In folial crowns, the intensity of IP3R1 staining in Purkinje neurons (arrows) or the molecular layer (ML) did not appear different in the PMCA2+/+ (A) and PMCA2−/− mice (B). However, the staining pattern was different in the wild type versus the knockout mouse. Immunoreactivity was mostly diffuse and punctate in the molecular layer (ML) of the wild type cerebellum and concentrated along the dendritic branches in the knockout mouse. C) The difference in IP3R1 staining in Purkinje cell clusters located along the fissure (white arrowheads) versus those in the folial crowns (black arrowheads) is noticeable in a low magnification picture of a section through the cerebellum of a knockout mouse. Note that the differences are most pronounced in the molecular layer. As in the case of IP3R1, the intensity of Homer 3 staining was strong in the folial crowns of both the wild type (D) and PMCA2-null mouse (E). However, a change in the pattern of the staining could be observed in the (ML). Note that the staining is diffuse in the ML of the wild type cerebellum whereas most immunoreactivity is localized to dendritic branches in the ML of the knockout mouse. The inset in figure E is a higher magnification picture showing the punctate staining in dendritic branches. F) Low magnification picture of a section through the cerebellum of a knockout mouse showing the difference in staining intensity in the ML and somata of Purkinje neurons located along a fissure (white arrowheads) as compared to folial crowns (black arrowheads). In some regions, indicated by the asterix, immunostaining was completely suppressed. GCL: granule cell layer. n=4, Bar represents 60 μm for A, B, D and E and 200 μm for C and F.
Immunostaining with the anti-Homer 3 antibody in the cerebellum of the PMCA2-null and wild type mice revealed an overall pattern similar to that of IP3R1. In the wild-type mouse, the antibody labeled the somata of Purkinje neurons and the molecular layer, evenly throughout the cerebellum. The staining was diffuse in the molecular layer, although some apical dendrites and dendritic branches could be discerned (Fig. 3C). As in the case of IP3R1, the staining intensity decreased in somata and dendrites of some Purkinje cell subpopulations located close to the cerebellar fissures of the PMCA2-null mouse, (Figs. 3D and 4F). On the contrary, strong immunoreactivity could still be detected in Purkinje neurons located in the folial crowns of the PMCA2−/− and PMCA2+/+ cerebellum and the molecular layer containing the dendrites of these cells (Figs. 4D and E). However, in these regions, the staining pattern in the molecular layer of the knockout mouse had a different appearance than the wild type littermates. In contrast to the diffuse staining observed in the molecular layer of the PMCA2+/+ mouse cerebellum, punctate Homer 3 immunostaining clearly revealed apical dendrites and many branches of the dendritic tree in the PMCA2-null mouse. As reported before, Homer 3 antibody did not stain cells of other layers including granule neurons or glia (Xiao et al., 1998).
To determine whether the decrease in Homer 3 and IP3R1 staining could be due to extensive dendritic loss, we labeled adjacent sections with an antibody against MAP2, a dendritic marker, which visualized a complex dendritic network both in wild type and PMCA2-null mouse cerebellum (Figs. 3E and F, respectively). In the wild type mouse. MAP2 antibody stained weakly the Purkinje cell bodies and strongly many fine processes in the molecular layer. In the cerebellum of the PMCA2-null mouse, in addition to fine processes, many apical dendrites and their main branches showed strong MAP2 immunoreactivity. These results support the notion that the ~40% decrease in the levels Homer 3 and IP3R1 are probably not due to extensive loss of dendrites although some decrease due to loss of dendritic spines cannot be ruled out.
To ascertain that the decrease in mGluR1, Homer 3 and IP3R1 is not due to generic reductions in Purkinje proteins, we stained cerebellar sections with antibodies against total and non-phosphorylated neurofilament H (NFH) (Fig. 5). In contrast to the aforementioned proteins, both total and, in particular, non-phosphorylated (NFH) levels show a pronounced increase in the cell bodies and dendrites of Purkinje neurons (Figs. 5A-F), ruling out the possibility of nonspecific decreases in total Purkinje proteins. Importantly, the increase in non-phosphorylated NFH further supports the concept of molecular abnormalities in Purkinje cells of the PMCA2-null mouse as it suggests defects in the cytoskeleton, a possibility that requires further investigation. Interestingly, these latter results are in conceptual agreement with our previous report showing an increase in non-phosphorylated NFH staining in neuronal cultures treated with a PMCA inhibitor (Kurnellas et al., 2005). The increase in NFH was not observed in other brain regions such as the substantia nigra (Figs. 5G and H) or the hippocampus (not shown), indicating regional specificity. Thus, in addition to other alterations, lack of PMCA2 may cause cytoskeletal abnormalities in Purkinje neurons.
Fig. 5.
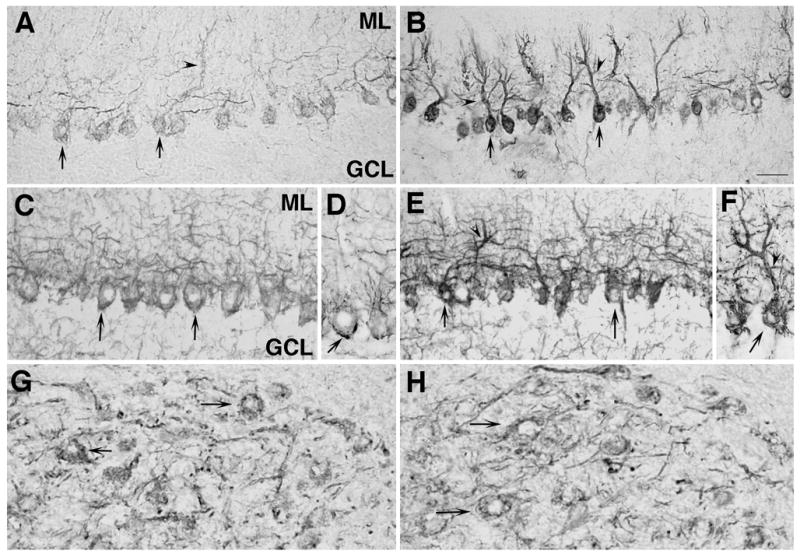
Alterations in the levels of total and non-phosphorylated neurofilament H immunoreactivity in Purkinje neurons of the PMCA2-null mouse. A) Non-phosphorylated NFH is low in somata of Purkinje cells (arrows) and in the molecular layer (ML) containing Purkinje dendrites in wild type mice (arrowheads). B) A dramatic increase in non-phosphorylated NFH immunostaining is observed both in cell bodies (arrows) and apical dendrites (arrowheads) of Purkinje neurons in the cerebellum of PMCA2-null mice. Similarly, total NFH is lower in Purkinje neurons of the wild type mouse (C and D, arrows show cells in two different regions) and is increased in somata (E and F, arrows) and dendrites (arrowheads) of the PMCA2-null mouse. The increase in NFH immunoreactivity occurred throughout the cerebellum. Non-phosphorylated NFH staining is not altered in other brain regions including the substantia nigra of the wild type (G) or knockout (H) mouse. Arrows point at some cells immunopositive for non-phosphorylated NFH. GCL: granule cell layer. n=4, Bar represents 150 μm for A and B, 100 μm for C, E, G and H and 60 μm for D and F.
PMCA2 coimmunoprecipitates with Homer 3, IP3R1 and mGluR1
Homer 3, IP3R1 and PMCA2 are highly enriched in dendrites and spines of the cerebellum (Stauffer et al., 1997; Satoh et al., 1990; Xiao et al., 1998). Previous investigations indicated that mGluR1-Homer-IP3R form a complex (Xiao et al., 1998; Tu et al., 1998) and that these proteins are co-localized in the same dendritic compartment of Purkinje neurons (Sandona et al., 2003). Co-localization of PMCA with IP3R1 and Homer 1b/c has also been shown (Sandona et al., 2003).
To determine whether PMCA2 is physically linked to IP3R1 and Homer 3, we performed coimmunoprecipitation studies by use of wild type cerebellar homogenates and an antibody against Homer 3. Subsequently, we probed the Western blots with antibodies against PMCA2, IP3R1 and mGluR1. We detected bands at the appropriate molecular weights. Thus, PMCA2 coimmunoprecipitates with Homer 3, IP3R1 and mGluR1, indicating a physical interaction between all four proteins (Fig. 6). We further corroborated these findings, by performing reciprocal studies, in which we immunoprecipitaed cerebellar extracts with an antibody against PMCA2 and probed the Western blots with antibodies against mGluR1, IP3R1 and Homer 3 (Fig. 6). As before, we detected bands at the expected molecular weights. These findings suggest that PMCA2 is a component of the mGluR1-Homer 3-IP3R1 signaling complex in the cerebellum.
Fig. 6.
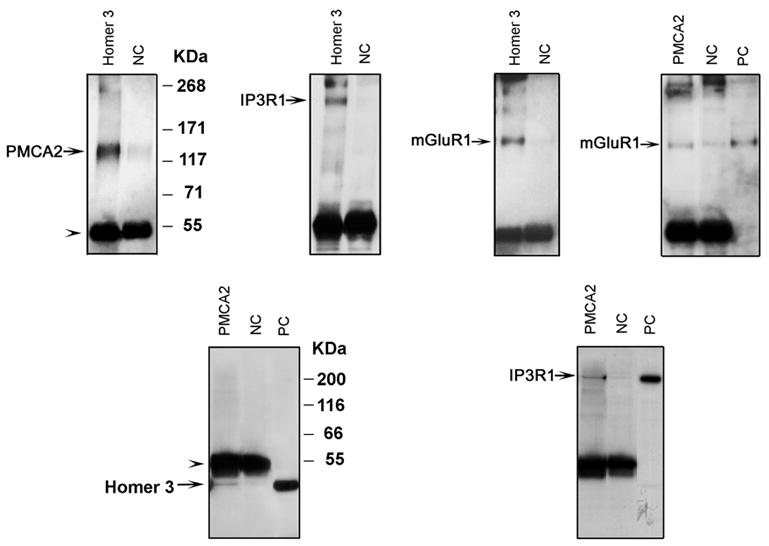
Coimmunoprecipitation of PMCA2 with mGluR1, Homer 3 and IP3R1 from mouse cerebellum. The upper panel shows pictures of 3–8% Tris-acetate gels and the lower panel are pictures of 10% Bis-tris gels, which were used to separate lower molecular weight proteins such as Homers from the ~60 kDa (arrowheads) rabbit IgG heavy chain, described previously (Xiao et al., 1998). The cerebellar extracts were immunoprecipitated either with an anti-Homer 3 or anti-PMCA2 antibody. The antibody used for immunoprecipitation is indicated above each lane. The blots were probed with antibodies against PMCA2 (~129 kDa), IP3R1, Homer 3 or mGluR1, which detected a band at the expected molecular weight (arrows). NG: is negative control, normal rabbit serum or an unrelated polyclonal antibody as indicated in Methods. PC: is positive control, cerebellum extracts.
DISCUSSION
The present study demonstrates specific molecular alterations in Purkinje neurons of the PMCA2-null mouse. The decrease in mGluR1 and IP3R1 in Purkinje somata and dendrites of the PMCA2-null mouse may be particularly important as both receptors play essential roles in cerebellar function including motor coordination, a form of synaptic plasticity called long term depression (LTD), associative learning and synapse elimination during development (Linden et al., 1991; Shigemoto et al., 1994; Ichise et al., 2000; Kishimoto et al., 2002). In fact, motor dyscoordination is observed in mGluR1-deficient as well as IP3R1 mutant mice (Aiba et al., 1994; Ogura et al., 2001). Both LTD and associative learning are impaired in the mGluR1 knockout mouse whereas synaptic plasticity is dependent on IP3-mediated release of Ca2+ in dendritic spines (Inoue et al., 1998; Miyata et al., 2000). Importantly, our studies indicate that PMCA2, which we previously found to be co-localized with mGluR1 in the molecular layer (Kurnellas et al., 2006), may be a constituent of the mGluR1 signaling complex. This is indicated by the immunoprecipitation studies showing association of PMCA2 with mGluR1, Homer 3 and IP3R1. In addition to changes in mGluR1 signaling pathway, we observed increases in total and especially non-phospohorylated NFH immunoreactivity in Purkinje somata and dendrites, which could reflect cytoskeletal anomalies. The combination of the different molecular changes together with more subtle alterations in dendritic morphology (Kozel et al., 1998) could perturb Purkinje cell function in the PMCA2-null mouse.
Lack of PMCA2 might affect Purkinje neurons in various ways. PMCA2 protein is highly concentrated in dendritic spines where the precise control of calcium balance, critical for the proper postsynaptic signaling of Purkinje neurons, occurs (Stauffer et al., 1997). PMCAs have been implicated not only in the maintenance of basal calcium levels but also in the regulation of local Ca2+ signaling in cells (Penniston and Enyedi, 1998; Garcia and Strehler, 1999). Therefore, deficiency in PMCA2 could lead to deregulation of Purkinje cell signaling due to local calcium dyshomeostasis in dendritic spines. However, the studies of Fierro et al. (1998) indicated that the contribution of PMCAs to calcium clearance in Purkinje neurons is modest as compared to that of the Na+/Ca2+ exchanger and SERCA pumps. These investigators reported that at low intracellular calcium concentrations, the contribution of all PMCAs to calcium clearance in Purkinje cells is 21% whereas at higher calcium levels, it is only 6%. Therefore, in addition to calcium dyshomeostasis, aberrance in additional mechanisms could disturb cerebellar function in the PMA2-null mouse. For example, the decrease in the levels of mGluR1 and its downstream effectors might reduce the effectiveness of mGluR1 signaling in Purkinje neuron-parallel fiber synapses. The 40% decline in the expression of mGluR1 alone may not be enough to cause cerebellar deficits in the PMCA2-null mouse. In fact, previous investigations have shown that mGluR1 heterozygous mice do not exhibit motor deficits or differences in synaptic responses as compared to the wild type controls (Conquet et al., 1994). Moreover, Ichise et al. (2000) showed that introduction of mGluR1 to Purkinje neurons of the mGluR1 knockout mouse can restore normal behavior and function even when the expression of the metabotropic receptor is much lower as compared to the wild type. However, in the case of the PMCA2-null mice, the decreases are not confined only to mGluR1 but are also detected in effectors downstream to this receptor. Consequently, the cumulative effect of reductions in the levels of mGluR1, IP3R1 and additional elements in the signaling complex such as Homer proteins, might weaken Purkinje cell signaling leading to cerebellar dysfunction.
The decrease in mGluR1, IP3R1 and Homer 3 levels could be due in part to downregulation of protein expression and at some extent, the result of dendritic loss. Our immunocytochemical studies using the anti-IP3R1 and anti-Homer 3 antibodies clearly show a weaker staining intensity both in Purkinje somata and the molecular layer. Moreover, strong and dense MAP2 immunoreactivity in cerebellar sections obtained from the same wild type and knockout mice indicates that the reduction in IP3R1 and Homer 3 staining intensity cannot be solely explained by extensive dendritic loss, supporting further the notion of a drop in protein expression. However, Kozel et al. (1998) reported a 17% decrease in the thickness of the molecular layer, which was attributed to reduced dendritic arborization. Our studies do not rule out the possibility that some of the changes observed are due, in part, to more subtle alterations in dendritic morphology such as diminished arborization and spines. It is worth noting that a decrease in the number of the parallel fibers, granule cell axons projecting on Purkinje cells, could also account for the reduction in the thickness of the molecular layer, as the density of granule neurons is diminished in the cerebellum of the PMCA2-null mouse (Kozel et al., 1998). This could alter the number of synaptic contacts between parallel fibers and Purkinje neuron dendritic spines, as observed in another mouse, the Wriggle Mouse Sagami, which carries a spontaneous mutation in the PMCA2 gene (Inoue et al., 1993). Thus, reductions in synaptic contacts between parallel fibers and Purkinje dendrites, where mGluR1 activation occurs, could affect the expression and function of the receptor. We found that the distribution of IP3R1 and Homer 3 immunoreactivity in folial crowns of the PMCA2-null mouse appears different than wild type controls. This might either be due to developmental modifications in dendrites, as seen in the Wriggle Mouse Sagami, or relocalization of these proteins along the dendritic shaft.
The results indicating association of PMCA2 with mGluR1 and its downstream effectors are in agreement with other studies showing formation of clusters between PMCA and Homer 1b/c or IP3R1 in the postsynaptic densities of Purkinje cell dendritic spines and shafts (Sandona et al., 2003) and co-localization of mGluR1 with PMCA2 in the molecular layer (Kurnellas et al., 2006). PMCA can couple with Homers via its PDZ (95 kDa postsynaptic protein/discs-large/ZO-1)-binding domain (Sandona et al., 2003; Sgambato-Faure et al., 2006), which is a protein-protein interaction motif. Homer 1b/c and Homer 3 contain sequences suggestive of PDZ domains although other studies indicate stronger homology to N-terminal EVH1 (Enabled/Vasp homology) domains (Satoh et al., 1990; Xiao et al., 1998; Kato et al., 1998). PDZ or EVH1 allow the binding of ligands such as group 1 metabotropic receptors and IP3Rs (Xiao et al., 1998; Brakeman et al., 1997; Tu et al., 1998; Kato et al., 1998). A C-terminal coiled-coil domain enables the formation of homo- and heteromultimeric complexes. Although Homers have only one ligand binding site, multimerization can link proteins such as mGluR1 or PMCA2 which are localized to the plasma membrane and IP3Rs found on the endoplasmic reticulum.
Our studies showing a reduction in IP3R1 and Homer 3 immunoreactivity primarily in clusters of Purkinje neurons along the cerebellar fissures of the PMCA2−/− mice indicate that lack of PMCA2 affects different Purkinje subpopulations in a distinct manner. This is not surprising because a vast number of studies demonstrated that Purkinje cells are not homogenous throughout the cerebellum. This is indicated by the expression patterns of many genes, which show regional heterogeneity (Hawkes and Leclerc, 1989; Hawkes and Turner, 1994; Sotelo and Wassef, 1991; Dusart et al., 1994; Oberdick et al., 1998), the alterations in the expression patterns of some proteins in pathological conditions including injury (Dusart et al., 1994), the pattern of Purkinje cell degeneration in mouse models of Niemann-Pick type C disease (Sarna et al, 2003) and the disparity in the rate of Purkinje neuron loss in the anterior versus posterior cerebellum of the mutant leaner mouse (Herrup and Wilczynski, 1982). The pattern of differential IP3R1 and Homer 3 staining in the PMCA2-null mouse cerebellum neither follow the classical parasagittal stripe and patch patterns reported previously nor correlate with the distribution of PMCA2 staining in the cerebellum of the wild type mouse which appears to be mostly uniform in various regions. Nevertheless, the present findings suggest that distinct Purkinje subpopulations are affected differently by the lack of PMCA2. It remains to be determined whether these selective changes in IP3R1 and Homer 3 immunoreactivities are the consequence of altered connectivity in the cerebellum of the PMCA2-null mouse.
In summary, the studies described here suggest that PMCA2 is a component of the mGluR1-Homer 3-IP3R1 signaling complex. Our findings raise the possibility that perturbations in the expression of mGluR1 and its downstream effectors as well as abnormal increases in NFH in Purkinje neurons might be a cause of cerebellar dysfunction in the PMCA2-null mouse. It is worth noting that cerebellar pathology occurs in several neurological diseases including ataxia and multiple sclerosis (Black et al., 2000; Muller et al., 2000; Hickman et al., 2001; Downey et al., 2002; Saab et al., 2004; Li et al., 2004; Pantano et al., 2005). Therefore, our findings might have relevance to these conditions, a possibility that requires further investigations.
Experimental Methods
Animals
PMCA2+/+ and PMCA2−/− mice have been previously described (Kozel et al., 1998; Kurnellas et al., 2005). PMCA2−/− mice have a normal life span similar to wild type littermates but are smaller in size. All animal protocols were performed according to institutional guidelines.
Immunocytochemistry
Mice were anesthetized by injection of Ketamine/Xylazine and perfused with saline followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were dissected out, postfixed, cryoprotected in 10 and 20% sucrose and frozen on dry ice. Ten micron sections obtained from brains of PMCA−/− and PMCA2+/+ littermates were mounted side by side on the same slide. The sections were blocked with 0.25% Triton, 1.5% or 30% normal horse serum in PBS for one hour and incubated with an antibody against non-phosphorylated NFH (SMI-32 ; 1:6000, Sternberger Monoclonal, Berkeley, CA) or total NFH (1:800, Sigma, St. Louis, MO) for 24 hours at 4°C or with an antibody against Homer 3 (1:3000, a generous gift of Dr. P. F. Worley; Johns Hopkins University, Baltimore, MD), IP3R1 (1:500, Calbiochem, San Diego, CA) or MAP2 (1:1000, Chemicon, Temecula, CA) for 48 hours at 4°C. The sections were then washed with PBS and incubated with the appropriate secondary antibody (1:100). Signal was visualized by use of avidin-biotin complex (Vector, Burlingame, CA) and 3,3’-diaminobenzidine (DAB, Sigma). The sections were then dehydrated in alcohol and coverslipped.
Western blot analysis
PMCA2+/+ and PMCA2−/− mice were sacrificed by exposure to CO2. Cerebella were dissected out and homogenized on ice in eppendorf tubes with a motorized pestle in 200 μl lysis buffer containing 100 mM HEPES (pH 8.0), 150 mM sodium chloride, 0.02% sodium azide, 0.1% sodium dodecylsulfate, 1% IgepalCA 630, 0.5% deoxycholic acid, 0.2 mM phenylmethylsulfonyl fluoride, 2 μg/ml aprotinin, 2 μg/ml leupeptin, 2 μg/ml pepstatin, and 50mM sodium fluoride. The protease inhibitors were purchased from Sigma. The homogenate was mixed by vortexing for 15–30 sec and kept on ice for 15 sec. This procedure was repeated 4 times after which the homogenate was sonicated in an ultrasonic bath for 15 sec, at lowest frequency, placed on ice for 30 min and centrifuged at 14,000 X g for 20 min at 4°C. The supernatant was collected and aliquots were stored at −80°C until use. Protein samples for the assessment of mGluR1 were prepared as above, except homogenates were frozen and thawed at 37°C three times and centrifuged at 6,000 X g for 1 min at 4°C (Zu et al., 2004). Total protein concentrations were determined utilizing the BCA protein quantification kit (Pierce, Rockford, IL) according to the manufacturer’s instructions. Ten μg of total protein obtained from individual cerebella was loaded on gels. Depending on the molecular weight of the protein to be analyzed, 4–20% and 6% Novex Tris-glycine or 3–8% NuPAGE Novex Tris-acetate gels (Invitrogen, Carlsbad, CA) were used. Denaturing electrophoresis was performed for 90 min at 125 V with Tris-glycine gels and 60 min at 150 V with Tris-acetate gels. The protein was then transferred onto a polyvinylidene difluoride (PVDF) membrane for 90 min at 25 V for Tris-glycine gels and 60 min at 30 V for Tris-acetate gels. Adequate transfer of proteins onto PVDF membranes was ascertained by staining with BLOT-FastStain (Chemicon), according to manufacturer’s instructions. Chemiluminescent immunodetection was employed using a Western Breeze kit (Invitrogen), according to manufacturer’s instructions. The dilutions of the primary antibodies were as follows: anti-Homer 3 (1:20,000), anti-Homer 1b/c (1:10,000, Dr. P. F. Worley), anti-mGluR1 (1:2500, BD Biosciences, San Diego, CA), anti-IP3R1 (1:2000), anti-α-tubulin (1:20,000, Sigma) and anti-GAPHD (1:10000, Calbiochem). Signal was visualized by exposure of membranes to Hyperfilm ECL (GE Healthcare, Piscataway, NJ) or by use of EpiChemi3 Darkroom Imaging System (UVP Bioimaging Systems, Upland, CA). Between each immunoblot, membranes were stripped with Re-Blot recycling kit (Chemicon), according to manufacturer’s instructions. Bands were quantified using the Un-Scan-It software (Silk Scientific, Orem, UT). Values were normalized to housekeeping proteins α-tubulin or GAPDH to correct for experimental variations.
Immunoprecipitation
Wild type mouse cerebellum (~50 mg) was homogenized in 300 or 1000 μl phosphate buffer saline (pH 7.4), 1% Triton X-100 and protease inhibitor cocktail (T-PBS). The homogenate was sonicated 3 times for 10 sec and then centrifuged at 16,000 X g for 20 min at 4°C. One hundred microliters of cerebellar extract was incubated with 3 μl of polyclonal antibody against Homer 3 or PMCA2 (Abcam, Cambridge, MA) for 4 hours or overnight at 4°C. Normal rabbit serum or an unrelated antibody (Calbindin D-28K ; Chemicon) served as negative control under the same experimental conditions. Twenty-five μl of Protein A Sepharose beads (GE Healthcare) was then added. The mixture was incubated for 2 hours at 4°C and centrifuged at 380 X g for 2 min at 4°C. The pellet was washed 3 times with T-PBS. Protein was eluted with 15 μl of SDS loading buffer. Western blots were performed on either 3–8% Tris-acetate or 10% Bis-tris gels (Invitrogen) as described above and probed with anti-PMCA2 (1:500, BD Biosciences), anti-Homer 3 (1:20000), anti-IP3R1 (1:2000) and anti-mGluR1 (1:2500) antibodies.
Acknowledgments
We are grateful to Dr. Paul F. Worley for providing the antibodies against Homer 1b/c and Homer 3. We thank Dr. Jian Cheng Tu and Dr. Tao Zu for advice with some of the techniques. This research was supported by NIH grants NS046363 to SE and NS046593 to HL.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aiba A, Kano M, Chen C, Stanton ME, Fox GD, Herrup K, Zwingman TA, Tonegawa S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–88. [PubMed] [Google Scholar]
- Black JA, Dib-Hajj S, Baker D, Newcombe J, Cuzner ML, Waxman SG. Sensory neuron- specific sodium channel SNS is abnormally expressed in the brains of mice with experimental allergic encephalomyelitis and humans with multiple sclerosis. Proc Natl Acad Sci U S A. 2000;97:11598–602. doi: 10.1073/pnas.97.21.11598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakeman PR, Lanahan AA, O'Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–8. doi: 10.1038/386284a0. [DOI] [PubMed] [Google Scholar]
- Burette A, Rockwood JM, Strehler EE, Weinberg RJ. Isoform-specific distribution of the plasma membrane Ca2+ ATPase in the rat brain. J Comp Neurol. 2003;467:464–76. doi: 10.1002/cne.10933. [DOI] [PubMed] [Google Scholar]
- Conquet F, Bashir ZI, Davies CH, Daniel H, Ferraguti F, Bordi F, Franz-Bacon K, Reggiani A, Materese V, Conde F, Collingridge GL, Crepel F. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature. 1994;372:237–43. doi: 10.1038/372237a0. [DOI] [PubMed] [Google Scholar]
- Downey DL, Stahl JS, Bhidayasiri R, Derwenskus J, Adams NL, Ruff RL, Leigh RJ. Saccadic and vestibular abnormalities in multiple sclerosis: sensitive clinical signs of brainstem and cerebellar involvement. Ann N Y Acad Sci. 2002;956:438–40. doi: 10.1111/j.1749-6632.2002.tb02849.x. [DOI] [PubMed] [Google Scholar]
- Dusart I, Morel MP, Sotelo C. Parasagittal compartmentation of adult rat Purkinje cells expressing the low-affinity nerve growth factor receptor: changes of pattern expression after a traumatic lesion. Neurosci. 1994;63:351–6. doi: 10.1016/0306-4522(94)90534-7. [DOI] [PubMed] [Google Scholar]
- Fierro L, DiPolo R, Llano I. Intracellular calcium clearance in Purkinje cell somata from rat cerebellar slices. J Physiol. 1998;510:499–512. doi: 10.1111/j.1469-7793.1998.499bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filoteo AG, Elwess NL, Enyedi A, Caride A, Aung HH, Penniston JT. Plasma membrane Ca2+ pump in rat brain. Patterns of alternative splices seen by isoform-specific antibodies. J Biol Chem. 1997;272:23741–7. doi: 10.1074/jbc.272.38.23741. [DOI] [PubMed] [Google Scholar]
- Garcia ML, Strehler EE. Plasma membrane calcium ATPases as critical regulators of calcium homeostasis during neuronal cell function. [Review] Front Biosci. 1999;4:D869–82. doi: 10.2741/garcia. [DOI] [PubMed] [Google Scholar]
- Hawkes R, Leclerc N. Purkinje cell axon collateral distributions reflect the chemical compartmentation of the rat cerebellar cortex. Brain Res. 1989;476:279–90. doi: 10.1016/0006-8993(89)91248-1. [DOI] [PubMed] [Google Scholar]
- Hawkes R, Turner RW. Compartmentation of NADPH-diaphorase activity in the mouse cerebellar cortex. J Comp Neurol. 1994;346:499–516. doi: 10.1002/cne.903460404. [DOI] [PubMed] [Google Scholar]
- Herrup K, Wilczynski SL. Cerebellar cell degeneration in the leaner mutant mouse. Neurosci. 1982;7:2185–96. doi: 10.1016/0306-4522(82)90129-4. [DOI] [PubMed] [Google Scholar]
- Hickman SJ, Brierley CM, Silver NC, Moseley IF, Scolding NJ, Compston DA, Miller DH. Infratentorial hypointense lesion volume on T1-weighted magnetic resonance imaging correlates with disability in patients with chronic cerebellar ataxia due to multiple sclerosis. J Neurol Sci. 2001;187:35–9. doi: 10.1016/s0022-510x(01)00519-6. [DOI] [PubMed] [Google Scholar]
- Hu J, Qian J, Borisov O, Pan S, Li Y, Liu T, Deng L, Wannemacher K, Kurnellas M, Patterson C, Elkabes S, Li H. Optimized Proteomic Analysis of a Mouse Model of Cerebellar Dysfunction Using Amine-Specific Isobaric Tags. Proteomics. 2006 Jun 26; doi: 10.1002/pmic.200600026. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichise T, Kano M, Hashimoto K, Yanagihara D, Nakao K, Shigemoto R, Katsuki M, Aiba A. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288:1832–5. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- Inoue T, Kato K, Kohda K, Mikoshiba K. Type 1 inositol 1,4,5-trisphosphate receptor is required for induction of long-term depression in cerebellar Purkinje neurons. J Neurosci. 1998;18:5366–73. doi: 10.1523/JNEUROSCI.18-14-05366.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue Y, Matsumura Y, Inoue K, Ichikawa R, Takayama C. Abnormal synaptic architecture in the cerebellar cortex of a new dystonic mutant mouse, Wriggle Mouse Sagami. Neurosci Res. 1993;16:39–48. doi: 10.1016/0168-0102(93)90007-d. [DOI] [PubMed] [Google Scholar]
- Kano M, Hashimoto K, Kurihara H, Watanabe M, Inoue Y, Aiba A, Tonegawa S. Persistent multiple climbing fiber innervation of cerebellar Purkinje cells in mice lacking mGlur1. Neuron. 1997;18:71–9. doi: 10.1016/s0896-6273(01)80047-7. [DOI] [PubMed] [Google Scholar]
- Kato A, Ozawa F, Saitoh Y, Fukazawa Y, Sugiyama H, Inokuchi K. Novel members of the Vesl/Homer family of PDZ proteins that bind metabotropic glutamate receptors. J Biol Chem. 1998;273:23969–75. doi: 10.1074/jbc.273.37.23969. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Fujimichi R, Araishi K, Kawahara S, Kano M, Aiba A, Kirino Y. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. Eur J Neurosci. 2002;16:2416–24. doi: 10.1046/j.1460-9568.2002.02407.x. [DOI] [PubMed] [Google Scholar]
- Knöpfel T, Grandes P. Metabotropic glutamate receptors in the cerebellum with a focus on their function in Purkinje cells. [Review] Cerebellum. 2002;1:19–26. doi: 10.1007/BF02941886. [DOI] [PubMed] [Google Scholar]
- Kozel PJ, Friedman RA, Erway LC, Yamoah EN, Liu LH, Riddle T, Duffy JJ, Doetschman T, Miller ML, Cardell EL, Shull GE. Balance and hearing deficits in mice with a null mutation in the gene encoding plasma membrane Ca2+-ATPase isoform 2. J Biol Chem. 1998;273:18693–6. doi: 10.1074/jbc.273.30.18693. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Lee AK, Szczepanowski K, Elkabes S. Role of plasma membrane calcium ATPase isoform 2 in neuronal function in the cerebellum and spinal cord. Ann NY Acad Sci. 2006 doi: 10.1196/annals.1387.025. In Press. [DOI] [PubMed] [Google Scholar]
- Kurnellas MP, Nicot A, Shull GE, Elkabes S. Plasma membrane calcium ATPase deficiency causes neuronal pathology in the spinal cord: a potential mechanism for neurodegeneration in multiple sclerosis and spinal cord injury. FASEB J. 2005;19:298–300. doi: 10.1096/fj.04-2549fje. Epub 2004 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehotsky J, Kaplan P, Racay P, Mezesova V, Raeymaekers L. Distribution of plasma membrane Ca2+ pump (PMCA) isoforms in the gerbil brain: effect of ischemia-reperfusion injury. Neurochem Int. 1999;35:221–7. doi: 10.1016/s0197-0186(99)00062-5. [DOI] [PubMed] [Google Scholar]
- Lehotsky J, Kaplan P, Murin R, Raeymaekers L. The role of plasma membrane Ca2+ pumps (PMCAs) in pathologies of mammalian cells. [Review] Front Biosci. 2002;7:D53–84. doi: 10.2741/A769. [DOI] [PubMed] [Google Scholar]
- Li Y, Chiaravalloti ND, Hillary FG, Deluca J, Liu WC, Kalnin AJ, Ricker JH. Differential cerebellar activation on functional magnetic resonance imaging during working memory performance in persons with multiple sclerosis. Arch Phys Med Rehabil. 2004;85:635–9. doi: 10.1016/j.apmr.2003.07.016. [DOI] [PubMed] [Google Scholar]
- Linden DJ, Dickinson MH, Smeyne M, Connor JA. A long-term depression of AMPA currents in cultured cerebellar Purkinje neurons. Neuron. 1991;7:81–9. doi: 10.1016/0896-6273(91)90076-c. [DOI] [PubMed] [Google Scholar]
- Miyata M, Finch EA, Khiroug L, Hashimoto K, Hayasaka S, Oda SI, Inouye M, Takagishi Y, Augustine GJ, Kano M. Local calcium release in dendritic spines required for long-term synaptic depression. Neuron. 2000;28:233–44. doi: 10.1016/s0896-6273(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Muller DM, Pender MP, Greer JM. A neuropathological analysis of experimental autoimmune encephalomyelitis with predominant brain stem and cerebellar involvement and differences between active and passive induction. Acta Neuropathol (Berl) 2000;100:174–82. doi: 10.1007/s004019900163. [DOI] [PubMed] [Google Scholar]
- Nicot A, Kurnellas M, Elkabes S. Temporal pattern of plasma membrane calcium ATPase 2 expression in the spinal cord correlates with the course of clinical symptoms in two rodent models of autoimmune encephalomyelitis. Eur J Neurosci. 2005;21:2660–70. doi: 10.1111/j.1460-9568.2005.04086.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicot A, Ratnakar PV, Ron Y, Chen CC, Elkabes S. Regulation of gene expression in experimental autoimmune encephalomyelitis indicates early neuronal dysfunction. Brain. 2003;126:398–412. doi: 10.1093/brain/awg041. [DOI] [PubMed] [Google Scholar]
- Oberdick J, Baader SL, Schilling K. From zebra stripes to postal zones: deciphering patterns of gene expression in the cerebellum. Trends Neurosci. 1998;21:383–90. doi: 10.1016/s0166-2236(98)01325-3. [DOI] [PubMed] [Google Scholar]
- Ogura H, Matsumoto M, Mikoshiba K. Motor discoordination in mutant mice heterozygous for the type 1 inositol 1,4,5-trisphosphate receptor. Behav Brain Res. 2001;122:215–9. doi: 10.1016/s0166-4328(01)00187-5. [DOI] [PubMed] [Google Scholar]
- Pantano P, Mainero C, Lenzi D, Caramia F, Iannetti GD, Piattella MC, Pestalozza I, Di Legge S, Bozzao L, Pozzilli C. A longitudinal fMRI study on motor activity in patients with multiple sclerosis. Brain. 2005;128:2146–53. doi: 10.1093/brain/awh549. [DOI] [PubMed] [Google Scholar]
- Penheiter AR, Filoteo AG, Croy CL, Penniston JT. Characterization of the deafwaddler mutant of the rat plasma membrane calcium-ATPase 2. Hear Res. 2001;162:19–28. doi: 10.1016/s0378-5955(01)00356-2. [DOI] [PubMed] [Google Scholar]
- Penniston JT, Enyedi A. Modulation of the plasma membrane Ca2+ pump. [Review] J Membr Biol. 1998;165:101–9. doi: 10.1007/s002329900424. [DOI] [PubMed] [Google Scholar]
- Saab CY, Craner MJ, Kataoka Y, Waxman SG. Abnormal Purkinje cell activity in vivo in experimental allergic encephalomyelitis. Exp Brain Res. 2004;158:1–8. doi: 10.1007/s00221-004-1867-4. [DOI] [PubMed] [Google Scholar]
- Sandona D, Scolari A, Mikoshiba K, Volpe P. Subcellular distribution of Homer 1b/c in relation to endoplasmic reticulum and plasma membrane proteins in Purkinje neurons. Neurochem Res. 2003;28:1151–8. doi: 10.1023/a:1024264025401. [DOI] [PubMed] [Google Scholar]
- Sarna JR, Larouche M, Marzban H, Sillitoe RV, Rancourt DE, Hawkes R. Patterned Purkinje cell degeneration in mouse models of Niemann-Pick type C disease. J Comp Neurol. 2003;456:279–91. doi: 10.1002/cne.10522. [DOI] [PubMed] [Google Scholar]
- Satoh T, Ross CA, Villa A, Supattapone S, Pozzan T, Snyder SH, Meldolesi J. The inositol 1,4,5,-trisphosphate receptor in cerebellar Purkinje cells: quantitative immunogold labeling reveals concentration in an ER subcompartment. J Cell Biol. 1990;111:615–24. doi: 10.1083/jcb.111.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgambato- Faure V, Xiong Y, Berke JD, Hyman SE, Strehler EE. The Homer-1 protein Ania-3 interacts with the plasma membrane calcium pump. Biochem Biophys Res Commun. 2006;343:630–7. doi: 10.1016/j.bbrc.2006.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M. Excitatory synapses. Glutamate receptors put in their place. Nature. 1997;386:221–223. doi: 10.1038/386221a0. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Abe T, Nomura S, Nakanishi S, Hirano T. Antibodies inactivating mGluR1 metabotropic glutamate receptor block long-term depression in cultured Purkinje cells. Neuron. 1994;12:1245–55. doi: 10.1016/0896-6273(94)90441-3. [DOI] [PubMed] [Google Scholar]
- Stahl WL, Eakin TJ, Owens JW, Jr, Breininger JF, Filuk PE, Anderson WR. Plasma membrane Ca(2+)-ATPase isoforms: distribution of mRNAs in rat brain by in situ hybridization. Brain Res Mol Brain Res. 1992;16:223–31. doi: 10.1016/0169-328x(92)90229-5. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Guerini D, Carafoli E. Tissue distribution of the four gene products of the plasma membrane Ca2+ pump. A study using specific antibodies. J Biol Chem. 1995;270:12184–90. doi: 10.1074/jbc.270.20.12184. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Guerini D, Celio MR, Carafoli E. Immunolocalization of the plasma membrane Ca2+ pump isoforms in the rat brain. Brain Res. 1997;748:21–9. doi: 10.1016/s0006-8993(96)01282-6. [DOI] [PubMed] [Google Scholar]
- Strehler EE, Zacharias DA. Role of alternative splicing in generating isoform diversity among plasma membrane calcium pumps. [Review] Physiol Rev. 2001;81:21–50. doi: 10.1152/physrev.2001.81.1.21. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Wassef M. Cerebellar development: afferent organization and Purkinje cell heterogeneity. Philos Trans R Soc Lond B Biol Sci. 1991;331:307–13. doi: 10.1098/rstb.1991.0022. [DOI] [PubMed] [Google Scholar]
- Street VA, McKee-Johnson JW, Fonseca RC, Tempel BL, Noben-Trauth K. Mutations in a plasma membrane Ca2+-ATPase gene cause deafness in deafwaddler mice. Nat Genet. 1998;19:390–4. doi: 10.1038/1284. [DOI] [PubMed] [Google Scholar]
- Thomas U. Modulation of synaptic signalling complexes by Homer proteins. [Review] J Neurochem. 2002;81:407–13. doi: 10.1046/j.1471-4159.2002.00869.x. [DOI] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF. Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors. Neuron. 1998;21:717–26. doi: 10.1016/s0896-6273(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Petralia RS, Yuan JP, Doan A, Breder CD, Ruggiero A, Lanahan AA, Wenthold RJ, Worley PF. Homer regulates the association of Group 1 metabotropic glutamate receptors with multivalent complexes of Homer-related synaptic proteins. Neuron. 1998;21:707–16. doi: 10.1016/s0896-6273(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Zu T, Duvick LA, Kaytor MD, Berlinger MS, Zoghbi HY, Clark HB, Orr HT. Recovery from Polyglutamine-Induced Neurodegeneration in Conditional SCA1 Transgenic Mice. J Neurosci. 2004;24:8853–61. doi: 10.1523/JNEUROSCI.2978-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


