Abstract
Until the 1990’s the 1,1,1-trichloro-bis-2,2′-(4chlorophenyl) ethane (DDT) was sprayed in the walls of the house in the along the Madeira River basin, Brazilian Amazon, a region well known by its large number of malaria cases. In the 1910, the relate of Oswaldo Cruz about health conditions in Madeira River region describes the presence of malaria in rates ranging until 100% of infected people in some localities. Data available in the literature points to the DDT contamination in fishes captured in Madeira River region. Fish is the major source of dietary protein to this people. DDT tends to accumulate in lipid rich tissues being eliminated by different events, including lactation. Considering the importance of the breast milk to the children feeding, the associated risks of DDT exposure via breast milk intake to children must be assessed. This is the main objective of this work: to analyse the presence of the p,p′-DDT and its metabolites p,p′-DDE and p,p′-DDD in 69 human milk samples and to estimate the intake of DDT and its metabolite in terms of total DDT (total DDT = p,p′-DDE+ p,p′-DDD+ p,p′-DDT). All sample showed contamination with DDT and its metabolites ranging from 25.4 to 9361.9 ng of total DDT / g of lipid (median=369.6 ng of total DDT / g of lipid) and 8.7 % of the Estimated Daily Intake (EDI), in terms of total DDT, was higher than the Acceptable Daily Intake proposed by the WHO. Key words: DDT, breast milk, children, organochlorine pesticide, fish.
1. Introduction
Breast milk is the most complete source of nutrients (proteins, carbohydrates, fat and vitamins), immune factors, and other important constituents linked to immune responses, including in the protection against infectious dieseases (Institute of Medicine, 1991; Oddy, 2001). Unfortunately, breast milk is not free of contaminants and its ingestion represents an important exposure pathway to organochlorine pesticides and other environmental and pharmaceutical chemicals to children (Berlin et al., 2002; Fitzgerald, et al., 2001; Koopman-Essembom et al., 1995; Patandin et al., 1999; Saver et al., 1994).
The presence of DDT and its metabolites is widely studied in many parts of the world in environmental samples, biota and humans. The interest in the DDT levels in many organisms and in humans is given by its adverse effects such as activity in estrogenic receptors (Daston et al., 1993; Gillesby and Sacarewsk, 1998; Guillette et al., 1995; Hoekstra et al., 2001), induction of spontaneous abortion (Hart et al., 1972; Palmer et al., 1972; Johnson et al., 1988; Korrick et al., 2001), and apoptosis in mononuclear cells (Pérez-Maldonado et al., 2004; Teoburi et al., 1998).
Fish consumption is considered an important source of DDT and other organochlorine pesticides to humans, since these compounds are frequently detected in a wide range of fish types from many parts of the word (Harris et al., 2001). Torres et al., (2002) and D’Amato et al., (2004) reported DDT contamination in various fish species from Madeira River. The presence of the DDT in these fishes can be explained by its widespread use in the Brazilian Amazon in the control of vector-borne diseases such as malaria. The Ministry of Health of Brazil reported that it ended the use of DDT in 1992 (Oliveira Filho, 1997), but still permitted its use in the control of leishmaniasis, that is also endemic in the Amazon region.
2. Material and methods
2.1. Samples
Samples of human milk (n=69) were collected between Porto Velho City and the locality of Axinim in two trips to the Madeira River in 2001 and 2002 totalling 20 localities. The sampling area is shown in the Figure 1. The milk samples were colleted in wide mouth glass flasks previously decontaminated with acetone and n-hexane and the milk samples were stored in the freezer until analysis. This study was previously approved in the Committee of Ethics in Research and conformed to meet high standards regarding human samples and other ethic guidelines (process n° 026/02 CEP-NESC/UFRJ).
Figure 1.
Sampling area.
2.2. Extraction method
The extraction procedure used was based in the Prapamontol and Stevenson (2001) method with certain modifications. The milk samples were warmed in a hot bath at 37°C before analysis until complete homogenisation. An aliquot of 1mL was added to 5mL of a solution containing ethyl acetate:acetone:methanol (1:2:2), homogenized by vortex mixing (1 minute), ultrasonic bath (20 minutes), and centrifuged for 15 minutes at 2000 RPM. To the supernatant was added 10mL of ultra pure water and the mix was passed through a C18 Solid Phase Extraction cartridge (C18 SPE) previously conditioned with n-hexane (2 x 1mL), ethyl acetate (2 x 1mL), methanol (2 x 1mL) and ultra pure water. The C18 SPE was washed twice with 1 mL of solution containing 25% of in water and kept in vacuum to dry completely. The DDT and its metabolites were eluted with 1mL of n-hexane, volume that was submitted to the cleanup step using Florisil SPE conditioned with of chlorine methylene (25mL), ethyl acetate (10mL), solution of 15% of petroleum ether in acetone (10ml), and n-hexane (10ml). After elution of the extracts the Florisil SPE was washed with 10mL of n-hexane and 5 mL of a solution containing 15% of petroleum ether in acetone. Both C18 and Florisil SPE were purchased by SUPELCO™ (USA). The combined was added 10μL of the standard dichloronaphtalene (DCN) and gently reduced to 1mL in flow of nitrogen. An aliquot of 3μL of the final extract was injected in a GC-ECD and the quantification was performed by internal standard method using DCN.
2.3. Quality control
Blanks were done in parallel of the samples and did not shown the presence of peaks in their chromatograms. The recovery was performed using spiked samples of breast milk. The used extraction method presented recovery of 88.4%, 102.6%, and 82.2% with coefficient of variation of 3.1%, 2.2%, and 2.7% for p,p′-DDE, p,p′-DDD, and p,p′-DDT respectively. The limit of detection was calculated as three times the standard deviation of the, and the obtained values for p,p′-DDE, p,p′-DDD, and p,p′-DDT were 0.0040ng/mL, 0.0340ng/mL, and 0.0040ng/mL respectively.
2.4. Estimative of the DDT daily intake
The estimative of the DDT daily intake by infant was performed using the calculated concentrations of total DDT in individual samples. The calculation of the Infant Daily Intake (IDI) was done according to Marien and Laflame (1995) that proposed the following equation: IDI = (BMLC x MC x PMF) / BW, where: BMLC = Breast Milk Lipid Concentration; MC = Milk Consumption, PMF = Percent Milk Fat, and BW = Body Weight of the nursing infants. The values used to the terms MC, PMF, and BW were estimated in 1kg/day, 4%, and 5 kg respectively, conform adopted in the work of Marien and Laflame (1995). The estimated values were compared with the proposed limit of 0.020mg of DDT/kg of body weight (WHO, 1984).
3. Results and discussion
3.1. Population data and diet assessment
The diet of the mothers was based in cassava flour, some fruits and fish according to the data of the applied questionnaire. Fishes represented the main source of proteins of the donor’s mothers, being consumed in all meals by 98% of the interviewed. The consumption of milk and meat (bovine, swine or poultry meat) was not declared in the same 98% of the applied questionnaires, a fact considered common for traditional Amazonian population. A additional point of view that should be considered is the absent of pesticide use in the Amazonian agriculture and no one mother had previous contact with DDT for agricultural use, a fact that was also identified in the questionnaires. The breast milk samples collected in the present work were obtained of mothers of communities from Madeira River basin. Some characteristic of the studied mothers are showed in the Table 1.
Table 1.
Age, parity, and number of the studied mothers.
| Range of age | Parity
|
n | |
|---|---|---|---|
| Range | Mean ±SD * | ||
| 15 – 20 | 1 – 3 | 1.9 ± 0.70 | 11 |
| 21 – 25 | 2 – 8 | 4 ± 1.89 | 15 |
| 26 – 30 | 1 – 11 | 5.01 ± 2.67 | 16 |
| 31 – 35 | 1 – 12 | 7.43 ± 3.03 | 14 |
| 36 – 40 | 2 – 12 | 6.40 ± 4.16 | 5 |
– SD: standard deviation
3.2 DDT status
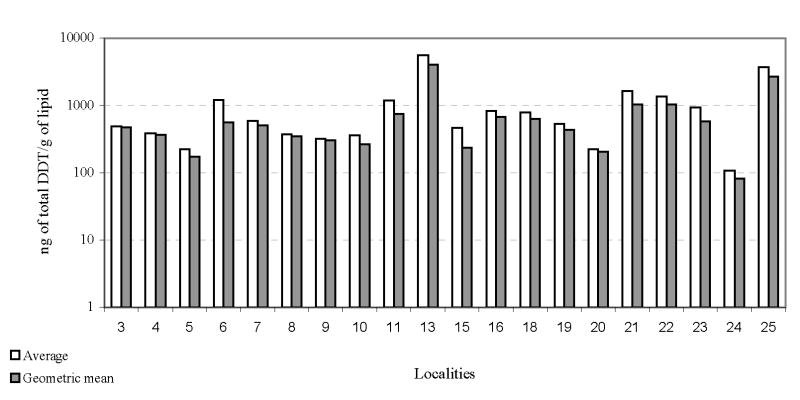
The chromatograms showed residues of DDT and its metabolites in all analysed samples. The concentration ranged from 10.7 a 7271.5ng/g of lipid for p,p′-DDE, from just a value lower than the limit of quantification to 400.7 ng/g of lipid for p,p′-DDD, from 3.0 to 2534.1 for p,p′-DDT, and 25.4 to 9361,9 to total DDT. The Figure 2 shows the distribution of average and geometric mean of the total DDT values in the different studied localities. The obtained geometric mean of the total DDT in the different studied localities ranged from 118.3 in Santa Rosa to 1005 ng of total DDT/g of lipid in Cachoeirinha. Geometric mean of total DDT/g of lipid of the localities showed a good correlation (r = 0.993) with the average values. The geometric mean/average ratio was 0.76.
Figure 2.
Total DDT in different localities of the Madeira River.
The highest value of total DDT (9361.9 ng of total DDT/g of lipid) was obtained of a primipara mother aging 27 years old. Parity is pointed as a factor in the organochlorine amounts in breast milk Harris et al. (2001). Lactation is potentially the most significant activity in the reduction of the stored organochlorine in the human body, once it was observed the decrease of these compounds during its course (Haggyard et al., 1973; Bakken and Siep, 1976; Rogan et al., 1986; Skare and Polder, 1990; Quinsey et al., 1996). Age has been noted as one of the most significant contributors of organochlorine pesticides in breast milk. The increase of the concentrations of DDT in breast milk with age was observed by previous studies (Stacey, et al., 1985; Mussalo-Rauhamaa et al., 1988; Dewailly, et al., 1996).
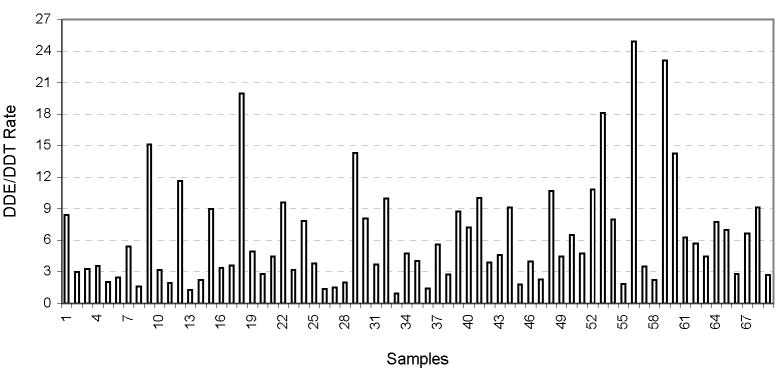
Conform expected the p,p′-DDT, p,p′-DDE, and p,p′-DDD did not occurred independently. The average of p,p′-DDE/p,p′-DDT ratio was 6.3, and the individual values are shown in the Figure 3. It means a non-recent use of the insecticide DDT, conform observed by Gladen et al. (1999) that obtained the DDE/DDT ratio of 7.1.
Figure 3.
p,p′-DDE/p,p′-DDT rates in individual milk samples from Madeira River.
There is sufficient evidence that the presence of the DDT and its metabolites in the analysed milk samples is due to diet rich in fish, once considered the Harris et al. (2001) statement that the consumption of contaminated food represents an important source of organochlorine pesticides to humans. The population living in the Madeira River region is characterised by high consumption of fish meat in their diet. The presence of DDT observed in previous works (Torres et al., 2002) and its high consumption can be the source of DDT in the studied human milk samples.
In 19 of the 20 localities presented total DDT contamination ranging from 118 to 771.4 ng of total DDT/g of lipid. Just one locality (Cachoeirinha) presented geometric mean for total DDT higher than 1000 ng of total DDT/g of lipid. The Table 2 presents the contamination expressed in geometric mean for all sampled localities.
Table 2.
Geometric means of the p,p′ DDT, p,p′ DDE. and p,p′ DDD and total DDT concentrations (values in ng / g of lipid), and number of samples collected in different localities.
| Localities | n | p,p′ DDT | p,p′ DDE | p,p′ DDD | Total DDT |
|---|---|---|---|---|---|
| Auxiliadora | 3 | 456.7 | 52.8 | 108.0 | 643.1 |
| Boa Ventura | 2 | 526.3 | 48.4 | 188.9 | 771.4 |
| Bom Jesus | 3 | 422.2 | 46.1 | 99.8 | 605.2 |
| Cachoeirinha | 6 | 1005.0 | 54.0 | 163.9 | 1005.0 |
| Caiçara | 2 | 421.9 | 37.5 | 110.8 | 615.1 |
| Carapanatuba | 2 | 513.4 | 18.6 | 42.7 | 588.4 |
| Carará | 4 | 266.5 | 37.0 | 43.4 | 376.3 |
| Espírito Santo | 4 | 206.7 | 26.7 | 38.8 | 284.8 |
| Livramento | 2 | 277.9 | 67.1 | 84.8 | 447.9 |
| Moanense | 5 | 216.9 | 26.8 | 34.5 | 433.8 |
| Paquiquí | 3 | 181.2 | 26.9 | 19.5 | 134.4 |
| Porto Velho | 4 | 460.6 | 27.3 | 88.7 | 619.4 |
| Remanso | 2 | 383.1 | 34.1 | 67.7 | 283.6 |
| Rosarina | 2 | 315.1 | 39.7 | 156.4 | 554.6 |
| São Pedro | 3 | 336.5 | 36.1 | 96.5 | 512.7 |
| São Sebastião | 4 | 222.1 | 14.7 | 28.3 | 272.7 |
| Santa Rosa | 2 | 91.1 | 14.5 | 12.4 | 118.3 |
| Santo Antônio do Pau Queimado | 4 | 103.1 | 28.8 | 30.4 | 178.6 |
| Uricurituba | 4 | 132.5 | 16.7 | 27.6 | 183.4 |
| Val Paraíso | 2 | 264.2 | 44.5 | 42.2 | 356.1 |
3.3 Comparison of the DDT contamination with literature data
A large number of studies involving the DDT contamination in breast milk are available in the literature. The observed contamination in breast milk donned for mothers from Guatemala in the 1970’s (Olszyna-Marzys et al., 1973) presents values higher than the observed in the present study. The authors analyzed breast milk samples from La Bomba, Cerro Colorado, and El Rosario. The mean values in these locality were 2150 (ranging from 411 to 11500), 4070 (from 1570 to 12210), and 1840 ng of total DDT / mL (from 342 to 4970ng of total DDT / mL). The values of the individual samples of the Olszyna-Marzys et al (1973) work were higher than the calculated in the present study. The magnitude of the DDT contamination in the Olszyna-Marzys et al (1973) can be explained by its allowed agricultural use in the 1970 decade and the donor mothers were from cotton culture areas, where DDT contamination presented higher values than the obtained in areas without cotton culture.
Lara et al. (1982) studied DDT levels in 25 human milk samples from São Paulo State, Brazil since 1979 to 1981. The observed amounts of DDT ranged from 16 to 2610μg of DDT/l in whole milk basis with average value of 278 μg of DDT/l in this work. Values of 400, 65250, and 7175 ng of total DDT/g of lipid were obtained (to minimum, maximum, and average, respectively) when adjusted to lipid basis assuming 4% of lipid in whole milk. These values were higher than the observed in the present work, but is important to consider that DDT was banned in Brazilian Government in 1985, fact that could explain the amounts found in the occasion of this 1982 work.
Paumgartten et al. (2000) investigated the levels of DDT and its metabolites in mothers living in the urban area of Rio de Janeiro, Brazil. The mean values found were 180 ng of DDT/g of lipid, 1520 ng of DDE/g of lipid, 6 ng of DDD/g of lipid, and 1700ng of total DDT/g of lipid. These values of DDT, DDE, and total DDT were higher than the observed geometric means in the present study. In this work, only DDD presented level higher (534.3ng of DDD/g of lipid) than the observed by Paumgartten et al., (2000). Other DDT levels around the world are shown in the Table 3.
Table 3.
Data of ng of DDT/g of lipid in some Countries.
| Locality | DDT | DDE | DDD | Total DDT | Estimative | Reference |
|---|---|---|---|---|---|---|
| Brazil | 72 | 343.4 | 42.1 | 492.8 | Geometric mean | Present study |
| Brazil | 2009.3 | 7262.7 | 9266.6 | Mean | Lara et al., 1982 | |
| Brazil | 0.12 | 2.53 | 0.03 | Mean | Beretta and Dick, 1994 | |
| Brazil | 0.07 | 0.32 | 0.39 | Mean | Oliveira and Dores, 1998 | |
| China | 390 | 2480 | Mean | Wong et al., 2002 | ||
| China | 3330a | Geometric mean | Sun et al., 2005 | |||
| 1916b | ||||||
| Kazakhstan | 1955 | Mean | Lutter et al., 1998 | |||
| 1500 | Median | |||||
| Thailand | 169.4* | 69.4* | 208.8* | Median | Stuetz et al., 2001 | |
| 287.5* | 90.9* | 329.9* | Mean | |||
| Ucraine | 822 | 2457 | Median | Gladen et al., 1999 | ||
| USA | 217 | Mean | Kostiniak et al., 1999 |
- results originally in ng/mL of whole milk corrected to ng/g of lipid, adopting 3% of fat in milk.
– mothers from urban area.
– mothers from rural area.
The mean concentration of total DDT (p,p′ DDT+ p,p′ DDE+ p,p′ DDD) in samples of breast milk donned by Chinese women (Kunisue et al., 2004). The mean value (2100ng of total DDT/g of lipid) was higher than the contamination expressed in terms of geometric mean in milk samples from Madeira River. The authors hypothesized that population might be mainly exposed to DDT via seafood intake.
Some values of DDT contamination in developed countries such as Sweden and Germany presents a perceptible decrease in the DDT levels (Schade and Heinzow, 1998; Norén and Meironyté, 2000; Solomon and Weiss, 2002.). The principal explication to the phenomena is the prohibition of the DDT use in agriculture by these countries, mainly in the decade of 1970. Solomon and Weiss (2002) reported a decrease of 81% of the detectable residue levels of DDT in Germany. The same tendency of decrease in human milk contamination levels are not observed in Latin America by lack of contamination data that support this kind of comparison and by a latter prohibition of DDT in the developing countries.
3.4. Differences in total DDT concentration between primipara and multipara mothers
The observed geometric mean for DDT and its metabolites for premipara mother were 698.8 ng of p,p′ DDE /g of lipid, 32.5 ng of p,p′, DDD/g of lipid, 85.1 ng of p,p′, DDT /g of lipid, and 838.7 ng of total DDT /g of lipid . These values were higher than the calculated for multipara mothers that presented contamination of p,p′-DDE = 238.2; p,p′-DDD = 31.5, p,p′-DDT = 52.1, and total DDT = 346.7ng/g of lipid.
Japanese data contamination of DDT and its metabolites (Kunisue et al., 2006) were lower than the found in the samples collected from Madeira River region for both premipara and multipara mothers. The values of contamination to primipara p,p′-DDT(13 ng/g of lipid), p,p′-DDD (1.2 ng/g of lipid), and p,p′-DDE (330ng/g of lipid) and for multipara mothers the contamination of p,p′-DDT, p,p′-DDD, and p,p′-DDE were 10, 0.67, and 220 ng/g of lipid respectively. These values were lower than the obtained in the present study and a possible explication is that DDT was used in the Brazilian Amazon until the decade of 1990 in vector control of malaria. Another interesting study from Russia reports breast milk contamination with total DDT. The Russian mean concentrations of with total DDT (p,p′-DDE + p,p′-DDD + p,p′-DDT) in breast milk collected in Kargopol was 991 and 1065, in Severodvinsk were 1131 and 804, in Arkahangelsk were 1392 and 1086, and in Naryan-Mar were 1103 and 757 ng of total DDT/g of lipid for primipara and for multipara mothers respectively (Polder et al., 2003). These values, except the mean value for multipara mother from Naryan-Mar, were higher than the geometric means observed in milk samples collected in all localities from Madeira River region. Data of DDT contamination of primipara milk samples from Poland (Szyrwińska and Lulek, 2007) showed comparable mean values.
3.5. Correlations between total DDT in breast milk vs. age and vs. parity
It is reported that concentrations of organochlorine pesticides in human breast milk vary with factors such as age of the mother and parity (Harris et al., 2001; LaKind et al., 2001). No significant correlations were found to total DDT in function of age and parity in the present study. The correlation showed very low r2 (0.0078 and 0.0614 respectively), the same observed by other authors (Kunisue et al. 2004; Minh et al., 2004). Tanabe and Kunisue (2006) considered in their work that the absence of correlations between organochlorine compounds levels and age/parity of the donor mothers living in Asian developing countries cannot be clearly explained. The authors point as a possible reason that most woman in these countries may have many children during her life and the first infant is born at a young age of the mother, the same fact is observed in the mothers participating in the present study. In a study of organochlorine contaminants in 412 milk samples from Canada the authors observed low correlation between organochlorine levels and age of the mothers (Mes et al., 1993), the same observed in other works for organochlorine compounds and sons number (Vannuchi et al., 1992; Spicer e Kereu, 1993).
3.6. Estimated infant daily intake
With the results for total DDT, it was calculated an estimative of the Infant Daily Intake. The individual values of IDI ranged from 0.00023 to 0.8322 and the geometric mean was 0.00329 mg of total DDT/g of body weight/day. According to the calculated values of the children 8.7% presented daily intake of the sum of DDT exciding the Tolerable Daily Intake (TDI) of 0.020mg of total DDT/kg of body weight proposed by WHO (1984). Polder et al., (2003) estimated the IDI of the DDT for Russian children. Their results were lower than the observed in the present study, and ranged from 0.004 to 0.007 mg of DDT/g of body weight/day, and no one child exceeded the TDI proposed by WHO.
4. Conclusions
According to the obtained results that shown considerable DDT contamination and some individual estimated IDI higher than the TDI proposed by WHO, it is important to establish of a systematic DDT monitoring program in breast milk from Madeira River Basin as well as to all of the other Amazon rivers, since this contaminant is pointed out as a endocrine disruptor and may have also a negative impact upon the development of the child nervous system. It is also important to do more research to investigate and develop other parameters which may be indicative of any disturbance caused by DDT in the exposed children. These data should be discussed by the overall Amazonian society, by the scientific community and Official Public Health organisms in order to help to construct decisions in the future use of the DDT in the Madeira River Basin, that is still presenting large numbers of cases of malaria each year together with other diseases transmitted by insect vectors. Nevertheless, despite of the obtained data showing the prevalence of DDT contamination, breast feeding should not be discouraged since it warrants the most complete nutrient supply to children.
Acknowledgments
The authors are indebted to the reviewers for their valuable comments that helped us very much to improve the manuscript. We need also to acknowledge the technicians of CESTH/FIOCRUZ Laboratory located at Rio de Janeiro, Brazil. Antonio Azeredo was supported by Brazilian Research Council (CNPq process n° 142118/2001-0). João P. M. Torres is Level II Research Fellow of CNPq and is Advance Selikoff Fellow at the Mount Sinai School of Medicine and Queens College in New York and is partially supported by Grant 1 D43 TW00640 from the Fogarty International Center of National Institute of Health of the United States of America (NIH-USA).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bakken AF, Siep M. Insecticides in human breast milk. Acta Pædiatr Scand. 1976;65:535–539. doi: 10.1111/j.1651-2227.1976.tb04928.x. [DOI] [PubMed] [Google Scholar]
- Beretta M, Dick T. Organochlorine Compounds in Human Milk, Porto Alegre, Brazil. Bull Environ Contam Toxicol. 1994;53:357–360. doi: 10.1007/BF00197226. [DOI] [PubMed] [Google Scholar]
- Berlin JRCM, Kacew S, Lawrence R, Lakind JS, Campbell R. Criteria for chemical selection for programs on human milk surveillance and research for environmental chemicals. J Toxicol Environ Health Part A. 2002;65:1839–1851. doi: 10.1080/00984100290071748. [DOI] [PubMed] [Google Scholar]
- Cruz OG. Madeira-Mamoré railway company. Considerações geraes sobre as condições sanitárias do Rio Madeira pelo Dr. Oswaldo Gonçalves Cruz. Papelaria Americana, Rio de Janeiro. 1910 [Google Scholar]
- D’Amato C, Torres JPM, Malm O, Bastos W, Cláudio L, Markowitz S. DDT in fishes from four different Amazon sites: exposure assessment for breast feeding infants. Organohalogen Compd. 2004;66:2483–2490. [Google Scholar]
- Danston GP, Gooch JW, Shuey DL, Nikiforow AI, Fico TA, Gorsuch JW. Environmental estrogens and reproductive health: a discussion of the human and environmental data. Reprod Toxicology. 1997;11:465–481. doi: 10.1016/s0890-6238(97)00014-2. [DOI] [PubMed] [Google Scholar]
- Dewailly E, Ayotte P, LaLiberté C, Weber JP, Gingras S, Nantel AJ. Polychlorinated biphenyls (PCB) and dichlorodiphenyl dichloroethylene (DDE) concentrations in the breast milk of women in Quebec. Am J Public Health. 1996;86:1241–1246. doi: 10.2105/ajph.86.9.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EF, Brix KA, Hwang SA, Deres DA, Bush B, Cook K, Worswick P. The association between local fish consumption and DDE, mirex, and HCB concentrations in the breast milk of Mohawk women at Akwesasne. J Exposure Anal Environ Epidemiol. 2001;11:381– 388. doi: 10.1038/sj.jea.7500180. [DOI] [PubMed] [Google Scholar]
- Gillesby BE, Zacharenski TR. Exoestrogens: mechanisms of action and strategies for identification and assessment. Environ Toxicol Chem. 1998;17:3–14. [Google Scholar]
- Gladden BC, Monaghan SC, Lukianova EM, Hulchiy OP, Shyryak-Nyzhnyk ZA, Sericano JL, Little RE. Organochlorine in breast milk from two cities in Ucraine. Environ Health Perspect. 1999;107:459–462. doi: 10.1289/ehp.99107459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillette JA, Crain DA, Rooney AA, Pickford DB. Organization versus activation: the role of endocrine disrupting contaminants (EDCs) during embryonic development in wildlife. Environ Health Perspect. 1995;103:157–164. doi: 10.1289/ehp.95103s7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggyard SB, Brown WH, Stull JW, Whiting FM, Kemberling SR. DDT and DDE content of human milk in Arizona. Bull Environ Contam Toxicol. 1973;9:169–172. doi: 10.1007/BF01684998. [DOI] [PubMed] [Google Scholar]
- Harris CA, Woolridge MW, Hay AWM. Factors affecting the transfer of organochlorine pesticides residues to breastmilk. Chemosphere. 2001;43:243–256. doi: 10.1016/s0045-6535(00)00149-1. [DOI] [PubMed] [Google Scholar]
- Hart MM, Whang-Peng J, Sieber SM, Fabro S, Adamson RH. Distribution and effects of DDT in the pregnant rabbit. Xenobiotica. 1972;2:567–574. doi: 10.3109/00498257209111084. [DOI] [PubMed] [Google Scholar]
- Hekstra PF, Burnison BK, Nehli T, Muir DCG. Enantiomer specific activity of o,p-DDT with human strogen receptor. Toxicology Letters. 2001;125:78–81. doi: 10.1016/s0378-4274(01)00410-6. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Nutrition during lactation. Washington, D.C.: National Academy Press; 1991. [Google Scholar]
- Johnson DC, Kogo H, Sam M, Dey SK. Multiple estrogenic action of o,p-DDT: initiation and maintenance of pregnancy in the rat. Toxicology. 1988;53:79–87. doi: 10.1016/0300-483x(88)90238-7. [DOI] [PubMed] [Google Scholar]
- Koopman-Esseboom C, Huisman M, Weisglas-Kuperus N, Boersma ER, Touwen BC, Sauer PJ. Results of the Dutch study on PCB and dioxin induced neurotoxicity in children. Neurotoxicology. 1995;16:752–759. [Google Scholar]
- Korrick AS, Chen C, Damokosh AI, Ni J, Liu X, Cho S, Altshul L, Ryan L, Xu X. Association of DDT with Spontaneous Abortion: A Case-Control Study. Annals Epidemiol. 2001;11:491–496. doi: 10.1016/s1047-2797(01)00239-3. [DOI] [PubMed] [Google Scholar]
- Kostyniak PJ, Stinson C, Greizerstein HB, Vena J, Buck G, Mendola P. Relation of Lake Ontario fish consumption, lifetime lactation, and parity to breast milk polychlorobiphenyl and pesticide concentrations. Environ Res. 1999;80:166–174. doi: 10.1006/enrs.1998.3939. [DOI] [PubMed] [Google Scholar]
- Kunisue T, Someya M, Kayama F, Jin Y, Tanabe S. Persistent organochlorines in human breast milk collected from primiparae in Dalian and Shenyang, China. Environ Poll. 2004;131:381–392. doi: 10.1016/j.envpol.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Kunisue T, Muraoka M, Ohtake M, Sudaryanto A, Minh NH, Ueno D, Higaki Y, Ochi M, Tsydenova O, Kamikawa S, Tonegi T, Nakamura Y, Shimomura H, Nagayama J, Tanabe S. Contamination status of persistent organochlorines in human breast milk from Japan: Recent levels and temporal trend. Chemosphere. 2006;64:1601–1608. doi: 10.1016/j.chemosphere.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Lakind JS, Berlin CM, Naiman DQ. Infant exposure to chemicals in breast milk in the United States: What we need to learn from a breast milk monitoring program. Environm Health Prespect. 2001;109:75–88. doi: 10.1289/ehp.0110975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara WH, Barreto HHC, Inomata ONK. Resíduos de pesticida organoclorados em leite humano, São Paulo, Brasil, 1979–1981. Rev Inst Adolfo Lutz. 1982;42:45–52. [Google Scholar]
- Marien K, Laflamme DM. Determination of a tolerable daily intake for consumers of DDT contaminated fish from the lower Yakima River, Washington. Risk Anal. 1995;15:709–717. doi: 10.1111/j.1539-6924.1995.tb01343.x. [DOI] [PubMed] [Google Scholar]
- Mes J, Davies DJ, Doucet J, Weber D, Mcmullen E. Levels of chlorinated hydrocarbons residues in Canadian human breast milk and their relationship with to some characteristics of the donors. Food Addit Contam. 1993;10:429–441. doi: 10.1080/02652039309374166. [DOI] [PubMed] [Google Scholar]
- Minh NH, Someya M, Minh TB, Kunisue T, Iwata H, Watanabe M, Tanabe S, Viet PH, Tuyen BC. Persistent organochlorine residues in human breast milk from Hanoi and Hochiminh City, Vietnam: contamination, accumulation kinetics and risk assessment for infants. Environ Poll. 2004;129:431–441. doi: 10.1016/j.envpol.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Mussalo-Rauhamaa Pyysalo H, Antervo K. Relation between the content of organochlorine compounds in Finnish human milk and the characteristics of mothers. J Toxicol Environ Health. 1988;25:1–19. doi: 10.1080/15287398809531185. [DOI] [PubMed] [Google Scholar]
- Norén K, Meironyté D. Certain organochlorine and organobromine contaminants in Swedish human milk in perspective of past 20–30 years. Chemosphere. 2000;40:1111–1123. doi: 10.1016/s0045-6535(99)00360-4. [DOI] [PubMed] [Google Scholar]
- Oddy WH. Breastfeeding against illness and infection in infant and children: a review of evidence. Breastfeed Rev. 2001;9:11–18. [PubMed] [Google Scholar]
- Olea-Serrano N, Fernandéz-Cabrera MF, Pulgar-Encinas R, Olea-Serrano F. Endocrine disrupting chemicals. Harmful substances and how to test them. Cad Saúde Pública. 2002;18:489–494. doi: 10.1590/s0102-311x2002000200013. [DOI] [PubMed] [Google Scholar]
- Oliveira Filho AM. General overview on vector-control in relation to the insecticide Pollution in Brazil. International Workshop on Organic Micropollutants in the Environment, IBCCF-UFRJ. Note no. 1097 AB-Dlo; Ministry of Agriculture, The Netherlands. 1997. [Google Scholar]
- Oliveira MAG, Dores EFGC. Níveis de praguicidas organoclorados no leite materno de uma população de Cuiabá – Mato Grosso. R Ecotoxicol E Meio Ambiente. 1998;8:77–90. [Google Scholar]
- Olszyna-Marzys AE, Campos M, Favar MT, Thomas M. Residuos de plaguicidas clorados em la leche humana en Guatemala. Bol Ofic Sanit Panam. 1973;74:93–107. [PubMed] [Google Scholar]
- Palmer K, Green S, Legator M. Dominant lethal study of p,p-DDT in rats. Food Cosmet Toxicol. 1973;11:53–62. doi: 10.1016/0015-6264(73)90061-8. [DOI] [PubMed] [Google Scholar]
- Patandin S, Dagnelie PC, Mulder PGH, Op De Coul E, Van Der Veen JE, Weisglas-Kuperus N, Sauer PJJ. Dietary exposure to polychlorinated biphenyls and dioxins from infancy until adulthood: a comparison between breast-feeding, toddler and long-term exposure. Environ Health Perspect. 1999;107:45– 51. doi: 10.1289/ehp.9910745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paumgartten FJR, Cruz CM, Chahoud I, Palavinskas R, Mathar W. PCDDs, PCDFs, PCBs, and other organochlorine Compounds in human milk from Rio de Janeiro, Brazil. Environ Res A. 2000;83:293–297. doi: 10.1006/enrs.2000.4062. [DOI] [PubMed] [Google Scholar]
- Perez-Maldonado IN, Diaz-Barriga F, de la Fuente H, Gonzales-Amaro R, Calderón J, Yânes L. DDT induces apoptosis in human mononuclear cells in vitro and is associated with increased apoptosis in exposed children. Environ Res. 2004;94:38–46. doi: 10.1016/s0013-9351(03)00112-9. [DOI] [PubMed] [Google Scholar]
- Polder A, Odland JO, Tkachev A, Føreid S, Savinova TN, Skaare JU. Geographic variation of chlorinated pesticides, toxaphenes and PCBs in human milk from sub-arctic and arctic locations in Russia. Sci Total Environ. 2003;306:179–195. doi: 10.1016/S0048-9697(02)00492-8. [DOI] [PubMed] [Google Scholar]
- Quinsey PM, Donohue DC, Cumming FJ, Ahocas JT. The importance of measured intakes in assessing exposure of breast–fed infants to organochlorine. Eur J Clin Nutr. 1996;50:438–442. [PubMed] [Google Scholar]
- Rogan WJ, Gladen BC, McKenney JD, Carreras N, Hardy P, Thullen J, Tingelstad J, Tully M. Polychlorinated biphenils (PBC) and dichlorodiphenyl dichloroethene (DDE) in human milk: effects of maternal factors and previous lactation. Am J Public Health. 1986;76:172–177. doi: 10.2105/ajph.76.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer PJ, Huisman M, Koopman-Esseboom C, Morse DC, Smits-van Prooije AE, van der Berg KJ, Tuinstra LG, Van Der Paauw CG, Boersma ER, Weisglas-Kuperus N, Lammers JH, Kulig BM, Brouwer A. Effects of polychlorinated biphenyls (PCBs) and dioxins on growth and development. Hum Exp Toxicol. 1994;13:900–906. doi: 10.1177/096032719401301213. [DOI] [PubMed] [Google Scholar]
- Schade G, Heinzow B. Organochlorine pesticides and polychlorinated biphenyls in human milk of mothers living in northern Germany: Current extent contamination, time trend from 1986 to 1997 and factors that influence the level of contamination. Sci Total Environ. 1998;215:31–39. doi: 10.1016/s0048-9697(98)00008-4. [DOI] [PubMed] [Google Scholar]
- Skare JU, Polder A. Polychlorinated biphenyls and organochlorine pesticides in milk of Norwegian women during lactation. Arch Environ Contam Toxicol. 1990;19:640–645. doi: 10.1007/BF01183978. [DOI] [PubMed] [Google Scholar]
- Solomon GM, Weiss PM. Chemical contaminants in breast milk: Time trends and regional variability. Environ Health Perspect. 2002;110:A339–A347. doi: 10.1289/ehp.021100339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer PE, Kereu RK. Organochlorine insecticide residues in human breast milk from a remote area in Papua New Guinea. Bull Environm Contam Toxicol. 1993;50:540–546. doi: 10.1007/BF00191243. [DOI] [PubMed] [Google Scholar]
- Stacey CI, Perriman WS, Whitney S. Organochlorine pesticide residues in human milk: Western Australia 1979–1980. Arch Environ Health. 1985;40:102–108. doi: 10.1080/00039896.1985.10545898. [DOI] [PubMed] [Google Scholar]
- Stuetz W, Prapamontol T, Erhardt JG, Classenand HG. Organochlorine pesticide residues in human milk of a Hmong hill tribe living in Northern Thailand. Sci Total Environ. 2001;273:53–60. doi: 10.1016/s0048-9697(00)00842-1. [DOI] [PubMed] [Google Scholar]
- Sudaryanto A, Kunisue T, Tanabe S, Niida M, Hashim M. Persistent organochlorine compounds in human breast milk from mothers living in Penang and Kedah Malaysia. Arch Environm Contam Toxicol. 2005;49:429–437. doi: 10.1007/s00244-004-0208-8. [DOI] [PubMed] [Google Scholar]
- Sun SJ, Zhao JH, Koga M, Ma YX, Liu DW, Nakamura M, Liu HJ, Horiguchi H, Clark GC, Kayama F. Persistent organic pollutants in human milk in women from urban and rural areas in northern China. Environ Res. 2005;99:285–293. doi: 10.1016/j.envres.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Szyrwińska K, Lulek J. Exposure to specific polychlorinated biphenyls and some chlorinated pesticides via breast milk in Poland. Chemosphere. 2007;66:1895–1903. doi: 10.1016/j.chemosphere.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Teoburi O, Rhouma KB, Sakli M. DDT induces apoptosis in rat thymocites. Bull Environ Contam. 1998;61:216–233. doi: 10.1007/s001289900751. [DOI] [PubMed] [Google Scholar]
- Torres JPM, Pfeiffer WC, Markowitz S, Pause R, Malm O, Japenga J. Dichlorodiphenyltrichloroethane in soil, River sediment, and fish in the Amazon in Brazil. Environ Res Section A. 2002;88:134–139. doi: 10.1006/enrs.2001.4312. [DOI] [PubMed] [Google Scholar]
- Vannuchi MTO, Antunes LAF, Pinotti MHP. Resíduos de psticidas organoclorados em leite materno no município de Londrina. Semina Ci Biol. 1992;13:52–57. [Google Scholar]
- WHO Pesticides residues in food. Evaluation 1984, v.67. DDT. Joint Meeting of the Food and Agricultural Organization of the United Nation and World Health Organization; Geneva. [Google Scholar]
- Wong CKC, Leung KM, Poon BHT, Lan CY, Wong MH. Organochlorine hydrocarbons in human breast milk collected in Hong Kong and Guangzhou. Arch Environ Contamin Toxicol. 43:364–372. doi: 10.1007/s00244-002-1210-7. [DOI] [PubMed] [Google Scholar]