Abstract
Objective
To determine the prevalence and predictors of abnormal liver enzyme levels in ambulatory young women with anorexia nervosa (AN).
Study design
In this cross-sectional study of 53 females with AN, serum concentrations of liver enzymes and hormones were measured. Anthropometric, dietary, and body composition information was collected. Correlational analyses were performed between liver enzyme concentrations and these variables.
Results
Elevated alanine aminotransferase (ALT) and gamma-glutamyltranspeptidase (GGT) levels were found in 14 subjects (26%) and 5 subjects (9%), respectively. ALT and GGT were inversely correlated with body mass index (r = −0.27 to −0.30, p ≤ 0.049) and percentage body fat (r = −0.36 to −0.47, p ≤ 0.007), but showed no relationship with lean body mass. Subjects with percentage body fat < 18% had higher ALT levels than those above this threshold (median 26.5 vs. 18.0 U/L, p = 0.01). Liver enzyme concentrations did not correlate with dietary variables, except for GGT and percentage of calories from protein (r = 0.28, p = 0.04).
Conclusions
Serum ALT and GGT concentrations are inversely related to adiposity in young women with AN. Future studies are needed to determine if these liver enzyme elevation signify unrecognized, clinically relevant liver disease.
Keywords: eating disorders, adolescents, liver, malnutrition, alanine aminotransferase, gamma-glutamyltranspeptidase
Elevated liver enzyme levels are frequently detected in clinical evaluations of young women with anorexia nervosa (AN). Previous case reports have described concentrations of alanine aminotransferase (ALT) greater than 132 times the upper limit of normal in hospitalized patients [1–5]. These abnormalities were attributed to acute liver hypoperfusion from hypovolemia and/or cardiac dysfunction [1–2]. Less severe ALT elevations have been described in ambulatory patients with AN [6], but the etiology and risk factors for this milder form of liver inflammation remain unclear.
Hepatocellular injury from non-alcoholic fatty liver disease (NAFLD) has been questioned as a cause of these enzyme elevations in AN, and disorders of malnutrition [7, 8]. NAFLD represents a spectrum of liver disease ranging from steatosis to cirrhosis, and is characterized by the accumulation of excess lipid within hepatocytes. Disturbances in hormonal regulation or nutrition may increase the risk for NAFLD. For example, insulin resistance, occasionally seen in AN [9–10], increases peripheral fatty acid mobilization and decreases hepatic oxidation of fatty acids. Although the relationship between insulin resistance and NAFLD has been well-described in obese and normal-weight individuals [11], it has not been explored in AN. Similarly, specific alterations in the dietary composition of adolescents with AN may lead to NAFLD. Higher carbohydrate and lower fat intake, commonly consumed by adolescents with AN [12–13], are associated with an increased risk for hepatic inflammation in obesity [14], but have not been studied in AN.
In this study, we sought to determine the prevalence and predictors of abnormal liver enzyme levels in ambulatory young women with AN. We hypothesized that individuals with more severe disease, as reflected by lower body mass index (BMI) and body fat, would be more likely to exhibit elevated ALT and gamma-glutamyltranspeptidase (GGT) levels. We also hypothesized that insulin resistance would be a predictor of abnormal ALT and GGT concentrations. We predicted that there would be a significant association between macronutrient caloric distribution and liver enzyme concentrations.
Methods
Design
This study was a cross-sectional evaluation of data obtained from the baseline visit of a clinical bone density trial.
Subjects
Adolescents and young women with AN were recruited from the Children’s Hospital Boston Eating Disorders Program, and met the following inclusion criteria: 1) age 13 – 30 years, and at least 2 years following menarche; 2) diagnosis of AN based on Diagnostic and Statistical Manual of Mental Disorders-IV criteria [15]; and 3) primary amenorrhea, or secondary amenorrhea for at least 3 months. Exclusion criteria included: 1) hormone replacement therapy (e.g., estrogen, oral contraceptives) within the past month; 2) presence of disease known to affect bone metabolism (e.g., Cushing’s syndrome, diabetes mellitus, skeletal dysplasia, etc.); and 3) use of glucocorticoids or other medications known to affect bone metabolism within the previous 3 months. All subjects who participated in the baseline visit of the clinical trial were included in the current study. The young women recruited for participation in the trial had all been diagnosed with AN by their clinical providers. However, the subjects had been in medical treatment for varying lengths of time (and thus had varying degrees of weight restoration) by the time of their baseline study visit. All subjects were amenorrheic at the time of the baseline visit. An additional 165 patients were approached: 127 chose not to participate and 38 were deemed ineligible. All participants gave written informed consent according to the guidelines of the Committee on Clinical Investigation at Children’s Hospital Boston.
Study Protocol
At the baseline visit, all subjects underwent a physical examination conducted by a single study physician in the outpatient division of the General Clinical Research Center, Children’s Hospital Boston. A structured interview was conducted by a single study co-investigator to obtain pertinent medical history and information about alcohol and medication use. Height (cm) and weight (kg) were measured in a standardized fashion using the same calibrated scale and stadiometer (Perspective Enterprises, Kalamazoo, Michigan), after the subject voided, and while dressed in a hospital gown. BMI was calculated by the formula: BMI = Weight (kg) / [Height (m)]2. For each subject ≤ 20 years old, percentage ideal body weight (% IBW) was determined using the 2002 Center for Disease Control Growth Charts and the formula: % IBW = 100 × [Patient BMI (kg/m2) / Median BMI for age (kg/m2)] [16]. For each subject > 20 years old, % IBW was determined by the formula: % IBW = 100 × [Patient BMI (kg/m2) / Median BMI for adults (21.7 kg/m2)] [17]. Total body dual-energy X-ray absorptiometry (DXA) scans were obtained with a Hologic 4500 Delphi scanner (Hologic, Inc., Waltham, Massachusetts ) for analysis of body composition, including body fat mass (g), body fat percentage, and lean body mass (g).
Dietary information was collected by a registered dietician, and included 3 separate 24-hour dietary recalls obtained on 3 non-consecutive days within a 3-week interval. The first dietary recall was conducted in person at the baseline weekday study visit, and the second and third recalls were conducted through unannounced telephone calls on a weekday and weekend, respectively. The information was collected using the Nutrition Data System for Research (NDS-R) software (versions 4.05_33, 5.0_35, and 2005, Nutritional Coordinating Center, University of Minnesota, Minneapolis, Minnesota). Final calculations of daily nutrient intake were performed using NDS-R version 2005. In order to compare each subject’s reported intake with recommended nutrient values, the dietary reference intake (DRI) for adequate intake (AI) was used for vitamin D, vitamin K, and calcium intake, while the DRI for recommended dietary allowance (RDA) was used for carbohydrate, protein, total vitamin A activity, vitamin E, vitamin C, phosphorus, magnesium, iron, zinc, and copper intake [18].
Subjects had venous blood drawn from an antecubital vein between 8:30 – 10:00 a.m. following an overnight fast. Serum samples were assayed in the Children’s Hospital Boston Core Laboratory for levels of liver enzymes [ALT, AST, gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (AP)], total bilirubin, direct bilirubin, and glucose by photometric analysis and indirect potentiometry. Insulin, cortisol, dehydroepiandrosterone-sulfate (DHEA-S), triiodothyronine (T3), thyroxine (T4), and thyroid stimulating hormone (TSH) levels were measured by electrochemiluminescence. Total estradiol was measured by radioimmunoassay and free and total testosterone levels by liquid chromatography/mass spectroscopy at Esoterix Laboratories, Inc. (Calabasas Hills, California). The homeostasis model assessment of insulin resistance (HOMA) score was calculated from the fasting serum insulin and glucose levels [HOMA = (fasting insulin concentration (µU/mL) × fasting glucose concentration (mmol/L)) / 22.5], and used as a measure of insulin resistance [19].
Statistical Analysis
Mean macro- and micronutrient intake levels were compared with established DRI thresholds for females [18], and mean macronutrient intake distributions (expressed as a % of total calories) compared with the median values of the DRI ranges using Wilcoxin Signed Ranks tests. Bivariate relationships among anthropometric variables, body composition measurements, hormonal concentrations, dietary variables, and serum liver enzymes were determined by Pearson correlational analyses. Serum liver enzymes and hormone levels were compared between subgroups divided on the basis of body composition using the Mann-Whitney U test; this conservative non-parametric approach was chosen based on the relatively small subgroups of interest. Specific bivariate correlates were entered into multiple linear regression models to determine the relationship between body composition measurements, hormonal concentrations, and liver enzymes, controlling for weight and duration of amenorrhea. All statistical analyses were performed using SPSS software (SPSS, Inc, Chicago, Illinois). Two-tailed tests for significance were used, and a p-value < 0.05 was considered statistically significant. Data are presented as mean ± SD unless otherwise indicated.
Results
Description of Study Population
Clinical, anthropometric, and body composition data were obtained from all participants (Table I). None of the subjects had a known history of liver disease or any external signs of liver disease on physical examination. Three young women were receiving oral contraceptives at the time of study recruitment. The oral contraceptives were discontinued at a minimum of 1 month prior to the baseline study visit. Three subjects had primary amenorrhea, and had a full endocrinologic evaluation prior to study enrollment which revealed no etiology for primary amenorrhea other than malnutrition; all 3 subjects had bone ages of ≥ 15 years. These 6 subjects were excluded from calculations of duration of amenorrhea. Five subjects were not able to fast overnight because of concern for hypoglycemia. These non-fasting measurements of insulin and glucose were excluded from the analyses, and HOMA was not calculated.
Table 1.
Clinical, Anthropometric, and Body Composition Data of 53 Young Women with AN
| Variable | Mean ± SD | Range |
|---|---|---|
| Age (y) | 18.5 ± 2.9 | 13.3 - 27.0 |
| Height (cm) | 164.5 ± 7.1 | 148.3 - 180.1 |
| Weight (kg) | 48.7 ± 6.0 | 37.4 - 67.9 |
| BMI (kg/m2) | 18.0 ± 1.6 | 14.8 - 22.9 |
| Lean body mass (g) | 36767.3 ± 6300.8 | 4029.2 - 47855.2 |
| Body fat mass (g) | 8551.5 ± 2872.6 | 3694.4 - 17428.8 |
| Percentage body fat (%) | 17.7 ± 4.7 | 8.6 - 27.4 |
| Percentage IBW (%) | 85.4 ± 8.2 | 68.0 - 110.2 |
| Duration of AN (mo) | 22.8 ± 26.8 | 1 - 132 |
| Duration of amenorrhea (mo) (n = 47) | 18.7 ± 23.2 | 3 - 144 |
| Percentage of subjects < 85% IBW (%) | 49.1 | |
|
Percentage of subjects reporting any alcohol consumption (%) |
13.2 Frequency = 2.9 drinks/week |
|
|
Percentage of subjects reporting regular weekly exercise (%) |
69.8 Frequency = 6.6 hours/week |
Nutritional Data
Nutritional data were obtained from all participants (Table II). Subjects were divided into two subgroups based on age in order to allow for appropriate comparison of each subject’s reported nutritional intake to the age-appropriate DRI for females. One subject was excluded from the nutritional analyses due to the lack of appropriate reference range for her age (13 years). Reported mean intake of all micronutrients except vitamin E was greater than the DRI for both age groups. Vitamin E intake was significantly lower than the DRI in young women ages 14 to 18 years (median 10.3 vs. 15.0 mg, p = 0.009) and 19 to 27 years (median 9.0 vs. 15.0 mg, p = 0.03). The percentage of calories consumed from fat in subjects ages 14 to 18 years was significantly lower than the median of the DRI range (median 23.8 vs. 30.0 %, p = 0.004). Distributions of all other macronutrients were within the DRI ranges for both age groups.
Table 2.
Average Reported Daily Dietary Intake of Subjects with AN
| Variable | Subjects ages 14 – 18 (n = 25) | DRI for females ages 14 – 18 | Subjects ages 19 – 27 (n = 27) | DRI for females ages 19 – 30 | % Subjects meeting the DRI (n = 52) |
|---|---|---|---|---|---|
| Calories (kcal/d) | 1946.9 ± 723.6 | ND | 1645.6 ± 643.9 | ND | ND |
| Average calories per weight (kcal/kg) | 41.0 ± 14.3 | ND | 34.8 ± 14.6 | ND | ND |
| Fat (g/d) | 54.4 ± 30.7 | ND | 50.2 ± 27.0 | ND | ND |
| Carbohydrate (g/d) | 189.8 ± 111.2 | 130 | 237.7 ± 97.0 | 130 | 92.3 |
| Protein (g/d) | 89.9 ± 34.3 | 46 | 76.1 ± 35.7 | 46 | 86.5 |
| Percentage calories from fat (%) | 23.7 ± 9.0 | 25 – 35 | 26.3 ± 9.7 | 20 – 35 | 57.7 |
| Percentage calories from carbohydrate (%) | 60.4 ± 9.0 | 45 – 65 | 59.3 ± 12.8 | 45 – 65 | 90.4 |
| Percentage calories from protein (%) | 19.1 ± 5.0 | 10 – 30 | 18.3 ± 4.7 | 10 – 35 | 96.2 |
| Total vitamin A activity (Retinol Activity Equivalents, µg) | 1027.5 ± 560.6 | 700 | 1041.9 ± 475.2 | 700 | 73.1 |
| Vitamin D (calciferol, µg) | 7.8 ± 4.7 | 5 | 6.1 ± 5.0 | 5 | 61.5 |
| Vitamin E (Total alpha-tocopherol, mg) | 11.7 ± 10.3 | 15 | 11.5 ± 7.4 | 15 | 26.9 |
| Vitamin K (phylloquinone, µg) | 141.5 ± 177.9 | 75 | 140.9 ± 149.4 | 90 | 57.7 |
| Vitamin C (ascorbic acid, mg) | 140.6 ± 74.5 | 65 | 137.1 ± 116.0 | 75 | 76.9 |
| Calcium (mg) | 1316.4 ± 636.8 | 1300 | 1092.2 ± 552.1 | 1000 | 55.8 |
| Phosphorus (mg) | 1590.1 ± 626.1 | 1250 | 1326.0 ± 566.8 | 700 | 75.0 |
| Magnesium (mg) | 382.3 ± 172.3 | 360 | 350.3 ± 137.7 | 310 | 57.7 |
| Iron (mg) | 22.6 ± 14.8 | 15 | 19.2 ± 9.7 | 18 | 51.9 |
| Zinc (mg) | 13.2 ± 6.5 | 9 | 13.7 ± 9.1 | 8 | 75.0 |
| Copper (µg) | 1528.7 ± 685.3 | 890 | 1488.9 ± 608.4 | 900 | 80.8 |
Data presented as mean ± SD
ND = not determined
Liver Enzyme and Hormonal Data
Serum liver enzyme and hormonal data were obtained from all participants (Table III). Testosterone and estradiol levels were compared with reference ranges used by Esoterix Laboratories. All other laboratory values were compared to established normative data used by our institution [20]. In this sample, 14 subjects (26%) had an elevated ALT (89.9 ± 64.8 U/L), 10 subjects (19%) had an elevated AST, 5 subjects (9%) had an elevated GGT (67.3 ± 21.5 U/L), and 3 subjects (6%) had an elevated AP. None of the subjects with elevated ALT had an AST to ALT ratio > 2:1. Nineteen subjects (41%) had low fasting insulin levels, while none had fasting hyperinsulinemia. The mean calculated HOMA score was 0.7 ± 0.5. None of the subjects had a HOMA > 3.16, a threshold considered diagnostic of insulin resistance in an adolescent population [21]. T3 and T4 levels were low in 28 subjects (57%) and 5 subjects (10%), respectively. TSH concentrations were normal for all subjects.
Table 3.
Liver Enzyme and Hormonal Data of Subjects with AN
| Laboratory Variable | Mean ± SD | Range | Normal Reference Range |
|---|---|---|---|
| ALT (U/L) | 40.0 ± 45.4 | 9 – 240 | 3 – 30 |
| AST (U/L) | 36.1 ± 26.4 | 16 – 187 | 2 – 40 |
| GGT (U/L) | 23.3 ± 21.0 | 8 – 112 | 8 – 35 |
| AP (U/L) | 74.1 ± 31.6 | 43 – 187 | 3 –120 |
| Total bilirubin (mg/dL) | 0.50 ± 0.26 | 0.1 – 1.5 | 0.3 – 1.2 |
| Direct bilirubin (mg/dL) | 0.11 ± 0.05 | 0.0 – 0.3 | 0.0 – 0.4 |
| Cortisol (µg/dL) | 16.0 ± 6.4 | 1.6 – 34.9 | 5 – 25 |
| Insulin (µU/mL) (n = 48) | 3.5 ± 2.2 | 0.2 – 8.9 | 3 – 12 |
| Glucose (mg/dL) (n = 48) | 75.9 ± 8.1 | 50 – 90 | 70 – 100 |
| T3 (ng/dL) | 85.8 ± 24.2 | 45 –142 | 86 – 153 |
| T4 (µg/dL) | 6.5 ± 1.1 | 4.7 – 9.4 | 5.2 – 10.9 |
| DHEAS (µg/dL) | 199.7 ± 99.0 | 51.1 – 545.0 | 45 – 380 |
| Total estradiol (ng/dL) | 3.3 ± 5.8 | 0.2 – 37.0 | 3.0 – 10.0 |
| Free testosterone (pg/mL) | 2.0 ± 1.0 | 0.1 – 5.8 | 1.1 – 6.3 |
| Total testosterone (ng/dL) | 22.5 ± 8.6 | 9 – 49 | 10 – 55 |
Predictors of Abnormal Liver Enzymes
Of the 14 subjects with abnormal ALT, only 1 (8%) reported any alcohol consumption, with a frequency of 1 drink per week. Six subjects (43%) were receiving antidepressants, including fluoxetine (n = 3), sertraline (n = 2), and escitalopram (n=2). One subject was receiving a benzodiazepine (lorazepam). Although subgroups were too small to compare formally using statistical tests, in the total group the frequency of elevated ALT was 14% in users and 28% in non-users of alcohol, and 24% in users and 30% in non-users of antidepressants.
Correlational analyses between ALT, GGT, and continuous anthropometric, body composition, hormonal and nutritional variables were performed. The percentage of calories consumed as protein was significantly correlated with GGT levels (r = 0.28, p = 0.04). There were otherwise no relationships noted between macronutrient intake and distribution variables and liver enzyme levels, nor between micronutrient intake (including vitamin E) and liver enzyme levels.
Measures of insulin resistance and cortisol did not correlate with ALT. However, an inverse trend was noted between GGT and both insulin (r = −0.28, p = 0.06) and HOMA (r = −0.29, p = 0.05). Similarly, a positive trend between cortisol and GGT approached statistical significance (r = 0.26, p = 0.06). There was a significant inverse relationship between GGT and T3 (r = −0.32, p = 0.03), but no other statistically significant relationships between liver enzymes and other hormonal variables.
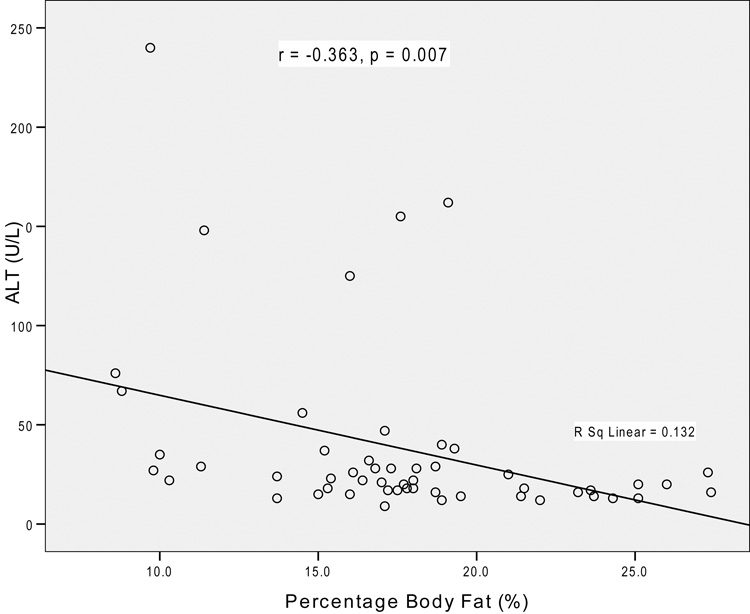
Age, duration of illness, and duration of amenorrhea were not associated with liver enzyme concentrations. As hypothesized, there was a significant inverse relationship between BMI and ALT (r = r = −0.27, p = 0.049), as well as GGT (r = −0.30, p = 0.03). In addition, there were significant inverse correlations between both liver enzyme levels and % IBW (r = −0.27 to −0.28, p ≤ 0.047), and % body fat (r = −0.36 to −0.47, p ≤ 0.007, Figure). In contrast, no significant relationships were found between lean body mass and the liver enzyme levels.
Figure 1. Relationship between ALT Concentration and Percentage Body Fat.
In multivariate models including duration of amenorrhea and weight as covariates, percentage body fat remained an independent predictor of both ALT (ß = −0.28, p = 0.05) and GGT (ß = −0.50, p = 0.001) concentrations. Along with duration of amenorrhea and weight, % body fat accounted for 20% and 24% of the variation in ALT and GGT concentrations, respectively. In addition, insulin (ß = −0.34, p = 0.047), HOMA (ß = −0.37, p = 0.04), cortisol (ß = 0.31, p = 0.04), and T3 (ß = −0.33, p = 0.03) were found to be independent predictors of GGT concentration in a multivariate model that also controlled for duration of amenorrhea and weight. Collectively, these variables accounted for 11% (insulin), 12% (HOMA), 11% (cortisol), and 12% (T3) of the variation in GGT concentration.
Body Fat Subgroup Analyses
Based on the results of initial correlational analyses, subjects were divided into 2 subgroups based on % body fat. Thirty subjects (57%) had a body fat percentage < 18% and were designated as having low body fat. Mean percentage body fat was significantly different between the 2 groups (14.6 vs. 21.7 %, p < 0.001). Mean age was not different between the subgroups. ALT values were significantly higher in the subjects with low compared with appropriate body fat (median ± SD = 26.5 ± 52.8 vs. 18.0 ± 30.6 U/L, p = 0.01). Although a similar trend was noted for GGT levels, the difference did not reach statistical significance (median ± SD = 16.0 ± 25.7 vs. 14.0 ± 9.4 U/L, p = 0.11). Subjects with low body fat had significantly lower total testosterone (median ± SD = 20.0 ± 5.0 vs. 24.0 ± 10.5 ng/dL, p = 0.02) and total estradiol levels (median ± SD = 1.3 ± 1.7 vs. 2.7 ± 8.0 ng/dL, p = 0.005) than subjects with appropriate percentage body fat. Concentrations of all other hormones were not different between the subgroups.
Discussion
In this study, we found that over one-quarter of participants had an elevated ALT concentration. This represents a prevalence greater than that reported previously (12%) in a sample with similar measures of body composition [6]. Subjects in the current study were younger (mean age 18.5 vs. 25.0 years), suggesting possible differences in liver injury susceptibility between adults and adolescents with AN.
One of our most compelling findings was that percentage body fat was inversely correlated with ALT and GGT in both bivariate and multivariate analyses. In contrast, no significant relationship was found between lean body mass and the liver enzyme levels. In bivariate analyses, we found that BMI was inversely correlated with levels of ALT and GGT. Ozawa et al. previously showed an inverse correlation between BMI and aminotransferase levels in severely malnourished hospitalized young women with AN (mean BMI 13.2 ± 1.3 kg/m2) [22]. Therefore, the relationship between BMI and liver inflammation in AN may be present across a wide range of BMIs. In addition, we found that the young women with a lower percentage body fat had significantly higher levels of ALT, accompanied by lower estradiol and testosterone concentrations. However, the higher ALT levels in this subgroup were not likely caused by steroid hormone abnormalities, as there were no relationships found between these hormones and the liver enzyme levels.
We had hypothesized that the subjects with more severe disease, as reflected by lower BMI and percentage body fat, would exhibit an elevated ALT and GGT concentration. The strong inverse relationship between adiposity and liver enzyme levels supports this hypothesis. The liver enzyme elevation may reflect NAFLD-induced hepatocellular injury. Westerbacka et al found a significant inverse relationship between serum ALT levels and liver fat by proton spectroscopy in obese men and women [23]. Although speculative, patients with lower adiposity may have increased susceptibility to NAFLD due to lower levels of adiponectin, an adipocytokine that increases fatty acid oxidation and decreases inflammation [24]. Adiponectin, although not measured in this study, has been shown to be low in NAFLD [25] and in some patients with AN [26]. The relationship between low adiponectin and NAFLD has been described in overweight patients [25], but has not been examined in AN. In addition to low adiposity, young women with AN may have hormonal or nutritional abnormalities that facilitate the development of NAFLD.
We had predicted that insulin resistance would be a risk factor mediating NAFLD-related liver enzyme abnormalities. However, in contrast to our a priori hypotheses, none of the subjects in our sample exhibited evidence of insulin resistance. In contrast, 41% of the sample had low insulin levels, and there were unexpected inverse relationships between serum insulin, HOMA, and GGT in both the bivariate and multivariate models. Although an oral glucose tolerance test or a hyperinsulinemic euglycemic clamp test would have represented more definitive methods for evaluating insulin resistance, previous work employing these techniques has yielded conflicting results about insulin resistance in AN. Low, normal, and high fasting and stimulated insulin levels have been reported in AN [27]. Due to the low prevalence of insulin resistance in our sample, we could not explore definitively the relationship between insulin resistance variables[H1] and liver enzymes. However, there were inverse trends noted between insulin, HOMA, and GGT concentrations, and positive trends between cortisol and GGT in bivariate analyses. In addition, T3 was negatively correlated with GGT. In multivariate analyses controlling for duration of amenorrhea and weight, insulin, HOMA, cortisol, and T3 were found to predict independently GGT levels. Both elevated cortisol and low serum T3, the latter a characteristic feature of the sick euthyroid syndrome, have been documented in young women with AN [28], and may serve as markers of disease severity. Taken together, these results suggest that abnormal liver enzyme levels are related not only to low adiposity, but also to disease severity in AN.
We predicted that low dietary fat and high carbohydrate intake would be related to elevated liver enzyme levels in AN, as demonstrated previously in obesity [14]. Although the percentage of calories consumed as fat was lower than the DRI range in the ages 14 – 18 years subgroup, macronutrient intake and distribution variables did not correlate with ALT. Only the percentage of calories consumed as protein was significantly correlated with GGT. Interestingly, the reported dietary intake of the subjects in this sample revealed less severe nutritional deficiencies than those noted in previous studies of adolescents and adults with AN [12–13]. Of the micronutrients analyzed, only vitamin E intake for both age groups was significantly less than the age-specific DRI. Musso et al found that patients with non-alcoholic steatohepatitis had lower dietary vitamin E intake than age-, BMI-, and sex-matched controls [29]. Based on the hypothesis that vitamin E, as an antioxidant, may confer protection against hepatic damage from lipid peroxidation and free oxygen radical species, Lavine et al treated children with NAFLD with vitamin E, which led to a reduction in ALT levels [30]. Debate exists as to whether vitamin E deficiency is a cause or effect of hepatic injury. Although vitamin E intake and liver enzyme levels were not correlated with one another in our sample, the low intake of vitamin E in the setting of liver enzyme abnormalities merits further research.
Use of hepatotoxic substances is another possible etiology of the observed elevations in ALT. However, only 1 of the 14 subjects with elevated ALT reported any alcohol consumption, and the level was far lower than that expected to cause liver injury. None of the 14 subjects had an AST:ALT ratio > 2:1, an indicator of alcoholic liver disease [31], and none reported receiving medications known to cause hepatotoxicity. Six of the 14 subjects were receiving antidepressants, but no medication which have shown a clear relationship with abnormal liver enzymes.
Study limitations deserve acknowledgement and consideration. Our study subjects were recruited from an eating disorders program, and thus were more likely to be engaged in active treatment, which includes regular visits with a physician and nutritionist. In addition, subjects were recruited as part of a clinical trial. Therefore, our results may not be generalizable to all ambulatory young women with AN. Our data were observational, and thus were compared were normative data only. The study did not include direct questions about family history of liver disease, probing questions regarding previous hepatotoxic drug use, hepatic imaging studies, liver biopsies, or extensive serum tests to explore specific causes of liver disease. We were not able to compare formally the frequency of elevated ALT in the subgroups that did and did not use alcohol or antidepressants given the small cell sizes in these subgroups. Finally, nutritional data were obtained by self-report, and patients with AN have a tendency to over-report their nutritional intake [12]. It is possible that this phenomenon led to an over-estimation of the nutrient intake of our sample. However, our primary study outcome variables were objective rather than subjective in nature.
Our study examined the relationships among nutritional data, hormonal variables, measures of body composition and liver enzyme levels in ambulatory adolescents and young women with AN. We found a strong inverse relationship between adiposity and ALT and GGT concentrations. We also found a relationship between hormonal markers of disease severity (e.g., low T3, elevated cortisol, low insulin, and low HOMA) and GGT levels. These findings are of potential concern, as they may signify previously unrecognized, clinically relevant liver disease in AN. The results of this exploratory study will hopefully pave the way for more definitive prospective studies, which may better delineate the etiology and duration of the liver enzyme abnormalities, as well as their clinical significance with respect to liver pathology.
Acknowledgments
We gratefully acknowledge our patients and their families; the expert nursing care of the General Clinical Research Center at Children’s Hospital Boston; contributions of the Children’s Hospital Boston Core Laboratory; and the excellent technical assistance of Suzanne Muggeo, Jessica Sexton, Katie Clegg, Christine Clark, and Nicolle Quinn.
Sources of financial support: NIH grants RO1 HD 43869, 5T32 HD 043034-02, and M01-RR-2172 to the Children’s Hospital Boston General Clinical Research Center; and a grant from the US Department of Defense (US Army, Bone, and Military Readiness Program)
List of Abbreviations Used
- AN
anorexia nervosa
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- GGT
gamma glutamyltranspeptidase
- AP
alkaline phosphatase
- T3
triiodothyronine
- T4
thyroxine
- TSH
thyroid stimulating hormone
- DHEA-S
dehydroepiandrosterone-sulfate
- HOMA
homeostasis model assessment of insulin resistance
- IBW
ideal body weight
- BMI
body mass index
- DXA
dual-energy X-ray absorptiometry
- NAFLD
non-alcoholic fatty liver disease
- DRI
dietary reference intake
- AI
adequate intake
- RDA
recommended dietary allowance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflicts of Interest: No authors have conflicts of interest.
References
- 1.De Caprio C, Alfano A, Senatore I, Zarrella L, Pasanisi F, Contaldo F. Severe acute liver damage in anorexia nervosa: Two case reports. Nutrition. 2006;22:572–575. doi: 10.1016/j.nut.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Di Pascoli L, Lion A, Milazzo D, Caregaro L. Acute liver damage in anorexia nervosa. Int J Eat Disord. 2004;36:114–117. doi: 10.1002/eat.20002. [DOI] [PubMed] [Google Scholar]
- 3.Rivera-Nieves J, Kozaiwa K, Parrish CR, Iezzoni J, Berg CL. Marked transaminase elevation in anorexia nervosa. Dig Dis Sci. 2000;45:1959–1963. doi: 10.1023/a:1005642601144. [DOI] [PubMed] [Google Scholar]
- 4.Furuta S, Ozawa Y, Maejima K, Tashiro H, Kitahora T, Hasegawa K, et al. Anorexia nervosa with severe liver dysfunction and subsequent critical complications. Internal Medicine. 1999;38:575–579. doi: 10.2169/internalmedicine.38.575. [DOI] [PubMed] [Google Scholar]
- 5.Komuta M, Harada M, Ueno T, Uchimura Y, Inada C, Mitsuyama K, et al. Unusual accumulation of glycogen in liver parenchymal cells in a patient with anorexia nervosa. Internal Medicine. 1998;37:678–682. doi: 10.2169/internalmedicine.37.678. [DOI] [PubMed] [Google Scholar]
- 6.Miller K, Grinspoon S, Ciampa J, Hier J, Herzog D, Klibanski A. Medical findings in outpatients with anorexia nervosa. Arch Intern Med. 2005;165:561–566. doi: 10.1001/archinte.165.5.561. [DOI] [PubMed] [Google Scholar]
- 7.Tajiri K, Shimizu Y, Tsuneyama K, Sugiyama T. A case report of oxidative stress in a patient with anorexia nervosa. Int J Eat Disord. 2006;39:616–618. doi: 10.1002/eat.20326. [DOI] [PubMed] [Google Scholar]
- 8.McLean AE. Hepatic failure in malnutrition. Lancet. 1962;280:1292–1294. doi: 10.1016/s0140-6736(62)90847-4. [DOI] [PubMed] [Google Scholar]
- 9.Pannacciulli N, Vettor R, Milan G, Granzotto M, Catucci A, Federspil G, et al. Anorexia nervosa is characterized by increased adiponectin plasma levels and reduced nonoxidative glucose metabolism. J Clin Endocrinol Metab. 2003;88:1748–1752. doi: 10.1210/jc.2002-021215. [DOI] [PubMed] [Google Scholar]
- 10.Franssila-Kallunki A, Rissanen A, Ekstrand A, Eriksson J, Saloranta C, Widen E, et al. Fuel metabolism in anorexia nervosa and simple obesity. Metabolism. 1991;40:689–694. doi: 10.1016/0026-0495(91)90085-b. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G, Brizi M, Morselli-Labate A, Bianchi G, Bugianesi E, McCullough A, et al. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 12.Hadigan C, Anderson E, Miller K, Hubbard J, Herzog D, Klibanski A, et al. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eat Disord. 2000;28:284–292. doi: 10.1002/1098-108x(200011)28:3<284::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 13.Misra M, Tsai P, Anderson E, Hubbard J, Gallagher K, Soyka L, et al. Nutrient intake in community-dwelling adolescent girls with anorexia nervosa and in healthy adolescents. Am J Clin Nutr. 2006;84:698–706. doi: 10.1093/ajcn/84.4.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Solga S, Alkhuraishe A, Clark J, Torbenson M, Greenwald A, Diehl A, et al. Dietary composition and nonalcoholic fatty liver disease. Digestive Diseases and Sciences. 2004;49:1578–1583. doi: 10.1023/b:ddas.0000043367.69470.b7. [DOI] [PubMed] [Google Scholar]
- 15.American Psychiatric Association. text revision (DSM-IV-TR) 4th edition. Washington, DC: American Psychiatric Press, Inc; 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 16.Kuczmarski R, Ogden C, Grummer-Strawn L, Flegal K, Guo S, Wei R, et al. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 17.World Health Organization. Physical status: the use and interpretation of anthropometry. Geneva: World Health Organization; Report of a WHO Expert Committee. WHO Technical Report Series 854. 1995 [PubMed]
- 18.Otten J, Hellwig JP, Meyers LD, editors. The Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press; 2006. [Google Scholar]
- 19.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and ß -cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 20.Soldin S, Brugnara C, Wong E, editors. Pediatric Reference Ranges. Washington, DC: AACC Press; 2003. [Google Scholar]
- 21.Keskin M, Kurtoglu S, Kendirci M, Atabek M, Cevat Y. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. J Pediatrics. 2005;115:500–503. doi: 10.1542/peds.2004-1921. [DOI] [PubMed] [Google Scholar]
- 22.Ozawa Y, Shimizu T, Shishiba Y. Elevation of serum aminotransferase as a sign of multiorgan-disorders in severely emaciated anorexia nervosa. Intern Med. 1998;37:32–39. doi: 10.2169/internalmedicine.37.32. [DOI] [PubMed] [Google Scholar]
- 23.Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Hakkinen A, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–1369. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 24.Czaja M. Liver injury in the setting of steatosis: Crosstalk between adipokine and cytokine. Hepatology. 2004;40:19–22. doi: 10.1002/hep.20328. [DOI] [PubMed] [Google Scholar]
- 25.Pagano C, Soardo G, Esposito W, Fallo F, Basan L, Donnini D, et al. Plasma adiponectin is decreased in nonalcoholic fatty liver disease. European Journal of Endocrinology. 2005;152:113–118. doi: 10.1530/eje.1.01821. [DOI] [PubMed] [Google Scholar]
- 26.Tagami T, Satoh N, Usui T, Yamada K, Shimatsu A, Kuzuya H. Adiponectin in anorexia nervosa and bulimia nervosa. J Clin Endocrinol Metab. 2004;89:1833–1837. doi: 10.1210/jc.2003-031260. [DOI] [PubMed] [Google Scholar]
- 27.Casper R. Carbohydrate metabolism and its regulatory hormones in anorexia nervosa. Psychiatry Research. 1996;62:85–96. doi: 10.1016/0165-1781(96)02984-8. [DOI] [PubMed] [Google Scholar]
- 28.Boyar RM, Hellman LD, Roffwarg H, Katz J, Zumoff B, O'Connor J, Bradlow HL, Fukushima DK. Cortisol secretion and metabolism in anorexia nervosa. N Engl J Med. 1977;296:190–193. doi: 10.1056/NEJM197701272960403. [DOI] [PubMed] [Google Scholar]
- 29.Musso G, Gambino R, De Michieli F, Cassader M, Rizzetto M, Durazzo M, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 30.Lavine JE. Vitamin E treatment of nonalcoholic steatohepatitis in children: a pilot study. J Pediatr. 2000;136:734–738. [PubMed] [Google Scholar]
- 31.Cohen J, Kaplan M. The SGOT/SGPT ratio: an indicator of alcoholic liver disease. Dig Dis Sci. 1979;24:835–838. doi: 10.1007/BF01324898. [DOI] [PubMed] [Google Scholar]