Abstract
Strengthening of cell-matrix adhesions in response to applied force has been well-documented. However, while implied by various lines of evidence, the force-mediated strengthening of cell-cell adhesions has not been directly demonstrated. In the current study, we present results consistent with force strengthening in adherens junctions, obtained by application of different force profiles to VE-cadherin coated magnetic beads attached to endothelial cells. When force is ramped from a low to high value over time, fewer beads detach than with the immediate application of high force. Cells treated with cytochalasin D or lacking Ena/VASP activity show similar levels of detachment relative to controls, but force strengthening is lost. Further, cells overexpressing VASP show stronger adhesion in response to low and high force, but adhesion weakening in response to ramped forces. These results indicate that force-mediated adhesion strengthening occurs in endothelial adherens junctions and that dynamic VASP activity is necessary for this process.
Keywords: Adherens junction, adhesion strengthening, actin, VE-cadherin, Ena/VASP
Introduction
Cell-cell junctions help to organize cells during development, differentiation, and wound healing, while loss of appropriate cell-cell adhesion is associated with a loss of differentiation and an invasive phenotype in tumorigenic cells [1-4]. These functions are regulated, in part, by force transmission between cells, as evidenced by abnormal development of load-bearing tissues such as the heart in the absence of key adhesion molecules [5, 6]. In addition, tension across adherens junctions appear to play a role in functions ranging from maintenance of epithelial barrier function and polarity [7, 8] to cell survival and proliferation [9, 10].
Cell-cell adhesions, like the somewhat more familiar cell-matrix adhesions, are dynamic structures that allow cells to interact with their external environment by providing biochemical signals as well as mechanical linkages between the outside and inside of a cell. Adherens junctions are defined by homophilic binding of transmembrane cadherins molecules on adjacent cells. Intracellularly, cadherins bind to numerous molecules involved in signaling, which provides the mechanical link to the actin cytoskeleton, again analogous to focal adhesions [11].
Given their functional similarity, it is not surprising that focal adhesions and adherens junctions share many linker molecules (e.g. vinculin, zyxin, VASP) and involvement of the force-responsive G proteins, Rho, Rac, and Cdc42 in formation and stabilization [12], while molecules such as α-catenin and β-catenin, are specific to adherens junctions [9, 13]. Both adherens junctions and focal adhesions follow a similar process of maturation involving actin polymerization and recruitment of intracellular linker proteins upon an initial binding event [14-17]. Structures resembling adherens junctions are formed when cadherin-coated beads are allowed to bind to cells, as evidenced by recruitment of the corresponding cadherins, β-catenin, F-actin, and Mena (a member of the Ena/VASP family) in various cells [18, 19]. Further it appears that a functional cadherin cytoplasmic tail, actin dynamics, and acto-myosin tension are all needed for formation of cell-cell junctions and to maintain their strength [19-21].
Once cadherins are engaged, adherens junctions exhibit time-dependent adhesion strengthening, much like focal adhesions. For example, while it took ∼20 nN to separate E-cadherin transfected cells after 30s of contact, a separation force of ∼200 nN was required after 1h of contact although the contact area remained virtually unchanged [16]. Disruption of actin dynamics and/or polymerization had no effect on the early separation force but decreased the force needed to separate the cells at later times [16]. Similarly, disruption of actin in endothelial cells, which express VE-cadherin, increased the number of VE-cadherin coated beads which detached under applied force [19]. Though there are many compositional similarities between focal adhesions and adherens junctions, both strengthen similarly with time, and whereas ample evidence points to focal adhesion strengthening with applied force [22], there is only indirect evidence that force-mediated adhesion strengthening occurs in adherens junctions [9].
Vasodilator-stimulated phosphoprotein (VASP) may be of particular interest in force-mediated adhesion strengthening of adherens junctions as it plays an important role in adherens junction formation and actin organization in epithelial cells [18, 23] and recent reports also suggest that Ena/VASP is necessary for the maintenance and strengthening of adherens junctions [8]. VASP localizes to cell-cell junctions, cell-matrix adhesions, stress fibers, and the lamellipodia of migrating cells and its activity is required for appropriate adhesion formation and associated actin polymerization [18, 23, 24]. Mice lacking members of the Ena/VASP family of proteins have increased endothelial permeability causing fatal vascular leakage and hemorrhaging during development [8]. Further, overexpression of VASP not only enhances barrier function of endothelial cells in vitro, it also increases their force generation [8]; loss of Ena/VASP activity causes increased permeability and decreased force generation [8].
The first goal of the present study was to use a VE-cadherin coated magnetic bead system to ask whether endothelial adherens junctions exhibit force-mediated adhesion strengthening by applying varying levels of force to beads coated with VE-cadherin and bound to the cells. Based on the available data on the roles of Ena/VASP in cell-cell junctions, we hypothesized that loss of Ena/VASP activity would result in a loss of adherens junction force strengthening. Further, based on the observation that VASP overexpression decreases endothelial permeability but increases force generation, we hypothesized that overexpression of VASP would enhance the strength of adherens junctions and also force strengthening. Our results indicate that cadherin contacts do indeed strengthen in response to applied force, and that changing Ena/VASP activity affects this process.
Materials and Methods
Cells
Human umbilical vein endothelial cells (HUVECs, Cascade Biologics, Portland, OR) were maintained in standard media (EBM-2, Lonza, Allendale, PA). HUVECs stably expressing GFP-VASP (VASP) or GFP-Mito-FPPPP (Mito) as previously described [8] were obtained from Drs. Craig Furman and Frank Gertler (MIT); GFP-VASP appears to be analogous to endogenous VASP with respect to localization and functionality, while GFP-Mito-FPPPP sequesters endogenous Ena/VASP proteins, effectively abrogating activity [8, 25]. For all experiments, cells (passage 5-7) were plated at subconfluence in collagen-coated 35-mm tissue culture petri dishes (10,000-20,000 cells per dish) and allowed to fully attach and recover (12-24 hours).
Magnetic Beads
The bead coating procedure was modified from Baumgartner et al. [26]. Briefly, paramagnetic beads coated in protein A (2.8 μm diameter, Dynal/Invitrogen, Carlsbad, CA) were rinsed in sodium phosphate buffer (100 mM, pH 8.1, from monosodium phosphate and disodium phosphate), incubated in 0.1 mg/ml Fc/VE-cadherin chimeric protein (calculated to correspond occupation of 25% of binding sites, R&D Systems, Minneapolis, MN) in sodium phosphate buffer for 3 minutes. Beads were then washed 3 times in triethanolamine (200 mM, pH 8.2, Sigma, St. Louis, MO), incubated in 5.4 mg/ml dimethyl pimelimidate dihydrochloride in triethanolamine to crosslink, washed twice for 30 minutes with 100 mM Tris (pH 7.5), and then 3 times in phosphate buffered saline (PBS, with calcium and magnesium). After coating, beads were stored in PBS for up to 3 months.
Magnetic trap
The magnetic trap was built as previously described [27]. Briefly, a CMI-C steel cylinder (161 mm-long and 20 mm diameter) was machined to have a 25° chiseled tip (44 mm long, 250 μm wide). The 72 mm-long core was wrapped in eight layers of 18-gauge enamel-coated copper wire to create a magnetic coil with ∼400 total turns. Current was provided to the coil by a power supply (PSP-603, Instek, Taiwan). The trap was mounted on a micromanipulator to control its position and the micromanipulator and microscope (Zeiss Axiovert 200) were mounted on a pneumatic vibration isolation table to minimize forces from other sources.
Calibration was performed by tracking the movement of polystyrene beads suspended in dimethylpolysiloxane with a kinematic viscosity of 12,500 centistokes (0.98 g/cm3, DMPS-12M, Sigma) in response to currents applied to the trap in 0.5 A steps from 1.0 to 3.5 A. Bead motion was recorded by a high-speed camera (25 fps, PCO.1200, PCO, Germany) at 50X magnification and then analyzed with particle-tracking software written in MATLAB (Mathworks, Natick, MA, software generously provided by Dr. Jan Lammerding) to obtain velocities. For a given velocity (u), force (F) was calculated using Stokes' equation, F = 3 πμDu, where μ is dynamic viscosity and D is bead diameter. Force vs. position data were then fit to the phenomenological power law, F = axb + c. The coefficients (n = 3 trials for 1.0 A and 3.5 A, n =1 trial for intermediate current values) were averaged to give current-force-distance relationships used for experiments.
Bead detachment experiments
Approximately 10,000 VE-cadherin coated beads were added to adherent HUVECs in 1.2 mL media and placed in the incubator (∼10 beads/mm2). In drug studies, cytochalasin D was added to culture media (200 nM) during bead incubation; this concentration has previously been shown to disrupt the actin cytoskeleton and remove most actin bundles [28]. After 45 minutes in the incubator, cell dishes were placed on a preheated aluminum plate connected to resistive heaters on the microscope stage to maintain the media at 37°C. To test for bead adhesion, cells which were not in contact with other cells and with only one magnetic bead associated with them were identified under the microscope and force of <1 pN was applied for 5s (current of 1.0 A at a distance of approximately 250 μm from the bead); beads that did not appear to move in response to this force were considered sufficiently attached for the experiment.
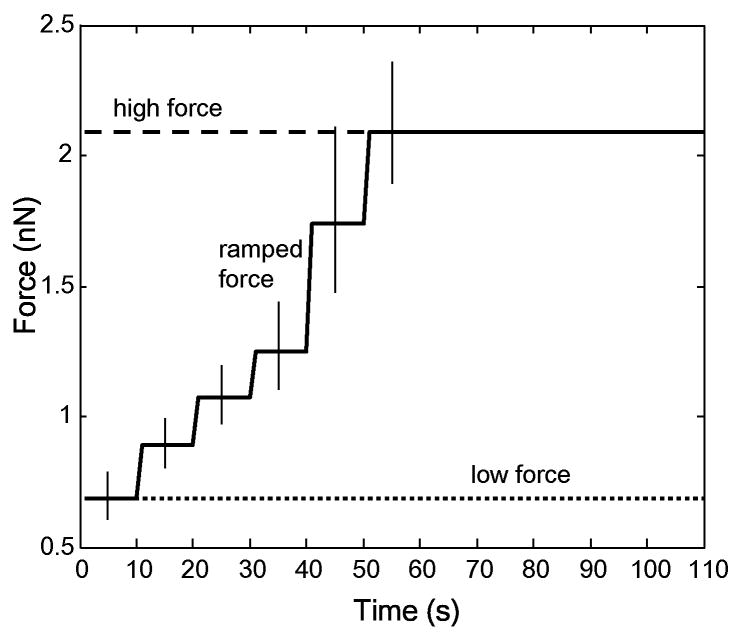
For adhesion studies, magnetic trap tip was brought parfocal to and 30 μm away from a bead and one of three force conditions was applied: 1) low force of 0.7 nN (via application of 1.0 A current) for 110 seconds, 2) high force of 2.1 nN (via application of 3.5 A current) for 110 seconds, or 3) ramped force from 0.7 to 2.1 nN via a 0.5 A increment in current every 10 seconds over 50 (corresponding to 0.7, 0.9, 1.1, 1.2, 1.7, and 2.1 nN) and then held at 2.1 nN for 60 seconds (Fig. 1). Fisher's one-tailed exact test was used to examine significance of differences between detachment rates. The application of currents for the specified periods of time was controlled by MATLAB via RS-232 serial communication. The attachment state of each bead was noted throughout the course of the experiment, with the time of detachment recorded in cases where detachment occurred; detached beads were easily identified and rapidly moved towards the tip. Subsequent tests were conducted on cells that were at least 500 μm away from previously tested cells to avoid confounding pre-stressing forces from previous tests. Each dish was used for a maximum of 30 minutes to minimize the effects of lowered CO2. Beads coated only with Protein A were unable to withstand 0.7 nN of force, indicating specific binding of VE-cadherin coated beads.
Figure 1.
Low, high, and ramped force profiles used for cell studies. Different force levels were obtained by holding the magnetic trap tip 30 μm from a bead attached to a cell and choosing currents based on trap calibration. The low and high force profiles (dotted and dashed lines respectively) were application of 0.7 nN or 2.1 nN for 110 s (corresponding to 1.0 A and 3.5 A of current, respectively). The ramped profile increased from 0.7 nN to 2.1 nN by increasing the current 0.5 A every 10s (corresponding step values are 0.7, 0.9, 1.1, 1.2, 1.7, and 2.1 nN) for 60s and then holding at 2.1 nN for the remaining 50s. For each of the six different force levels, error bars show forces correlating to a ±5 μm difference from 30 μm in placement of the magnetic trap. Force-current relationships are based on bead calibration in dimethylpolysiloxane (see Methods).
Results and Discussion
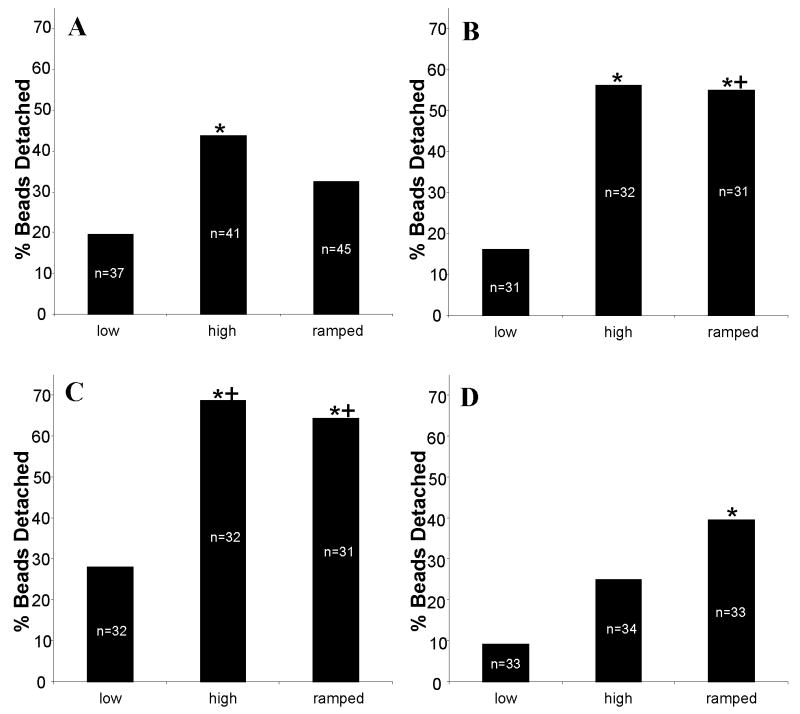
Magnetic beads coated with VE-cadherin were allowed to settle and bind to human umbilical vein endothelial cells (HUVECs) for 45 minutes and then force was applied to individual beads via a magnetic trap. Low (0.7 nN), high (2.1 nN), and ramped (0.7 to 2.1 nN) force profiles were applied (Fig. 1) in order to assess whether force-mediated adhesion strengthening was occurring (n > 30 beads per condition). As expected, a greater number of beads detached when high force (44%, p<0.005) versus low force (19%) was applied for 110 s (Fig. 2a). Greater than 99% of beads that detached did so within the first 50s of force application (not shown). If adherens junctions exhibit force-mediated adhesion strengthening, ramping the force from low to high should strengthen the binding between the cell and bead and result in a decrease in detachment with application of high force. Consistent with this prediction, fewer beads detached (32%, not significant), relative to high force conditions, when force was ramped from low to high levels over 50 s and then held at 2.1 nN for 60 s (Fig. 2a).
Figure 2.
Percent detachment of VE-Cadherin coated beads on control (A), cytochalasin D treated (B), Ena/VASP inactivated (Mito, C), or VASP overexpressing (D) HUVEC in response to low (0.7 nN), high (2.1 nN), and ramped (from 0.7 nN to 2.1 nN over 50s of applied force). A decrease in the number of beads detaching between high and ramped force conditions would be consistent with force strengthening (A). Disruption of the actin cytoskeleton or inactivation of Ena/VASP blocks force strengthening (B, C). While overexpression of VASP appears to slightly increase basal binding strength, it also leads to weaker adhesions in the presence of ramped force (D). For a given cell type, significant difference to low force, determined with Fisher's one-tailed exact test (p<0.005), is indicated with (*). Significant difference (p<0.05) from the same force profile for control HUVECs is indicated with (+).
The level of force required to detach beads can be compared with other experiments. For the present study, if we assume that ∼5% of the bead surface area is in contact with the cell [29], the force applied to the VE-cadherin mediated adhesion is ∼0.6-1.7 nN/μm2 for low to high force profiles. Though different cell systems and different cadherins may exhibit a range of binding strengths, this value is of the same order as the ∼4 nN/μm2 required to separate cells transfected to express E-cadherin after 1 hour (comparable to the 45 minute adhesion time in the present study) [16]. It is also in the range of traction forces measured by N-cadherin expressing cells (∼5 nN) on polydimethylsiloxane posts [30]. These comparisons support the idea that mature adherens junction type adhesions are forming between the endothelial cells and the VE-cadherin coated beads in our studies.
An intact actin network and functional dynamics are necessary for force-mediated adhesion strengthening of focal adhesions [22] and for time-dependent strengthening of adherens junctions [16]. Thus, we predicted that disrupting actin by treating cells with cytochalasin D (200 nM) would reduce force-mediated adhesion strengthening of adherens junctions. With cytochalasin D treatment, 16% of beads detached in response to low force (Fig. 2B). The observation that similar numbers of beads under control conditions detached at low force suggests that basal adhesion through VE-cadherin was not greatly affected by the application of cytochalasin D. Significantly more (56%, p<0.005) beads detached under high force with cytochalasin D treatment (Fig. 2B), consistent with previous reports [19]. However, adhesion strengthening appears to have been disrupted by cytochalasin D treatment as application of ramping force resulted in detachment of 55% of beads, similar to that seen with high force (Fig. 2B, p<0.005 relative to low force).
To study the role of Ena/VASP proteins in adhesion strengthening, HUVECs lacking VASP activity (Mito) or overexpressing VASP (VASP) were investigated. Consistent with increased permeability in vessels and cultured endothelial cells lacking VASP activity [8], bead detachment was increased in Mito cells relative to control HUVECs under all conditions (Fig. 2C). Twenty-eight percent, 69%, and 65% of beads detached from Mito cells under low, high, and ramped force profiles, respectively (high and ramped profiles both significantly different from low, p<0.05). The detachment profile for VASP cells appeared to be similar to that of cytochalasin-treated HUVECs, indicating that loss of VASP did not greatly disrupt the VE-cadherin mediated adhesion, but it also prevented adhesion strengthening.
Given that VASP overexpression decreases permeability [8], likely via increased cell-cell adhesion strength, we predicted that bead adhesion strength would be increased in VASP-overexpressing cells. Consistent with this, we observed that beads detachment was slightly decreased (p=0.054 for high force) relative to control cells in VASP-overexpressing cells under low (9%) or high (24%) force application (Fig. 2D). Based on this apparent increased basal adhesion strength, and the observation that loss of VASP activity inhibited adhesion strengthening, we hypothesized that overexpression of VASP would enhance the adhesion strengthening effect. However, somewhat surprisingly, under ramped force conditions VASP overexpression led to a further increase in bead detachment (39%, Fig. 2D, significantly different from low force, p<0.005). Under these conditions, VASP overexpression appears to have reversed the cells' ability to respond to dynamic changes in force application and led to a weakening of adhesion with ramping force.
A similarly paradoxical effect of VASP has been seen in the context of studying the effect of VASP overexpression on fibroblast migration. VASP increased lamellipodial ruffling, typically thought to promote migration and presumably due to increased polymerization caused by VASP anti-capping activity [25]. However, it also caused a decreased average migration speed due to decreased persistence [25]. In the present study, it is possible that increased actin dynamics due to VASP activity, in combination with force on the adherens adhesion, leads to instability or a slower response which leads to the apparent adhesion weakening. This observation is intriguing given the increased expression of VASP seen in lung adenocarcinoma cells [31]; adhesion weakening in these cells could contribute to the loss of polarity and cell-cell contacts associated with a tumorigenic phenotype.
In summary, we demonstrate here an actin-dependent, VE-cadherin-mediated adhesion strengthening in HUVECs. Though only demonstrated for one specific cadherin, given the importance of tension in maintaining adherens junctions and tissue architecture [1, 6, 21], it is likely that adhesion strengthening occurs in adherens junctions containing other types of cadherins. We also expand upon previously published results showing that Ena/VASP proteins are involved in adherens junction formation [8, 23] by demonstrating that Ena/VASP activity is necessary for force strengthening of endothelial adherens junctions. However, as shown by the somewhat surprising results obtained with VASP overexpressing cells, the role of VASP in basal adhesion strength force strengthening may be complex.
Acknowledgments
The authors would like to thank Jan Lammerding and Hayden Huang for their assistance building the magnetic trap, David Quinn for his assistance with the microscope and camera setup, and Craig Furman and Frank Gertler for cells and stimulating discussion on Ena/VASP. This work was partially funded by a NIBIB research grant from the National Institutes of Health (EB003805).
References
- 1.Mege RM, Gavard J, Lambert M. Regulation of cell-cell junctions by the cytoskeleton. Curr Opin Cell Biol. 2006;18:541–548. doi: 10.1016/j.ceb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 2.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 3.Yap AS, Kovacs EM. Direct cadherin-activated cell signaling: a view from the plasma membrane. J Cell Biol. 2003;160:11–16. doi: 10.1083/jcb.200208156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zanetta L, Corada M, Grazia Lampugnani M, Zanetti A, Breviario F, Moons L, Carmeliet P, Pepper MS, Dejana E. Downregulation of vascular endothelial-cadherin expression is associated with an increase in vascular tumor growth and hemorrhagic complications. Thromb Haemost. 2005;93:1041–1046. doi: 10.1160/TH04-10-0680. [DOI] [PubMed] [Google Scholar]
- 5.Ko KS, McCulloch CA. Intercellular mechanotransduction: cellular circuits that coordinate tissue responses to mechanical loading. Biochem Biophys Res Commun. 2001;285:1077–1083. doi: 10.1006/bbrc.2001.5177. [DOI] [PubMed] [Google Scholar]
- 6.Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- 7.Braga VM, Del Maschio A, Machesky L, Dejana E. Regulation of cadherin function by Rho and Rac: modulation by junction maturation and cellular context. Mol Biol Cell. 1999;10:9–22. doi: 10.1091/mbc.10.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furman C, Sieminski AL, Kwiatkowski AV, Rubinson DA, Vasile E, Bronson RT, Fassler R, Gertler FB. Ena/VASP is required for endothelial barrier function in vivo. J Cell Biol. 2007;179:761–775. doi: 10.1083/jcb.200705002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- 10.Iurlaro M, Demontis F, Corada M, Zanetta L, Drake C, Gariboldi M, Peiro S, Cano A, Navarro P, Cattelino A, Tognin S, Marchisio PC, Dejana E. VE-cadherin expression and clustering maintain low levels of survivin in endothelial cells. Am J Pathol. 2004;165:181–189. doi: 10.1016/s0002-9440(10)63287-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 12.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114:1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 13.Vincent PA, Xiao K, Buckley KM, Kowalczyk AP. VE-cadherin: adhesion at arm's length. Am J Physiol Cell Physiol. 2004;286:C987–997. doi: 10.1152/ajpcell.00522.2003. [DOI] [PubMed] [Google Scholar]
- 14.Lotz MM, Burdsal CA, Erickson HP, McClay DR. Cell adhesion to fibronectin and tenascin: quantitative measurements of initial binding and subsequent strengthening response. J Cell Biol. 1989;109:1795–1805. doi: 10.1083/jcb.109.4.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallant ND. Mechanical Engineering. Georgia Institute of Technology; Atlanta: 2004. [Google Scholar]
- 16.Chu YS, Thomas WA, Eder O, Pincet F, Perez E, Thiery JP, Dufour S. Force measurements in E-cadherin-mediated cell doublets reveal rapid adhesion strengthened by actin cytoskeleton remodeling through Rac and Cdc42. J Cell Biol. 2004;167:1183–1194. doi: 10.1083/jcb.200403043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galbraith CG, Yamada KM, Sheetz MP. The relationship between force and focal complex development. J Cell Biol. 2002;159:695–705. doi: 10.1083/jcb.200204153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scott JA, Shewan AM, den Elzen NR, Loureiro JJ, Gertler FB, Yap AS. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol Biol Cell. 2006;17:1085–1095. doi: 10.1091/mbc.E05-07-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgartner W, Schutz GJ, Wiegand J, Golenhofen N, Drenckhahn D. Cadherin function probed by laser tweezer and single molecule fluorescence in vascular endothelial cells. J Cell Sci. 2003;116:1001–1011. doi: 10.1242/jcs.00322. [DOI] [PubMed] [Google Scholar]
- 20.Navarro P, Caveda L, Breviario F, Mandoteanu I, Lampugnani MG, Dejana E. Catenin-dependent and -independent functions of vascular endothelial cadherin. J Biol Chem. 1995;270:30965–30972. doi: 10.1074/jbc.270.52.30965. [DOI] [PubMed] [Google Scholar]
- 21.Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res. 2006;312:1637–1650. doi: 10.1016/j.yexcr.2006.01.031. [DOI] [PubMed] [Google Scholar]
- 22.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin- cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 23.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 24.Krause M, Dent EW, Bear JE, Loureiro JJ, Gertler FB. Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu Rev Cell Dev Biol. 2003;19:541–564. doi: 10.1146/annurev.cellbio.19.050103.103356. [DOI] [PubMed] [Google Scholar]
- 25.Bear JE, Svitkina TM, Krause M, Schafer DA, Loureiro JJ, Strasser GA, Maly IV, Chaga OY, Cooper JA, Borisy GG, Gertler FB. Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell. 2002;109:509–521. doi: 10.1016/s0092-8674(02)00731-6. [DOI] [PubMed] [Google Scholar]
- 26.Baumgartner W, Golenhofen N, Grundhofer N, Wiegand J, Drenckhahn D. Ca2+ dependency of N-cadherin function probed by laser tweezer and atomic force microscopy. J Neurosci. 2003;23:11008–11014. doi: 10.1523/JNEUROSCI.23-35-11008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lammerding J. Biological Engineering Division. MIT; Cambridge: 2004. [Google Scholar]
- 28.Chen J, Fabry B, Schiffrin EL, Wang N. Twisting integrin receptors increases endothelin-1 gene expression in endothelial cells. Am J Physiol Cell Physiol. 2001;280:C1475–1484. doi: 10.1152/ajpcell.2001.280.6.C1475. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Sylvan J, Jonas M, Barresi R, So PT, Campbell KP, Lee RT. Cell stiffness and receptors: evidence for cytoskeletal subnetworks. Am J Physiol Cell Physiol. 2005;288:C72–80. doi: 10.1152/ajpcell.00056.2004. [DOI] [PubMed] [Google Scholar]
- 30.Ganz A, Lambert M, Saez A, Silberzan P, Buguin A, Mege RM, Ladoux B. Traction forces exerted through N-cadherin contacts. Biol Cell. 2006;98:721–730. doi: 10.1042/BC20060039. [DOI] [PubMed] [Google Scholar]
- 31.Dertsiz L, Ozbilim G, Kayisli Y, Gokhan GA, Demircan A, Kayisli UA. Differential expression of VASP in normal lung tissue and lung adenocarcinomas. Thorax. 2005;60:576–581. doi: 10.1136/thx.2004.037622. [DOI] [PMC free article] [PubMed] [Google Scholar]