Abstract
Leishmaniasis is a tropical disease caused by Leishmania, eukaryotic parasites transmitted to humans by sand flies. Towards the development of new chemotherapeutic targets for this disease, biochemical and in vivo expression studies were performed on one of two M32 carboxypeptidases present within the Leishmania major (LmaCP1) genome. Enzymatic studies reveal that like previously studied M32 carboxypeptidases, LmaCP1 cleaves substrates with a variety of C-terminal amino acids - the primary exception being those having C-terminal acidic residues. Cleavage assays with a series of FRET-based peptides suggest that LmaCP1 exhibits a substrate length restriction, preferring peptides shorter than 9–12 amino acids. The in vivo expression of LmaCP1 was analyzed for each major stage of the L. major life cycle. These studies reveal that LmaCP1 expression occurs only in procyclic promastigotes – the stage of life where the organism resides in the abdominal midgut of the insect. The implications of these results are discussed.
Keywords: Leishmania, M32 carboxypeptidase, stage-specific expression, metalloprotease
INTRODUCTION
M32 carboxypeptidases are metalloproteases that exhibit a distinct overall fold and sequence – the most notable being a conserved HEXXH metal binding motif [1–3]. The first M32 member identified was isolated from thermophilic bacteria Thermus aquaticus (TaqCP) and was demonstrated to be a carboxypeptidase by steady state assays on a variety of peptide substrates [1]. Subsequent BLAST [4] searches have identified more than 300 M32 family members in species spanning all three kingdoms of life.
One of these other members is the M32 carboxypeptidase from the hyperthermophilic archaeon Pyrococcus furiosus (PfuCP). Among PfuCP’s notable features is an unusually high Topt (~100°C), and requirement for cobalt as its active site metal [2]. We recently determined the crystal structure of PfuCP providing the first picture for how M32 carboxypeptidases might cleave their peptide substrates [3]. The most striking feature of the PfuCP structure was the presence of a deep substrate groove spanning the length of one subunit. The presence of this unusual groove led us to hypothesize that M32 carboxypeptidases might exhibit a substrate length restriction - a property would have novel functional and mechanistic implications.
The M32 member we chose for our initially study of this issue was the Leishmania major M32 carboxypeptidase (LmaCP1) - one of two M32 carboxypeptidase genes in the L. major genome. Our hope was that in addition to helping us to address whether M32 carboxypeptidases exhibit a length restriction, the characterization of this enzyme would allow us explore the potential for M32 carboxypeptidases as targets for antiparasitic and antibacterial chemotherapies. LmaCP1 was also attractive as being the only eukaryotic M32 member identified at the time of our study. Recently, Trypanosoma cruzi (associated with Chagas disease) has also been shown to encode two M32 carboxypeptidases [9]. Both Leishmania and Trypanosoma are members of trypanosomatidae; a family of protozoan blood parasites transmitted by insects.
Human infection by Leishmania leads to leishmaniasis, a tropical disease afflicting over 12 million people worldwide (http://www.who.int/leishmaniasis/en/). Leishmaniasis has various forms depending on the tissues targeted: cutaneous (skin), mucosal (internal mucosal tissues), and visceral (internal organs) [10]. Leishmania are transmitted to humans by sand flies. In the sand fly, Leishmania live as promastigotes, an extracellular motile stage in which the cell adopts an elongated shape with a flagellar tail. When an infected sand fly takes a bloodmeal, the promastigotes are passed to the host’s bloodstream where they are eventually phagocytized by the host’s macrophages. Once in a macrophage, the Leishmania promastigotes transform into a distinct intracellular non-motile form called an amastigote - a form that is presumably better suited for survival in the macrophage. The amastigotes multiply in the macrophage, and when a different sand fly bites the now infected host, the amastigote-containing macrophages are taken up into the insect’s midgut together with the rest of the blood mass. Digestion of the macrophage releases the Leishmania amastigotes, at which point, the parasite begins its slow transformation back to a promastigote - ready to renew the life cycle again [11, 12].
Herein, we describe our progress on the characterization of the biochemical and functional properties of LmaCP1 in vivo and in vitro. We demonstrate that LmaCP1 exhibits a substrate length restriction and that it exhibits broad substrate specificity similar to previously characterized M32 carboxypeptidases. We also discuss LmaCP1’s potential limitations as a target of antibacterial chemotherapies based on its stage specific expression, and suggest a potential functional role for LmaCP1 and other M32 carboxypeptidases in pathogens.
MATERIAL AND METHODS
Cloning, Expression and Purification
The gene for one of the two M32 carboxypeptidases from L. major (GeneID:5650034) was amplified by PCR from the PAC clone P1046 (a generous gift from Prof. A. Ivens’s, Sanger Institute, United Kingdom). The gene was cloned into pET-28b(+) vector with an N-terminal His tag and overexpressed in E. Coli BL21 (DE3)-RP. In a typical preparation, 1 L of LB media containing 1% glucose, 17 µg/ml chloramphenicol, and 30 µg/ml kanamycin was inoculated with 20 mL of overnight culture and shaken at 30°C. Expression was induced by adding 0.1 mM IPTG when the absorbance reached 0.6 O.D (600 nm). The cells were harvested after 7 hours yielding ~2.2 g of cells that were stored at −80°C.
As the first step of the protein purification, the frozen cells were thawed on ice and resuspended in 20 mL of lysis buffer (50 mM Tris-HCl pH 8.0, 300 mM NaCl, 10 µM APMSF [(4-amidinophenyl)-methanesulfonyl fluoride]. To aid in breaking the cell wall, 20 mg of lysozyme was added and the solution was incubated on ice for 30 minutes. The cell suspension was then sonicated and cell debris removed by centrifugation at 15,000×g for 35 min at 4 °C. The supernatant was loaded onto a Ni-NTA column with loading buffer (50 mM Tris-HCl pH 8.0, 300 mM NaCl), and washed extensively with washing buffer (50 mM Tris-HCl pH 8.0, 300 mM NaCl, 25 mM imidazole). The His-tagged protein was collected with 40 ml of eluting buffer (50 mM Tris-HCl pH 8.0, 300 mM NaCl, 250 mM imidazole), and then concentrated and buffer exchanged with 50 mM Tris-HCl pH 8.0 buffer by ultrafiltration using a YM10 membrane. Aliquots of LmaCP1 were frozen with liquid nitrogen and stored at −80°C.
Amino acid specificity of LmaCP1 towards ZAX substrates
The activity of LmaCP1 was measured for a series of ZAX substrates (where ZA = benzyloxycarbonyl-protected alanine and X = one of the following amino acids: K, R, H, W, Y, F, I, V, A, M, S, N, or E). The reactions were carried out at 37°C by mixing 100 µL of substrate (in 0.1 M HEPES-NaOH pH 7.5) at concentrations ranging from 0 to 15 mM, with 4 µL of 2 µM LmaCP1 solution. Different time point readings were taken from 0 to 15 minutes. To determine the rate of cleavage, the amount of new amino groups formed was calculated using a ninhydrin assay [13]. The concentration of new amino groups formed was obtained by comparing the absorbance of the quenched reaction solutions with calibration curves prepared for the corresponding free amino acid. The cleavage rates at different substrate concentrations were used to obtain the Lineweaver-Burke plots from which the Vmax and KM were calculated.
FRET peptide length dependence assay
The amino acid N-3-(2,4-dinitrophenyl)-L-2,3-diamino propionyl (Dpa) was synthesized according to literature procedures [14]. Three FRET-based peptides of different lengths [8-mer (ASGK-Dpa-AAW), 12-mer (ASGKASGK-Dpa-AAW), and 16-mer (ASGKASGKASGK-Dpa-AAW)] were prepared by the batchwise Fmoc-polyamide method on a PS3 peptide synthesizer using pre-loaded Wang resin. After synthesis, the peptides were deprotected and cleaved from the resin by stirring with a mixture of 10 mL TFA, 0.75 g phenol, 0.5 ml thioanisol, 0.5 mL water and 0.25 mL ethanedithiol at rt for 1–2 hours and precipitated with methyl t-butyl ether at 2000×g for 10 minutes three times and dried in vacuo. The resulting white powder was purified by reverse-phase HPLC using a Vydac C18 column (10 mm × 250 mm) and acetonitrile-water mixtures containing 0.1% TFA (v/v). The final products were analyzed by positive ion-electrospray mass spectroscopy and then stored at −20°C.
Because the Dpa group quenches the inherent fluorescence of the C-terminal Trp, the cleavage of each peptide could be monitored by measuring the increase in the Trp fluorescence (λexcitation = 290 nm, λemission = 370 nm). The cleavage assays were performed with 150 µL of 0.3 mM substrate in 0.1M HEPES-NaOH pH 7.5 buffer and 1.2 µL of 0.17 mM enzyme solution at 37°C. The substrate length preferences of LmaCP1 could be determined by comparing the relative rate of cleavage of each peptide.
Preparation and isolation of L. major amastigotes
Following a procedure adapted from Chakrabarti et al. [15], two popliteal lymph nodes from mice with footpad lesions were used (6 to 8 weeks after infection with 2*106 L. major (LV39) promastigotes). To release the amastigotes, the mouse tissue was gently macerated in phosphate buffer solution (10 mM KH2PO4/K2HPO4 pH 7.0, 150 mM NaCl, 2 mM EDTA, and 5.5 mM glucose). The mixture was initially centrifuged at 200×g for 10 minutes at 20°C to remove unwanted larger aggregates, and then the supernatant was spun down at 3000×g for 10 min at 20°C to yield the pellet containing the L. major parasites. The desired amastigote cells were further purified via a density gradient. This involved reconstituting and dissolving the pellet in 4 mL of 45% Percoll solution. The density gradient was created by first adding 1.5 mL of 70% Percoll to the bottom of a centrifuge tube, layering the 4 mL of parasite solution followed by 4.5 mL of 25% Percoll solution on top, and then centrifuging the resulting sealed tube at 2500×g at 4°C for one hour. After centrifugation, three distinct layers were observed. Most of the Leishmania amastigotes were confined to the bottom layer (more than 90% of the cells present being amastigotes). This bottom layer was separated, and then diluted with 5 volumes of PBS buffer (0.9% NaCl, 10 mM sodium phosphate buffer pH 7.2) per volume of Percoll solution and centrifuged at 2450×g at 4°C for 10 min. The resulting pellet of amastigote cells was used for the subsequent protein extractions.
To obtain the soluble amastigote proteins, the pellet was washed by resuspension in PBS buffer (pH = 7.4) and then centrifuged at 500×g for 10 min. The supernatant was removed, and the cells were lysed by resuspension in 400 µL lysis buffer (10% Triton X-100, 1M Tris-HCl pH 8.0, 4 M NaCl, 10% glycerol, 2% Protease arrest, 2 mM NaVO4) and incubation on ice for 10 min. The cell debris was removed by centrifugation (15 min., 4°C, 14000 rpm) yielding the amastigote soluble protein extract which was subsequently stored at −80°C.
Preparation and isolation of L. major promastigotes
L. major (LV39) was maintained by serial passage of amastigotes inoculated subcutaneously into the shaven rumps of BALB/c mice [16]. Amastigotes isolated from the skin lesions of infected mice were grown to stationary phase promastigotes as described previously [17].
Preparation and separation of L. major procyclic and metacyclic promastigotes
To obtain isolated procyclic and metacyclic promastigotes, freshly harvested promastigotes were grown for six days in M199 tissue culture medium supplemented with 1% fetal calf serum. The separation was done using the peanut agglutinin (PNA) method [18]. The parasites were washed twice in 50 mL DMEM (Dulbecco’s minimal essential medium) followed by centrifugation (15 min, 4°C, 3000×g). The pellet was resuspended in DMEM and the parasite count was brought to 2*108/mL. PNA was added to the parasite solution to a final concentration of 75 µg/mL PNA. The parasite-PNA mixture was incubated for 30 min at room temperature. After incubation, the mixture was centrifuged (5 min., 4°C, 200×g). The metacyclic promastigotes were recovered from the supernatant and washed twice by centrifugation (15 min, 4°C, 3000×g). Similarly, the pellet with the procyclic promastigotes was washed twice by resuspension in DMEM buffer followed by centrifugation (15 min, 4°C, 3000×g). The soluble protein extract was obtained as described for the amastigote cells.
Analysis of LmaCP1 expression
Western blot analysis was performed as described previously [19].
RESULTS
Substrate length dependence of LmaCP1
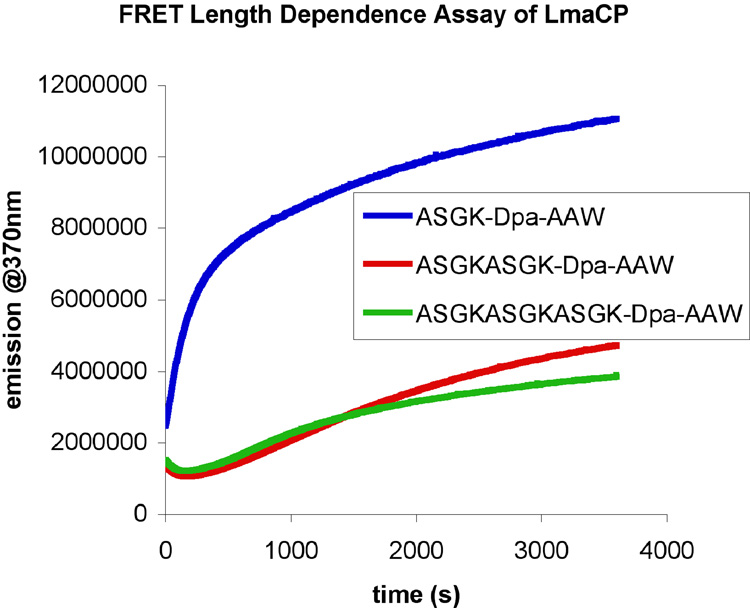
Based on the structure of PfuCP [3], we predicted that M32 carboxypeptidases might exhibit a substrate length restriction. To test this hypothesis, a set of three peptides of different lengths (8-mer, 12-mer, and 16-mer) were designed and synthesized to evaluate the length dependence of LmaCP1 using a FRET-based assay. Comparison of the activity of LmaCP1 for the three peptides (Fig. 1) reveals that the longer 12-mer and 16-mer peptides display a much slower rate of cleavage than the 8-mer peptide - consistent with the length restriction predicted from the PfuCP and LmaCP1 structures.
Fig. 1.
FRET data for LmaCP1 mediated cleavage of 8-mer, 12-mer, and 16-mer peptide substrates.
Amino acid specificity of LmaCP1 towards ZAX substrates
To facilitate a direct comparison of LmaCP1 specificity with TaqCP [21] and PfuCP [2], the same procedures and substrates were used. The activity of LmaCP1 towards a series of ZAX substrates was determined by monitoring the free amino groups produced using a ninhydrin assay. The Vmax and kcat/KM values are shown in Supporting Table 1. The data reveals that like PfuCP and TaqCP, LmaCP1 has broad substrate specificity for ZAX peptides containing a wide variety of C-terminal amino acids.
One difference exhibited by LmaCP1 as compared to its thermophilic (TaqCP) and hyperthermophilic (PfuCP) counterparts is its 100-fold lower activity at physiological temperature (37°C). Similar differences for other mesophilic and thermophilic counterparts, however, have been described in the literature [22]. Presumably, the rate of peptide hydrolysis is faster at higher temperatures due to the greater ease in overcoming the activation barrier at higher temperature. Consistent with this evaluation, the kinetic parameters of LmaCP1 compare well to other mesophilic proteases. The kcat (12 s−1) of LmaCP1 for the cleavage of ZAR substrate is within the range of values reported for a structural homologue, rat neurolysin (kcat ~ 1 to 15 s−1 depending on the substrate) [23], and a functional homologue, carboxypeptidase B (CPB) (kcat = 60 s−1 for the cleavage of Cbz-Gly Arg) [24].
Exploring the stage specific expression of LmaCP1 in L. major
Given the two distinct stages of Leishmania, amastigotes in the mammalian host and promastigotes in the sand fly vector, we decided to explore the stage specific expression of the LmaCP1 in L. major to see if would provide any clues into its function. Towards this end, amastigote and promastigote cells were grown and isolated. Western blotting was used to monitor expression of LmaCP1 using antibodies grown against purified LmaCP1. These studies revealed that LmaCP1 appears to be specifically expressed in promastigote cells (Fig. 2A). No LmaCP1 protein was oberved in amastigotes isolated from mice lymph cells. Notably, given the absence of LmaCP1 from amstigotes in our stage specific expression profiles, it is clear that LmaCP1 cannot play a role in antigen degradation within the mammalian host. Instead, presence of LmaCP1 in promastigotes suggests a functional role for LmaCP1 during life cycle of Leishmania in the sand fly.
Fig. 2.
Comparision of LmaCP1 expression in (A) L. major promastigotes and amastigotes; and in (B) procyclic and metacyclic promastigotes.
The stage-specific expression of LmaCP1 could be further narrowed by taking advantage of the fact that promastigotes undergo a series of transformations during their life cycle in the insect. When an insect bites an infected host, macrophages are taken in with the blood meal and fill the insect abdominal midgut. During the first several days (0–4), the amastigotes contained in the host macrophages slowly convert to procyclic promastigotes that are ideally suited for surviving in the toxic environment required to digest the blood meal in the insect abdominal midgut. The procyclic promastigotes divide rapidly. After 4–7 days, the procyclic promastigotes convert to nectomonads that attach their flagella to the epithelial cell microvilli and then slowly move up to the thoracic midgut where they transform to metacyclic promastigotes, which are the non-dividing infective form of Leishmania parasites [11, 12].
Utilizing procedures reported in the literature, infectious and noninfectious promastigotes were separated from the total promastigote sample using PNA agglutination [18]. The expression of LmaCP1 in infectious and noninfectious promastigotes was then compared by Western blotting. These data, shown in Fig. 2B, reveal that LmaCP1 is solely expressed in noninfectious procyclic promastigotes and not in infectious metacyclic promastigotes. These findings suggest that LmaCP1 is unlikely to play a role in infection of the host and is likely involved in a process in the sandfly midgut stage of the parasite.
DISCUSSION
A proposed role for LmaCP1 and other pathogenic M32 carboxypeptidases
Based on LmaCP’s biochemical properties, and its stage-specific expression, we suggest that LmaCP1 serves a role in peptide catabolism. Biochemical support for this possibility comes from LmaCP1’s demonstrated length restriction and broad substrate specificity. The 8-to-11-mer peptide preference of LmaCP1 would make it ideally suited to target the downstream products of proteasomes and other ATP-dependent proteases, while its broad specificity would impart it with the ability to cleave multiple peptide products. While one concern is that LmaCP1 has poor specificity towards substrates with certain C-terminal amino acids, this concern is potentially resolved by the presence of the second M32 carboxypeptidase in L. major (GENE_ID: 5654678), LmaCP2 (with a 54% identity, and 71% similarity to LmaCP1). In support of this possibility, recent studies have shown that Trypansoma cruzi harbors two M32 carboxypeptidases, TcMCP-1 and TcMCP-2 that exhibit broad but distinct substrate specificities and that have different stage specific expression [9]. We have recently shown that the C-terminal M3 dipeptidase, Escherichia coli oligopeptidase A (OdpA), is capable of cleaving the oligopeptidase products of E. coli Lon, HslUV, and ClpXP suggesting its role as a general protease downstream of these ATP-dependent proteases [25]. Given that OdpA is a structural homolog of M32 carboxypeptidase, it is also plausible that LmaCP1 evolved to degrade the dipeptide products of the related M3 dipeptidases to their single amino acids in Leishmania.
The stage specific expression of LmaCP1 is also consistent with its role in peptide degradation. When the parasites are in the abdominal midgut, soon after ingestion, they are confined by the peritrophic membrane (PM) that is secreted by the fly’s midgut epithelium, building a cylindrical compartment that completely surrounds the blood mass [26]. The PM consists of a network of chitin in a matrix composed of proteins and proteoglycans [27]. Initially the PM serves as protection for the promastigotes by delaying their encounter with the fly’s digestive enzymes. When promastigotes are ready to leave the abdominal midgut, they secrete several enzymes to break the PM leaving a food mass presumably rich in peptide nutrients. At this stage, the parasites are rapidly growing and dividing [28], and thus would have a strong demand for free amino acids. LmaCP1 working together with proteasomes, ATP-dependent proteases, and other peptidases could facilitate this growth by helping to facilitate the rapid catabolism of peptides and proteins to the single amino acids required for protein synthesis.
Intriguingly, analysis of the pathogenic organisms (Helicobacter pylori, Trypanosoma cruzi, Rickettsia rickettsii, Vibrio cholera, Yersina pestis) with M32 carboxypeptidase genes reveals that many also exist in digestive environments. Like L. major, T. cruzi, R. rickettsii, and Y. pestis all spend part of their life cycle in the stomach of their insect vector, while H. pylori, V. cholera, and Y. pestis infect the stomach and digestive systems in humans. This commonality suggests a potentially general role for pathogenic M32 carboxypeptidases in protein degradation.
Supplementary Material
ACKNOWLEDGEMENTS
These laboratories are supported by NIH grants GM061796 and AI51823 and NSC 93-2113-M-001-025 from Taiwan. We thank Dr. Rinku Jain for her helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- 1.Lee SH, Minagawa E, Taguchi H, Matsuzawa H, Ohta T, Kaminogawa S, Yamauchi K. Purification and characterization of a thermostable carboxypeptidase (carboxypeptidase Taq) from Thermus aquaticus YT-1. Biosci. Biotechnol. Biochem. 1992;56:1839–1844. doi: 10.1271/bbb.56.1839. [DOI] [PubMed] [Google Scholar]
- 2.Cheng TC, Ramakrishnan V, Chan SI. Purification and characterization of a cobalt-activated carboxypeptidase from the hyperthermophilic archaeon Pyrococcus furiosus. Protein Sci. 1999;8:2474–2486. doi: 10.1110/ps.8.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arndt JW, Hao B, Ramakrishnan V, Cheng T, Chan SI, Chan MK. Crystal structure of a novel carboxypeptidase from the hyperthermophilic archaeon Pyrococcus furiosus. Structure. 2002;10:215–224. doi: 10.1016/s0969-2126(02)00698-6. [DOI] [PubMed] [Google Scholar]
- 4.Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown CK, Madauss K, Lian W, Beck MR, Tolbert WD, Rodgers DW. Structure of neurolysin reveals a deep channel that limits substrate access. Proc. Natl. Acad. Sci. U. S. A. 2001;98:3127–3132. doi: 10.1073/pnas.051633198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Natesh R, Schwager SL, Sturrock ED, Acharya KR. Crystal structure of the human angiotensin-converting enzyme-lisinopril complex. Nature. 2003;421:551–554. doi: 10.1038/nature01370. [DOI] [PubMed] [Google Scholar]
- 7.Towler P, Staker B, Prasad SG, Menon S, Tang J, Parsons T, Ryan D, Fisher M, Williams D, Dales NA, Patane MA, Pantoliano MW. ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J. Biol. Chem. 2004;279:17996–18007. doi: 10.1074/jbc.M311191200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ray K, Hines CS, Coll-Rodriguez J, Rodgers DW. Crystal structure of human thimet oligopeptidase provides insight into substrate recognition, regulation, and localization. J. Biol. Chem. 2004;279:20480–20489. doi: 10.1074/jbc.M400795200. [DOI] [PubMed] [Google Scholar]
- 9.Niemirowicz G, Parussini F, Aguero F, Cazzulo JJ. Two metallocarboxypeptidases from the protozoan Trypanosoma cruzi belong to the M32 family, found so far only in prokaryotes. Biochem. J. 2007;401:399–410. doi: 10.1042/BJ20060973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herwaldt BL. Leishmaniasis. Lancet. 1999;354:1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 11.Sacks D, Kamhawi S. Molecular aspects of parasite-vector and vector-host interactions in leishmaniasis. Annu. Rev. Microbiol. 2001;55:453–483. doi: 10.1146/annurev.micro.55.1.453. [DOI] [PubMed] [Google Scholar]
- 12.Joshi PB, Kelly BL, Kamhawi S, Sacks DL, McMaster WR. Targeted gene deletion in L. major identifies leishmanolysin (GP63) as a virulence factor. Mol. Biochem. Parasitol. 2002;120:33–40. doi: 10.1016/s0166-6851(01)00432-7. [DOI] [PubMed] [Google Scholar]
- 13.Doi E, Shibata D, Matoba T. Modified colorimetric ninhydrin methods for peptidase assay. Anal. Biochem. 1981;118:173–184. doi: 10.1016/0003-2697(81)90175-5. [DOI] [PubMed] [Google Scholar]
- 14.Ng M, Auld DS. A fluorescent oligopeptide energy transfer assay with broad applications for neutral proteases. Anal. Biochem. 1989;183:50–56. doi: 10.1016/0003-2697(89)90170-x. [DOI] [PubMed] [Google Scholar]
- 15.Chakrabarti G, Basu A, Manna PP, Mahato SB, Mandal NB, Bandyopadhyay S. Indolylquinoline derivatives are cytotoxic to Leishmania donovani promastigotes and amastigotes in vitro and are effective in treating murine visceral leishmaniasis. J. Antimicrob. Chemother. 1999;43:359–366. doi: 10.1093/jac/43.3.359. [DOI] [PubMed] [Google Scholar]
- 16.Satoskar AR, Okano M, Connaughton S, Raisanen-Sokolwski A, David JR, M, Labow Enhanced Th2-like responses in IL-1 type 1 receptor-deficient mice. Eur. J. Immunol. 1998;28:2066–2074. doi: 10.1002/(SICI)1521-4141(199807)28:07<2066::AID-IMMU2066>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.Satoskar A, Okano M, David JR. γδ cells are not essential for control of cutaneous L. major infection in genetically resistant C57BL/6 mice. J. Infect. Dis. 1997;176:1649–1652. doi: 10.1086/517348. [DOI] [PubMed] [Google Scholar]
- 18.Sacks DL, Hieny S, Sher A. Identification of cell surface carbohydrate and antigenic changes between noninfective and infective developmental stages of L. major promastigotes. J. Immunol. 1985;135:564–569. [PubMed] [Google Scholar]
- 19.Bhardwaj N, Rosas LE, Lafuse WP, Satoskar AR. Leishmania inhibits STAT1-mediated IFN-gamma signaling in macrophages: increased tyrosine phosphorylation of dominant negative STAT1beta by Leishmania mexicana. Int. J. Parasitol. 2005;35:75–82. doi: 10.1016/j.ijpara.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Schut GJ, Brehm SD, Datta S, Adams MWW. Whole genome DNA microarray analysis of a hyperthermophile and an archaeon: Pyrococcus furiosus grown on carbohydrates or peptides. J. Bacteriol. 2003;185:3935–3947. doi: 10.1128/JB.185.13.3935-3947.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Taguchi H, Yoshimura E, Minagawa E, Kaminogawa S, Ohta T, Matsuzawa H. Carboxypeptidase Taq, a thermostable zinc enzyme, from Thermus aquaticus YT-1 - Molecular-cloning, sequencing, and expression of the encoding gene in Escherichia coli. Biosci. Biotechnol. Biochem. 1994;58:1490–1495. doi: 10.1271/bbb.58.1490. [DOI] [PubMed] [Google Scholar]
- 22.Cowan DA, Daniel RM, Morgan HW. The specific activites of mesophilic and thermophilic proteinases. Int. J. Biochem. 1987;19:741–743. doi: 10.1016/0020-711x(87)90092-9. [DOI] [PubMed] [Google Scholar]
- 23.Oliveira V, Campos M, Hemerly JP, Ferro ES, Camargo ACM, Juliano MA, Juliano L. Selective neurotensin-derived internally quenched fluorogenic substrates for neurolysin (EC 3.4.24.16): comparison with thimet oligopeptidase (EC 3.4.24.15) and neprilysin (EC 3.4.24.11) Anal. Biochem. 2001;292:257–265. doi: 10.1006/abio.2001.5083. [DOI] [PubMed] [Google Scholar]
- 24.Alter GM, Leussing DL, Neurath H, Vallee BL. Kinetic properties of carboxypeptidase B in solutions and crystals. Biochemistry. 1977;16:3663–3668. doi: 10.1021/bi00635a024. [DOI] [PubMed] [Google Scholar]
- 25.Jain R, Chan MK. Support for a potential role of E. coli oligopeptidase A in protein degradation. Biochem. Biophys. Res. Commun. 2007;359:486–490. doi: 10.1016/j.bbrc.2007.05.142. [DOI] [PubMed] [Google Scholar]
- 26.Pimenta PF, Turco SJ, McConville MJ, Lawyer PG, Perkins PV, Sacks DL. Stage specific adhesion of Leishmania promastigotes to the sandfly midgut. Science. 1992;256:1812–1815. doi: 10.1126/science.1615326. [DOI] [PubMed] [Google Scholar]
- 27.Walters LL, Irons KP, Guzman H, Tesh RB. Formation and composition of the peritrophic membrane in the sand fly, Phlebotomus perniciosus (Diptera: Psychodidae) J. Med. Entomol. 1993;30:179–198. doi: 10.1093/jmedent/30.1.179. [DOI] [PubMed] [Google Scholar]
- 28.Gossage SM, Rogers ME, Bates PA. Two separate growth phases during the development of Leishmania in sand flies: implications for understanding the life cycle. Int. J. Parasitol. 2003;33:1027–1034. doi: 10.1016/s0020-7519(03)00142-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.