Abstract
Objective
Inflammation is a potential mechanism to explain the accelerated atherosclerosis observed in HIV- and hepatitis C virus (HCV)–infected persons. We evaluated C-reactive protein (CRP) in HIV-infected and HIV/HCV-coinfected individuals in the era of effective antiretroviral (ARV) therapy.
Design
Cross-sectional study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) cohort and controls from the Coronary Artery Risk Development in Young Adults (CARDIA) study.
Methods
CRP levels were measured in 1135 HIV-infected participants from the FRAM cohort and 281 controls from the CARDIA study. The associations of HIV and HIV/HCV infection with CRP levels were estimated by multivariable linear regression.
Results
Compared with controls, HIV monoinfection was associated with an 88% higher CRP level in men (P < 0.0001) but with no difference in women (5%; P = 0.80) in multivariate analysis. CRP levels were not associated with ARV therapy, HIV RNA level, or CD4 cell count. Compared with controls, HIV/HCV coinfection was associated with a 41% lower CRP level in women (P = 0.012) but with no difference in men (+4%; P = 0.90). Among HIV-infected participants, HCV coinfection was associated with 50% lower CRP levels after multivariable analysis (P < 0.0001) in men and women. Greater visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) were strongly associated with CRP levels. Among HIV- infected participants, CRP levels were 17% (P < 0.001) and 21% (P = 0.002) higher per doubling of VAT and SAT; among controls, CRP levels were 34% (P < 0.001) and 61% (P = 0.009) higher, respectively.
Conclusions
In the absence of HCV coinfection, HIV infection is associated with higher CRP levels in men. HCV coinfection is associated with lower CRP levels in men and women.
Keywords: cardiovascular disease, C-reactive protein, hepatitis C virus, HIV, inflammation
Inflammation plays a key role in the pathogenesis of atherosclerosis.1 C-reactive protein (CRP) is currently the most widely used biomarker of inflammation, given its ability to be quantified in a highly sensitive assay.2,3 Elevated levels of CRP have been associated with cardiovascular disease (CVD), and the prognostic utility of CRP to predict cardiovascular risk beyond the Framingham Risk Score has been demonstrated.4–8
Higher rates of CVD have been associated with HIVand hepatitis C virus (HCV) infections.9–17 Furthermore, a recent analysis suggests that coinfection with HIV and HCV is associated with a higher adjusted odds ratio for the prevalence of CVD.18 One mechanism for this association may be inflammation, because chronic infections activate the immune system and result in a proinflammatory state.19–23 Yet, before the prognostic ability of CRP to predict CVD can be investigated in cohorts of HIV-infected people, fundamental questions remain about the relation between CRP levels and HIV infection. Although some of the factors associated with CRP in the absence of HIV infection are well known, such as obesity,24 few studies have investigated the relation between HIV and CRP levels in the era of effective antiretroviral (ARV) therapy. Furthermore, no study to date has directly compared CRP levels between large multiethnic cohorts with and without HIV infection. Therefore, we evaluated the association between HIV infection and CRP levels, and we investigated the contributors to CRP levels among HIV-infected persons in a nationally representative sample of HIV-infected subjects and in controls.
METHODS
The study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM) is a cross-sectional study designed to evaluate the prevalence and correlates of changes in fat distribution and metabolism in a representative sample of HIV-infected participants and controls in the United States. The methods have been described in detail previously.25,26 HIV-infected participants were recruited from 16 HIV or infectious disease clinics or cohorts between June 2000 and September 2002.27 Control participants were recruited from 2 centers from the Coronary Artery Risk Development in Young Adults (CARDIA) study.28,29 CARDIA participants were originally recruited in 1985 to 1986 as a population-based sample of healthy 18- to 30-year-old white and African American women and men to study cardiovascular risk factors longitudinally.29 The FRAM protocol was approved by institutional review boards at all sites.
Here, we analyze data from 1135 HIV-infected participants and 281 controls in the FRAM study who had CRP measurements. Of those, 905 HIV-infected participants and 255 controls had magnetic resonance imaging (MRI) assessment of adipose tissue performed. In analyses directly comparing the HIV-infected and control populations, 517 HIV-infected participants between the ages of 33 and 45 years old (to correspond with the age range of the control group) and those without recent opportunistic infections (OIs) were included. Additionally, 5 control subjects who were infected with HCV were excluded from analysis.
Measurements
CRP was measured in serum that had been previously frozen and stored at −70°C using the BNII nephelometer from Dade Behring (Deerfield, IL), which utilizes a particle-enhanced immunonephelometric assay. The interassay co-efficient of variation for this study ranges from 3.7% to 4.5%. High CRP was defined as >3 mg/L, based on the Centers for Disease Control and Prevention (CDC)/American Heart Association (AHA) guidelines;30 the effect of excluding CRP >10 mg/L was also analyzed.
HCV RNA testing was performed on frozen sera using the Bayer Versant 3.0 branched DNA (bDNA) assay (Leverkusen, Germany) in the entire cohort. CD4 lymphocyte count and percent, HIV RNA level in HIV-infected participants, and other blood specimens were analyzed in a single centralized laboratory (Covance, Indianapolis, IN).
Height and weight were measured by standardized protocols, and adipose tissue volume was measured using MRI as previously described.31 Standardized questionnaires were used to determine demographic characteristics; medical history; risk factors for HIV; and use of alcohol, tobacco, and illicit drugs.25,29 Research associates interviewed subjects and reviewed medical charts regarding ARV medication use. A diagnosis of AIDS was made by history of OI or CD4 count <200.
Statistics
Characteristics of HIV-infected participants and controls were compared and tested for statistical significance using the Mann-Whitney U test for continuous variables and the Fisher exact test for categoric variables.
Multivariable linear regression analysis was used to investigate the association of HIV infection with CRP levels after adjustment for potential confounding factors. To test the validity of pooling men and women in this analysis, interactions between gender and other factors in the model were assessed and included in models if they had a P value <0.05. Because we saw substantial opposing effects of HIV and HCV on CRP levels in men, HIV-monoinfected and HIV/HCV-coinfected participants were compared separately with controls. In addition, we observed significant interactions of HIV and gender on CRP levels; thus, these analyses comparing HIV and controls were also gender stratified. Because of its skewed distribution, CRP was log-transformed in all linear regression analyses; results were back-transformed to produce estimated percentage differences in CRP. Potential confounders in the combined HIV and control analyses included demographic information (eg, gender, age, ethnicity), MRI measurements of visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), level of physical activity (inactive vs. active), current smoking status, current illicit drug use, less than adequate food consumption, and alcohol use (weekly vs. nonweekly use). Although 5 adipose tissue sites were screened as candidate predictors (VAT, lower trunk SAT, upper trunk SAT, arm SAT, and leg SAT), model fit was optimized by including VAT and total SAT. Adipose measurements were normalized by dividing by height-squared, analogous to body mass index (BMI), and results were back-transformed to 1.75 m of height.
To determine whether HIV monoinfection and HIV/HCV coinfection were associated with different CRP levels compared with controls, we performed stepwise linear regression. To avoid exclusion of observations that had missing data only on unselected candidate variables, we evaluated possible models individually rather than with an automated stepwise procedure. Age, race, and gender were forced into the models, and other candidates were included with a criterion of P ≤ 0.05 for entry and retention. The linearity assumption for each continuous predictor was tested. Confidence intervals were determined using the bias-corrected accelerated bootstrap method,32 with the P value defined as that minus the highest confidence level that still excluded 0. This was necessary because the outcome seemed to be non-Gaussian, even after log transformation.
A second set of multivariable regression analyses was performed only among HIV-infected subjects to determine the factors independently associated with CRP levels in HIV infection. In addition to the predictors listed previously, these models included HIV RNA level (log10) and CD4 cell count (log2) at the time of study visit. Also tested for inclusion in the model were HCV status (HCV RNA > 615), recent OI status, AIDS by CD4 cell count or OI, HIV duration, days since last OI, and HIV risk factors. In multivariable models controlling for these factors, we evaluated current use of each individual ARV drug and ARV class: nucleoside reverse transcriptase inhibitor (NRTI), nonnucleoside reverse transcriptase inhibitor (NNRTI), protease inhibitor (PI), and highly active antiretroviral therapy (HAART), as previously defined.26 Current use of each ARV, ARV class, and HAARTwas added to the adjusted model in a forward stepwise manner.
All analyses were conducted using the SAS system, version 9.1 (SAS Institute Inc., Cary, NC).
RESULTS
Subject Demographics
The demographic and clinical characteristics of the 1135 HIV-infected participants and 281 controls who had CRP levels measured and the factors that entered into the multivariable models are shown in Table 1 and are similar to those of the larger cohort. The factors presented include those previously reported to be associated with CRP and those that were included in the final models. More HIV-infected men were white, and more HIV-infected women were African American. Among controls, there were similar numbers of white and African American subjects by design. HIV-infected men and women were less physically active, less likely to consume alcohol, and more likely to smoke than controls. Although the rate of current crack or cocaine use was low in all groups, use was more common in HIV-infected women than in control women. Compared with control men, HIV-infected men had lower BMI and less SAT but similar amounts of VAT. Compared with control women, HIV-infected women had similar BMI and less SAT but greater VAT. HIV-infected women were also more likely to be postmenopausal.
TABLE 1.
Characteristics of HIV-Infected and Control Subjects, Stratified by Gender
| Men
|
Women
|
|||
|---|---|---|---|---|
| HIV+ | Control | HIV+ | Control | |
| n | 799 | 150 | 336 | 131 |
| Age [y], median (IQR) | 43 (38 to 49)† | 40 (37 to 43) | 41 (36 to 47) | 42 (37 to 44) |
| Race‡ | ||||
| White | 55%* | 53% | 33%* | 50% |
| African American | 33% | 47% | 55% | 50% |
| Other | 12% | 0 | 12% | 0 |
| Physical inactivity | 48%† | 25% | 39%* | 22% |
| No alcohol use | 25%* | 16% | 39%* | 26% |
| Current smoker | 42%† | 21% | 45%† | 13% |
| Current crack/cocaine | 6% | 4% | 8%* | 1% |
| BMI [kg/m2], median (IQR) | 24 (22 to 27)† | 27 (25 to 30) | 26 (22 to 31) | 28 (23 to 33) |
| VAT [L], median (IQR) | 1.8 (0.8 to 3.0) | 2.0 (1.1 to 3.0) | 1.4 (0.6 to 2.4)* | 1.1 (0.4 to 1.9) |
| Total SAT [L], median (IQR) | 10 (7 to 14)† | 15 (11 to 19) | 26 (17 to 37)* | 30 (20 to 40) |
| Menopausal | NA | 23%† | 7% | |
| Current hormonal contraceptive use | NA | 10% | 15% | |
| Current HRT use | NA | 10% | 5% | |
P < 0.05 vs. control;
P < 0.0001 vs. control.
P value for race testing difference in proportions between white and African American participants.
HRT indicates hormone replacement therapy; IQR, interquartile range; NA, not applicable.
HIV-infected men and women had similar duration of HIV infection, CD4 cell count, and proportion with a diagnosis of AIDS or a recent OI but differed in their prevalence of undetectable HIV RNA (Table 2). Within this cohort, 20% of men and 26% of women had HCV infection as measured by a detectable HCV RNA level. The prevalence of hepatitis B surface antigenemia (HBsAg+) was low in both genders but higher in HIV-infected men. Eighty percent of men and 69% of women were on HAART therapy; specific classes of ARVs are shown in Table 2.
TABLE 2.
Characteristics of HIV-Infected Men and Women
| HIV+ |
||
|---|---|---|
| Men | Women | |
| Duration HIV [y], median (IQR) | 8 (5 to 12) | 8 (6 to 11) |
| Current CD4 count [cells/μL], median (IQR) | 345 (219 to 523) | 361 (196 to 557) |
| HIV RNA level [1000 copies/mL], median (IQR) | 0.4 (0.4 to 10.7) | 0.5 (0.4 to 14.8) |
| Undetectable HIV RNA | 53%* | 46% |
| AIDS by CD4 cell count/OI | 73% | 73% |
| Recent OI | 2% | 4% |
| Days since OI, median (IQR) | 195 (25 to 865) | 120 (21 to 702) |
| HIV risk factors | ||
| Heterosexual | 10%† | 56% |
| IDU | 18% | 27% |
| MSM | 66% | 0 |
| Other | 6% | 17% |
| HCV RNA+ | 20%* | 26% |
| HBsAg+ | 8%† | 2% |
| ARV medications | ||
| Current HAART use | 80%† | 69% |
| Current NRTI use | 86%† | 76% |
| Current NNRTI use | 39% | 36% |
| Current PI use | 58%* | 47% |
P < 0.05 vs. women;
P < 0.0001 vs. women.
IDU indicates injection drug use; IQR, interquartile range; MSM, men who have sex with men.
CRP Levels by Gender, HIV, and HCV Status
Because we identified a statistically significant HIV/HCV-by-gender interaction (P = 0.002), we compared median CRP levels among control, HIV-monoinfected, and HIV/HCV-coinfected participants, stratified by gender (Fig. 1). For men and women, HIV-monoinfected participants had significantly higher median CRP levels compared with those participants with HIV/HCV coinfection.
FIGURE 1.
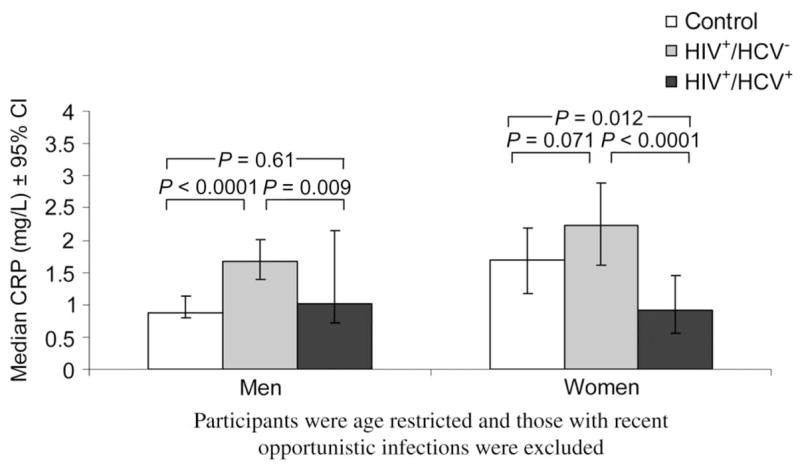
Median CRP levels by gender and HIV/HCV status.
The median CRP level was nearly twice as high in HIV-monoinfected men compared with control men (1.67 vs. 0.88; P < 0.0001); CRP levels were somewhat higher in HIV-monoinfected women compared with control women (2.22 vs. 1.70; P = 0.071). The median CRP level was lower in HIV/HCV-coinfected women compared with control women (0.92 vs. 1.70; P = 0.012), whereas levels were similar in HIV/HCV-coinfected and control men (1.01 vs. 0.88; P = 0.61).
After multivariable analysis (Table 3), in men, HIV monoinfection was associated with 88% higher CRP levels compared with controls; however, in women, HIV mono-infection was not associated with substantially higher CRP levels. In men, HIV/HCV coinfection showed no substantial association with higher CRP levels compared with controls; however, among women, HIV/HCV coinfection was associated with 41% lower CRP levels compared with controls.
TABLE 3.
Association of HIV and HIV/HCV Infection With CRP* Levels, Stratified by Gender
| HIV+/HCV− vs. Control
|
HIV+/HCV+ vs. Control
|
|||
|---|---|---|---|---|
| % Effect (95% CI) | P | % Effect (95% CI) | P | |
| Men† | ||||
| Unadjusted | 75 (38 to 116) | <0.0001 | 10 (−25 to 60) | 0.64 |
| Adjusted‡ | 88 (43 to 143) | <0.0001 | 4 (−35 to 58) | 0.90 |
| Women† | ||||
| Unadjusted | 34 (−0.9 to 84) | 0.055 | −43 (−62 to −17) | 0.006 |
| Adjusted‡ | 5 (−27 to 43) | 0.80 | −41 (−64 to −11) | 0.012 |
Outcome analyzed is log-transformed CRP, with results back-transformed to the original scale.
Participants were age restricted, and those with recent OIs were excluded.
Adjusted effects models control for HIV and HCV status, gender, age, ethnicity, lifestyle factors, visceral fat, and subcutaneous fat.
95% CI indicates 95% confidence interval.
Factors Associated With CRP
We used multivariable analysis to examine characteristics associated with CRP levels among HIV-infected participants (Table 4). Among the different possible models using the 5 depots of adipose tissue tested, the best-fitting model had VAT and total SAT as the only 2 predictors. Increased volume of VAT and SAT were strongly associated with higher CRP among the HIV-infected participants. These associations were even stronger among controls: 34% (P < 0.001) higher CRP per doubling of VAT and 61% higher CRP per doubling of SAT (P = 0.009). Among lifestyle factors, current use of tobacco, physical inactivity, current use of crack or cocaine, and nonuse of alcohol were associated with higher CRP levels. Although these lifestyle factors are linked to adipose tissue volume, their associations with CRP were independent of adipose tissue in this multivariable analysis.
TABLE 4.
Characteristics Associated With CRP* Among HIV-Infected Participants After Multivariable Adjustment
| % Effect (95% CI) | P | |
|---|---|---|
| Demographic factors | ||
| Female vs. male | −19 (−36 to 4) | 0.097 |
| African American vs. white | 21 (−2 to 50) | 0.068 |
| Hispanic vs. white | 21 (−8 to 62) | 0.18 |
| Other vs. white | −40 (−64 to 3) | 0.061 |
| Age (per decade) | 9 (−2 to 21) | 0.11 |
| Adipose tissue | ||
| VAT (log2) | 17 (8 to 26) | <0.0001 |
| Total SAT (log2) | 21 (7 to 36) | 0.002 |
| Lifestyle | ||
| Physical activity: inactive vs. active | 31 (12 to 56) | 0.002 |
| Nondrinker vs. drinker | 24 (3 to 49) | 0.024 |
| Current smoker vs. nonsmoker | 33 (15 to 59) | <0.0001 |
| Crack/cocaine use | 60 (10 to 132) | 0.012 |
| HIV-related | ||
| AIDS by CD4 cell count/OI | 25 (2 to 53) | 0.028 |
| Current HIV viral load (log10) | 5 (−5 to 16) | 0.35 |
| Current CD4 cell count (log2) | −2 (−9 to 7) | 0.67 |
| HCV (HCV RNA > 615) | −50 (−59 to −38) | <0.0001 |
Outcome analyzed is log-transformed CRP, with results back-transformed to the original scale.
Also tested for inclusion in the multivariable model were MRI measurements of adipose tissue volume from other sites, other lifestyle factors, and HIV-related factors, as described in more detail in the methods section.
Finally, we assessed factors related to HIV infection, such as current CD4 cell count, HIV viral load, ARV therapy, and prior diagnosis of AIDS (see Table 4). The diagnosis of AIDS had a statistically significant association with CRP, whereas CD4 cell count and viral load did not seem to have substantial effects. The median CRP was 1.7 among users of ARV therapy and 1.9 among nonusers (P = 0.94). No specific ARV therapy or ARV class had significant associations with CRP level. Coinfection with HCV was associated with 50% lower CRP after multivariable adjustment. This association was essentially unchanged by further adjustment for ALT, AST, and albumin concentrations and did not differ substantially by gender.
HIV Infection and High CRP Levels
Overall, HIV-infected men were more likely to have CRP levels in the CDC/AHA high-risk group (>3 mg/L) for CVD compared with control men (32% vs. 17%; P < 0.001). HIV-infected and control women had similar prevalence of high CRP levels (33% vs. 37%; P = 0.55). HIV-monoinfected women were twice as likely to have high CRP levels compared with HIV/HCV-coinfected women (43% vs. 21%; P = 0.0002). The difference in prevalence of high-risk CRP between HIV-monoinfected men and HIV/HCV-coinfected men did not reach statistical significance (33% vs. 28%; P = 0.26).
The prevalence of extremely high CRP levels (> 10 mg/L) was 7% in controls and 9% in HIV-infected subjects. Because extremely high CRP levels may obscure the prediction of coronary risk factors, exploratory analyses were conducted excluding or truncating extremely high CRP values (> 10 mg/L); little change in the results was seen, and the key finding that HCV coinfection is associated with lower CRP levels (P < 0.0001) persisted.
DISCUSSION
In this study, we compared CRP levels in subjects with HIV monoinfection and HIV/HCV coinfection with similarly aged controls. We found that adjusted CRP levels were substantially higher in HIV-monoinfected men compared with control men but that adjusted levels of CRP were similar in HIV-monoinfected and control women. There was no substantial association of CRP levels with ARV therapy, HIV RNA level, or CD4 cell count. We report the novel finding that HIV coinfection with HCV was associated with a 50% lower adjusted CRP level compared with HIV monoinfection in men and women, however.
Our finding that CRP levels are nearly twice as high among HIV-monoinfected men compared with control men may be of clinical importance. Nearly one third of the HIV-infected men had CRP levels >3 mg/L, which would be classified as high risk for CVD by the CDC/AHA guidelines. (The prevalence of CRP levels >3 mg/mL was not accounted for by extremely high levels of CRP [eg, >10 mg/L].) This degree of CRP elevation could be expected to increase cardiovascular risk by 50% based on evidence from the general population,33 although the prognostic role of CRP and CVD in HIV infection has yet to be validated. In women, HIV infection was not associated with substantially higher CRP levels after multivariable adjustment for confounding factors, including adipose tissue volume as measured by MRI. A previous study also found that CRP levels were elevated in HIV-infected women but that HIV was not a factor after adjusting for waist-to-hip ratio.34 That study did not report on the prevalence of HCV coinfection.
Our finding that HCV infection was associated with substantially lower CRP levels among HIV-infected subjects should be interpreted within the context of the cross-sectional design of this study. We cannot prove causality or define a mechanism from this study; however, interesting hypotheses emerge. One possibility is that HCV decreases production of CRP from the liver. In a study of patients on hemodialysis, infection with HCV was associated with a lower CRP/interleukin (IL)-6 ratio.35 This finding suggests a disturbance in the liver’s ability to respond to IL-6 with stimulation of CRP secretion. The concept that HCV prevents secretion of CRP is supported by in vitro studies of expression of HCV proteins in cultured hepatocytes, which causes inhibition of secretion of other liver proteins, such as apo B100,36 a protein whose circulating levels are also decreased in HCV infection.37 The association of HCV with decreased CRP was not affected by adjustment for measures of hepatotoxicity, however, such as levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), or albumin.
Our results and those among patients undergoing dialysis have potential clinical implications. Epidemiologic studies suggest an association between HCV infection and increased risk of CVD.37 Our data therefore raise the question as to whether CRP can predict CVD risk differently in patients with HCVor HIV/HCV coinfection. This is an important topic for future study.
A similar discordance has been found in patients with acromegaly, among whom CRP levels are significantly lower than those of controls despite acromegaly’s association with premature cardiovascular mortality.38 CRP levels increase toward the levels of controls when acromegaly is treated, yet the treatment reduces cardiovascular mortality risk. Conversely, CRP levels are relatively elevated in persons who are deficient in growth hormone and correct with growth hormone replacement therapy.39,40 These findings suggest that CRP may not be as good an indicator of CVD risk in persons with abnormal levels of growth hormone, because these conditions affect CRP independent of inflammation and atherosclerosis. Whether HCV infection poses a similar obstacle to the use of CRP as a cardiovascular risk factor awaits additional investigation.
The strengths of this study include a large population-based HIV cohort; a control group of similar age, gender, and racial composition; and the use of MRI for body composition analysis, which allowed important multivariable adjustments. This study does, however, have certain limitations, especially its cross-sectional design, which limits the ability to evaluate the direction and causality of the reported associations. Clearly, CRP levels could not have caused HIV and HCV infection; however, we cannot determine whether the participants with HIV and HIV/HCV coinfection would have had different CRP levels compared with the controls before their infection. Although we adjusted for other determinants of CRP levels, we cannot exclude the possibility of residual confounding. In addition, we may have had insufficient statistical power to detect associations of ARV use with CRP levels, particularly for agents that were used infrequently.
In conclusion, this study found that HIV/HCV coinfection was associated with substantially lower CRP levels among HIV-infected men and women. Only men with HIV monoinfection had substantially higher CRP levels than controls. Future epidemiologic studies should evaluate carefully the association of CRP with CVD in HIV-monoinfected and HIV/HCV-coinfected persons.
Acknowledgments
Supported by National Institutes of Health grants RO1-DK57508, HL74814, HL53359, and HL077499 and National Institutes of Health General Clinical Research Centers grants M01-RR00036, RR00051, RR00052, RR00054, RR00083, RR0636, and RR00865.
The funding agency had no role in the collection or analysis of the data.
APPENDIX
Conflicts of Interest
Drs. Wanke, Kotler, Lewis, Tracy, Tien, Heymsfield, Bacchetti, Scherzer, Grunfeld, and Shlipak received funding from the supporting grants.
Role of the Funder
The funder played no role in the conduct of the study, collection of the data, management of the study, analysis of data, interpretation of the data, or preparation of the manuscript. A representative of the funding agent participated in planning the protocol. As part of the standard operating procedures of the CARDIA study, the manuscript was reviewed at the National Heart, Lung, and Blood Institute, but no revisions were requested.
Sites and Investigators
University Hospitals of Cleveland (Barbara Gripshover, MD); Tufts University (Abby Shevitz, MD, and Christine Wanke, MD); Stanford University (Andrew Zolopa, MD, and Lisa Gooze, MD); University of Alabama at Birmingham (Michael Saag, MD, and Barbara Smith, PhD); John Hopkins University (Joseph Cofrancesco and Adrian Dobs); University of Colorado Heath Sciences Center (Constance Benson, MD, and Lisa Kosmiski, MD); University of North Carolina at Chapel Hill (Charles van der Horst, MD); University of California at San Diego (W. Christopher Mathews, MD, and Daniel Lee, MD); Washington University (William Powderly, MD, and Kevin Yarasheski, PhD); Veterans Affairs Medical Center, Atlanta (David Rimland, MD); University of California at Los Angeles (Judith Currier, MD, and Matthew Leibowitz, MD); Veterans Affairs Medical Center, New York (Michael Simberkoff, MD, and Juan Bandres, MD); Veterans Affairs Medical Center, Washington, DC (Cynthia Gibert, MD, and Fred Gordin, MD); St. Luke’s–Roosevelt Hospital Center (Donald Kotler, MD, and Ellen Engelson, PhD); University of California at San Francisco (Morris Schambelan, MD, and Kathleen Mulligan, PhD); Indiana University (Michael Dube, MD); Kaiser Permanente, Oakland (Stephen Sidney, MD); and University of Alabama at Birmingham (Cora E. Lewis, MD)
Data Coordinating Center
University of Alabama, Birmingham (O. Dale Williams, PhD, Heather McCreath, PhD, Charles Katholi, PhD, George Howard, PhD, Tekeda Ferguson, and Anthony Goudie)
Image Reading Center
St. Luke’s–Roosevelt Hospital Center (Steven Heymsfield, MD, Jack Wang, MS, and Mark Punyanitya)
Office of the Principal Investigator
University of California, San Francisco, Veterans Affairs Medical Center and the Northern California Institute for Research and Development (Carl Grunfeld, MD, PhD, Phyllis Tien, MD, Peter Bacchetti, PhD, Dennis Osmond, PhD, Andrew Avins, MD, Michael Shlipak, MD, Rebecca Scherzer, PhD, Mae Pang, RN, MSN, Heather Southwell, MS, RD, Erin Madden, MPH, and Yong Kyoo Chang, MS)
References
- 1.Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts WL. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: laboratory tests available to assess inflammation—performance and standardization: a background paper. Circulation. 2004;110:e572–e576. doi: 10.1161/01.CIR.0000148986.52696.07. [DOI] [PubMed] [Google Scholar]
- 4.Smith SC, Jr, Anderson JL, Cannon RO, 3rd, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the clinical practice discussion group. Circulation. 2004;110:e550–e553. doi: 10.1161/01.CIR.0000148981.71644.C7. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Hennekens CH, Buring JE, et al. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 6.Libby P, Ridker PM. Inflammation and atherosclerosis: role of C-reactive protein in risk assessment. Am J Med. 2004;116(Suppl 6A):9S–16S. doi: 10.1016/j.amjmed.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 8.Cushman M, Arnold AM, Psaty BM, et al. C-reactive protein and the 10-year incidence of coronary heart disease in older men and women: the Cardiovascular Health Study. Circulation. 2005;112:25–31. doi: 10.1161/CIRCULATIONAHA.104.504159. [DOI] [PubMed] [Google Scholar]
- 9.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 10.Vassalle C, Masini S, Bianchi F, et al. Evidence for association between hepatitis C virus seropositivity and coronary artery disease. Heart. 2004;90:565–566. doi: 10.1136/hrt.2003.018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishizaka N, Ishizaka Y, Takahashi E, et al. Association between hepatitis C virus seropositivity, carotid-artery plaque, and intima-media thickening. Lancet. 2002;359:133–135. doi: 10.1016/s0140-6736(02)07339-7. [DOI] [PubMed] [Google Scholar]
- 12.Ishizaka Y, Ishizaka N, Takahashi E, et al. Association between hepatitis C virus core protein and carotid atherosclerosis. Circ J. 2003;67:26–30. doi: 10.1253/circj.67.26. [DOI] [PubMed] [Google Scholar]
- 13.Volzke H, Schwahn C, Wolff B, et al. Hepatitis B and C virus infection and the risk of atherosclerosis in a general population. Atherosclerosis. 2004;174:99–103. doi: 10.1016/j.atherosclerosis.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Mary-Krause M, Cotte L, Simon A, et al. Increased risk of myocardial infarction with duration of protease inhibitor therapy in HIV-infected men. AIDS. 2003;17:2479–2486. doi: 10.1097/00002030-200311210-00010. [DOI] [PubMed] [Google Scholar]
- 15.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. J Acquir Immune Defic Syndr. 2003;33:506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 16.Holmberg SD, Moorman AC, Williamson JM, et al. Protease inhibitors and cardiovascular outcomes in patients with HIV-1. Lancet. 2002;360:1747–1748. doi: 10.1016/S0140-6736(02)11672-2. [DOI] [PubMed] [Google Scholar]
- 17.Bozzette SA, Ake CF, Tam HK, et al. Cardiovascular and cerebrovascular events in patients treated for human immunodeficiency virus infection. N Engl J Med. 2003;348:702–710. doi: 10.1056/NEJMoa022048. [DOI] [PubMed] [Google Scholar]
- 18.Freiberg MS, Cheng DM, Kraemer KL, et al. The association between hepatitis C infection and prevalent cardiovascular disease among HIV-infected individuals. AIDS. 2007;21:193–197. doi: 10.1097/QAD.0b013e3280118a0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fauci AS, Pantaleo G, Stanley S, et al. Immunopathogenic mechanisms of HIV infection. Ann Intern Med. 1996;124:654–663. doi: 10.7326/0003-4819-124-7-199604010-00006. [DOI] [PubMed] [Google Scholar]
- 20.Abbate I, Romano M, Longo R, et al. Endogenous levels of mRNA for IFNs and IFN-related genes in hepatic biopsies of chronic HCV-infected and non-alcoholic steatohepatitis patients. J Med Virol. 2003;70:581–587. doi: 10.1002/jmv.10433. [DOI] [PubMed] [Google Scholar]
- 21.Rimaniol AC, Zylberberg H, Zavala F, et al. Inflammatory cytokines and inhibitors in HIV infection: correlation between interleukin-1 receptor antagonist and weight loss. AIDS. 1996;10:1349–1356. doi: 10.1097/00002030-199610000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Stephensen CB, Marquis GS, Douglas SD, et al. Immune activation and oxidative damage in HIV-positive and HIV-negative adolescents. J Acquir Immune Defic Syndr. 2005;38:180–190. doi: 10.1097/00126334-200502010-00009. [DOI] [PubMed] [Google Scholar]
- 23.Khovidhunkit W, Kim MS, Memon RA, et al. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Bouter LM, McQuillan GM, et al. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 25.Tien PC, Benson C, Zolopa AR, et al. The study of Fat Redistribution and Metabolic Change in HIV Infection (FRAM): methods, design, and sample characteristics. Am J Epidemiol. 2006;163:860–869. doi: 10.1093/aje/kwj111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bacchetti P, Gripshover B, Grunfeld C, et al. Fat distribution in men with HIV infection. J Acquir Immune Defic Syndr. 2005;40:121–131. doi: 10.1097/01.qai.0000182230.47819.aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsue PY, Hunt PW, Sinclair E, et al. Increased carotid intima-media thickness in HIV patients is associated with increased cytomegalovirus-specific T-cell responses. AIDS. 2006;20:2275–2283. doi: 10.1097/QAD.0b013e3280108704. [DOI] [PubMed] [Google Scholar]
- 28.Hughes GH, Cutter G, Donahue R, et al. Recruitment in the Coronary Artery Disease Risk Development in Young Adults (CARDIA) study. Control Clin Trials. 1987;8(Suppl):68S–73S. doi: 10.1016/0197-2456(87)90008-0. [DOI] [PubMed] [Google Scholar]
- 29.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–1116. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 30.Myers GL, Rifai N, Tracy RP, et al. CDC/AHA Workshop on Markers of Inflammation and Cardiovascular Disease: Application to Clinical and Public Health Practice: report from the laboratory science discussion group. Circulation. 2004;110:e545–e549. doi: 10.1161/01.CIR.0000148980.87579.5E. [DOI] [PubMed] [Google Scholar]
- 31.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–E258. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 32.Efron BTR. An Introduction to the Bootstrap. London, UK: Chapman and Hall; 1993. [Google Scholar]
- 33.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 34.Dolan SE, Hadigan C, Killilea KM, et al. Increased cardiovascular disease risk indices in HIV-infected women. J Acquir Immune Defic Syndr. 2005;39:44–54. doi: 10.1097/01.qai.0000159323.59250.83. [DOI] [PubMed] [Google Scholar]
- 35.Nascimento MM, Bruchfeld A, Suliman ME, et al. Effect of hepatitis C serology on C-reactive protein in a cohort of Brazilian hemodialysis patients. Braz J Med Biol Res. 2005;38:783–788. doi: 10.1590/s0100-879x2005000500017. [DOI] [PubMed] [Google Scholar]
- 36.Domitrovich AM, Felmlee DJ, Siddiqui A. Hepatitis C virus nonstructural proteins inhibit apolipoprotein B100 secretion. J Biol Chem. 2005;280:39802–39808. doi: 10.1074/jbc.M510391200. [DOI] [PubMed] [Google Scholar]
- 37.Serfaty L, Andreani T, Giral P, et al. Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. J Hepatol. 2001;34:428–434. doi: 10.1016/s0168-8278(00)00036-2. [DOI] [PubMed] [Google Scholar]
- 38.Sesmilo G, Fairfield WP, Katznelson L, et al. Cardiovascular risk factors in acromegaly before and after normalization of serum IGF-I levels with the GH antagonist pegvisomant. J Clin Endocrinol Metab. 2002;87:1692–1699. doi: 10.1210/jcem.87.4.8364. [DOI] [PubMed] [Google Scholar]
- 39.Sesmilo G, Miller KK, Hayden D, et al. Inflammatory cardiovascular risk markers in women with hypopituitarism. J Clin Endocrinol Metab. 2001;86:5774–5781. doi: 10.1210/jcem.86.12.8087. [DOI] [PubMed] [Google Scholar]
- 40.Sesmilo G, Biller BM, Llevadot J, et al. Effects of growth hormone administration on inflammatory and other cardiovascular risk markers in men with growth hormone deficiency. A randomized, controlled clinical trial. Ann Intern Med. 2000;133:111–122. doi: 10.7326/0003-4819-133-2-200007180-00010. [DOI] [PubMed] [Google Scholar]


