Abstract
Cytotoxic T lymphocytes are critical for the control of respiratory syncytial virus infection in humans and mice. Recently, we identified a new H-2Kd restricted subdominant epitope in the RSV M2 protein. In this study, we investigated if modification of anchor residues at positions 2 and 9 in the dominant M282–90 epitope in the M2 protein would alter the CTL epitope dominance hierarchy following immunization with plasmid DNA encoding M2 proteins. We showed that immunogenicity of the subdominant epitope M2127–135 was enhanced when the anchor residues of the dominant epitope were mutated, suggesting that the immunodominant epitope induces a suppression of response to the subdominant epitope.
Keywords: Respiratory Syncytial Viruses, DNA vaccines, Epitopes, T-Lymphocyte
Introduction
Respiratory syncytial virus causes serious lower respiratory tract illness in infants, young children and the elderly [1–3]. To date, no vaccine has been approved for human use, and serious concerns have been raised about the previous experience with a formalin-inactivated respiratory syncytial virus (RSV) vaccine candidate in which vaccination was associated with enhanced disease [4–6]. Virus-specific cytotoxic T lymphocytes (CTLs) are critical for controlling RSV infection in mice and humans and thus are likely to be an important mediator for protection induced by a successful RSV vaccine. Previously, it has been shown that adoptively-transferred CD8+ CTLs can protect BALB/c mice from secondary infection by limiting viral replication in vivo [7]. However, the same CD8+ CTLs can contribute to RSV pathogenesis in the lungs and potentially lethal pulmonary disease in mice when administered in high doses [7]. Therefore, an intricate balance between controlling infection and aggravating disease by RSV-specific CD4+ and CD8+ T cells likely needs to be established when developing a safe and effective vaccine for humans.
CD8+ T cells recognize small antigenic peptides (8–10 amino acids) that are associated with major histocompatibility complex (MHC) class I molecules. For most viruses, although there are a number of potential motifs that are favorable for class I binding, only a handful of dominant epitopes are found. In humans, a number of HLA-restricted RSV T cell epitopes have been found in the nucleocapsid (N) protein, fusion (F) protein, the matrix 2 open reading frame 1 (M2-1) protein and the short hydrophobic (SH) protein [8–10]. In mice, the CD8+ T cell responses are highly focused on a few epitopes in the F, glycoprotein (G), matrix (M) and M2-1 proteins, possibly due to more limited breadth of MHC class I molecules in inbred animals [11–15]. A hierarchy in the immunogenicity of these RSV epitopes has been demonstrated [16, 17]. Epitopes that induce the most robust CTL responses are termed immunodominant epitopes, while those eliciting lower level or frequency of responses have been termed subdominant epitopes. The mechanisms governing this hierarchy are poorly understood but could be attributed to several factors such as (i) the kinetics and amount of antigen production, (ii) the efficiency of intracellular processing of antigenic peptide, (iii) the binding affinity of peptide to MHC class I and (iv) the T-cell receptor repertoire [18–22]. CTL responses from immunodominant epitopes have been reported to directly suppress the development of CTLs from subdominant epitopes through interferon-γ (IFN-γ) [23]. In individuals infected with human immunodeficiency virus (HIV), CTL responses targeting subdominant epitopes have been found to contribute to control of virus replication and escape [24, 25]. However, vaccine constructs that target multiple epitopes with different degrees of dominance failed to alter the inherited immunodominance hierarchy in animals [26].
We previously identified a RSV-specific, H-2Kd-restricted subdominant epitope in the M2-1 protein (M2127–135: VYNTVISYI) in BALB/c mice that is present in the same protein as the most dominant epitope in the virus for BALB/c mice (M282–90: SYIGSINNI) [27]. We found that there is a tissue-specific regulation of CD8+ T lymphocytes immunodominance in RSV infection that affects the ratio of cells specific for these two epitopes. In the current study, we sought to determine if the immunodominance hierarchy could be manipulated by vaccination with altered immunogens. We examined the immunogenicity of a DNA vaccine where the anchor residues of the dominant epitope (M282–90: SYIGSINNI) were mutated. A comparison of the responses by DNA vaccination to these two epitopes revealed a consistent immunodominance hierarchy as seen in infection model. The loss of the dominant T cell response from M2 mutant DNA immunization in BALB/c mice was compensated for by increased induction of subdominant responses. Furthermore, these subdominant T cells reduced viral replication in the vaccinated mice after RSV challenge. Taken together, this study shows promise for the approach of manipulating epitope hierarchy by depletion of an immunodominant epitope, and has implications for a rationale approach to T cell vaccine design.
Materials and Methods
Mice
Respiratory pathogen-free 6–8 week old female BALB/c (H-2d) mice were purchased from Harlan Sprague-Dawley Laboratory (Indianapolis, IN, USA). We adhered to the Guide for the Care and Use of Laboratory Animals of the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council in conducting the research described in this paper. All experiments were reviewed and approved by the Vanderbilt Institutional Animal Care and Use Committee. Animals were maintained in microisolator cages throughout the studies.
Cells and Virus
HEp-2 cells (ATCC no. CCL-23) were maintained in Opti-MEM I medium (Invitrogen) supplemented with 4 mM glutamine, 5 μg/ml amphotericin B, 50 μg/ml gentamicin, and 2% fetal bovine serum. The RSV wild-type strain A2 was grown in HEp-2 cell monolayer cultures, titrated, and stored in aliquots at −80 °C until use.
Peptide Design
Peptides for use in MHC-binding affinity assays were synthesized as previously described [27]. The peptides tested were based on the H-2d-restricted immunodominant peptide in the wild-type sequence of the RSV strain A2, a peptide spanning amino acids 82–90 and designated here M282–90 (SYIGSINNI), and a peptide spanning amino acids 127–135 and designated here M2127–135 (RVYNTVISY). Variant peptides and the rationale for them are shown in Table 1.
Table 1.
Peptide sequences used in these studies
| Peptide designation | Sequence | Rationale |
|---|---|---|
| M282–90 | SYIGSINNI | The wild-type epitope spanning amino acids 82–90 |
| M282–90ΔP2 | SRIGSINNI | Alteration of the anchor residue at position 2 (Y83R) |
| M282–90ΔP9 | SYIGSINNT | Alteration of the anchor residue at position 9 (I90T) |
| M282–90ΔP2ΔP9 | SRIGSINNT | Alteration of both anchor residues (Y83R and I90T) |
Peptide Synthesis
Peptides were derivatized with a monodisperse PEG685 linker (Quanta BioDesign), for improved solubility, using a standard coupling procedure. m-dPEG685 NHS ester (4 eq) and HOBt (4 eq) were dissolved in DMF and added to the N-Fmoc deprotected peptidyl resin. The coupling reaction was carried out overnight and its completeness was verified by the Kaiser test. Purified peptides were lyophilized (Labconco Freezezone 4.5) and stored at −80 °C until use. The peptides were synthesized and high-performance liquid chromatography-purified to more than 95% purity.
DNA vaccine design and preparation
We generated a plasmid DNA encoding the M2-1 protein of RSV wild-type strain A2 by cloning a sequence-optimized M2-1 cDNA into a mammalian cell expression vector that uses the CMV promoter. Gene optimization of the M2-1 open reading frame (605 bp) was performed using GeneOptimizer™ Software (GENEART GmbH, Regenburg, Germany). A codon-modified M2-1 gene was synthesized by GENEART. To generate the final wild-type DNA vaccine construct, the optimized M2-1 gene was subcloned between XhoI and BamHI restriction sites of pcDNA3.1 (Invitrogen). The wild-type plasmid (designated here M2-wt) contained both the H-2d-restricted immunodominant peptide spanning amino acids 82–90 (SYIGSINNI) and the subdominant epitope peptide spanning amino acids 127–135 (RVYNTVISY). A second DNA vaccine (designated here M2-mut) was generated that specified an altered sequence in the 82–90 immunodominant epitope region that contained two point mutations (Y83R and I90T) that eliminated the predicted anchor residues that are required for Kd binding for that epitope, while the subdominant epitope sequence was left unaltered. Plasmids encoding the M2-wt and M2-mut sequences were purified using the Endofree plasmid Giga kit (Qiagen, Hilden, Germany).
DNA immunization
Six-week-old female BALB/c mice were anesthetized with inhaled isoflurane (Aerrane, Baxter Healthcare Corporation, Deerfield, IL). Mice were immunized intramuscularly with 100 μg of RSV M2-wt or M2-mut plasmid DNA formulated in PBS divided into two 50 μg doses, one injected into each quadriceps. Immunization was performed at three time points, on day 0, day 14, and day 21.
RMA-S stabilization assay
RMA-S cells expressing Kd were kindly provided by Dr. E. Pamer (Memorial Sloan Kettering Cancer Center, New York, NY). The RMA stabilization assay was performed as described previously [19]. RMA-S cells were maintained in complete RPMI 1640 medium supplemented with 10% FBS, L-glutamine, 2-mercaptoethanol, HEPES, and gentamicin. RMA-S-Kd cells (106) were maintained at 26 °C for 18–24 h, pulsed with indicated amounts of peptide in duplicate for 45 min at 26 °C in a 5% CO2 atmosphere, and transferred to 37 °C for an additional 3 h. Cells were stained with biotinylated anti-Kd mAb (clone SF1-1,1. Pharmingen, San Jose, CA) followed by PE-conjugated streptavidin (Pharmingen), washed twice with FACS buffer, and analyzed by flow cytometry. The results were expressed as the mean fluorescence intensity (MFI) ratio. Percent MFI increase was calculated as follows: Percent MFI increase = (MFI with the given peptide-MFI without peptide)/(MFI without peptide) ×100.
Preparation of splenocytes
Mice were sacrificed by CO2 inhalation at varying time points, as indicated in the figures. The spleen was removed from each animal and placed separately into complete RPMI supplemented with 10% FBS, L-glutamine, 2-mercaptoethanol, HEPES, and gentamicin (designated R10 medium). The tissues were minced and ground through a sterile steel mesh to obtain a single-cell suspension. Cells were treated with red blood cell lysing buffer (Sigma-Aldrich, St-Louis, MO). Cells were counted and resuspended at the stated cell concentration for the appropriate in vitro assay.
ELISpot assay
96 well plates (MAIPSWU 10, Millipore, Bedford, MA) were coated with 10 μg/ml of anti-IFN-γ monoclonal antibody (clone A18, Mabtech, Stockholm, Sweden) in PBS (without Ca2+ or Mg2+) at 4 °C overnight. Plates then were washed with sterile PBS three times and blocked with 10% RPMI for at least 2 hours at room temperature. Peptides were added directly to wells in a volume of 50 μl, and then freshly isolated splenocytes were added at a concentration of 105 cells/well in 50 μl of R10 medium. The final concentration of the peptides in the screening assay was 10 μM. The plates were incubated for 18–20 h at 37 °C in 5% CO2. The plates then were washed, labeled with 2 μg/ml biotinylated anti-IFN-γ mAb (clone R4-6A2, Mabtech) in PBS, and incubated at room temperature for 3 h. After additional washes, avidin–peroxidase complex (Vector Laboratories, Burlingame, CA) was added to each well in PBS for 1 h at RT. The plates were washed, and IFN-γ producing cells were detected after a 4 min color reaction using 100 μl of AEC substrate (20 mg of 3-amino-9-ethylcarbazol [Sigma-Aldrich]) dissolved in 2.5 ml of dimethylformamide, diluted 1:20 in 47.5 ml of sodium-acetate buffer + 25 μl 30% H2O2. IFN-γ-producing cells were counted using an automated ELISpot reader system and ImmunoSpot 4 software (Cellular Technology Ltd, Cleveland, OH). Results were expressed as the number of spot-forming cells (SFC) per 106 input cells.
Intracellular Cytokine Staining (ICS) Assay and Tetramer Staining
To enumerate the number of IFN-γ-producing cells, intracellular staining was performed as previously described [28]. In brief, freshly isolated splenocytes (2 × 106) were left untreated or stimulated with individual peptides (1 μg/sample) or treated with PMA (10 ng/ml) and ionomycin (500 ng/ml) and costimulatory monoclonal antibodies anti-CD28 and anti-CD49d for 6 h at 37 °C in 5% CO2. Brefeldin A (10 μg/ml; Sigma) was added during the culture period to facilitate intracellular cytokine accumulation. Cell surface staining was performed followed by intracellular cytokine staining using the Cytofix/Cytoperm kit (BD Pharmingen, San Diego, CA) in accordance with the manufacturer’s protocol. For tetramer analysis, freshly isolated cells (2 × 106) in R10 medium were incubated with pre-titered, optimal amounts of H-2Kd M2 tetramers for 1h on ice followed by surface staining for CD3, CD4, and CD8.
The following antibodies were obtained from BD Pharmingen: anti-CD3 FITC, anti-CD4 PE-Cy7, anti-CD8 Cy7-allophycocyanin, and anti-IFN-γ PE. Tetramers were obtained from Beckman Coulter. The peptide epitopes used in these tetramers were SYIGSINNI (M282–90) and VYNTVISYI (M2127–135). Flow cytometry was performed using an LSRII cytometer (BD Immunocytometry Systems). Data analysis was performed using FlowJo software version 8.3 (Tree Star, San Carlos, CA).
Challenge, tissue collection, and plaque assay
Mice were challenged intranasally (i.n) with 106 PFU RSV strain A2 7 days after the third immunization. On day 4 after challenge, mice were sacrificed by CO2 inhalation. The lungs and nasal turbinates were harvested separately for virus quantification by plaque assay. Lung tissues were ground using a glass homogenizer in 3 ml of HBSS (Hanks Balanced Salt Solution) medium, and nasal turbinates were homogenized in 3 ml by grinding in a cold mortar and pestle with sterile sand. Homogenates were clarified by centrifugation in a tabletop centrifuge at 2,000 rpm at 4 °C for 10 min, and supernatants were frozen in cryovials at −80 °C until use. To determine RSV titers, 100 μl of 10-fold serial dilutions of the homogenates was added to confluent HEp-2 cell monolayer cultures in duplicate and cultured for 4 days under a semisolid methylcellulose overlay medium, followed by fixation with 80% cold methanol. Virus plaques were visualized by staining with immunoperoxidase using a cocktail of three anti-F protein mouse monoclonal antibodies.
Statistical analysis
Significant differences among groups were determined using Student t-test.
RESULTS
Binding affinity of MHC class I to peptides with altered anchor residues at position 2 and/or position 9
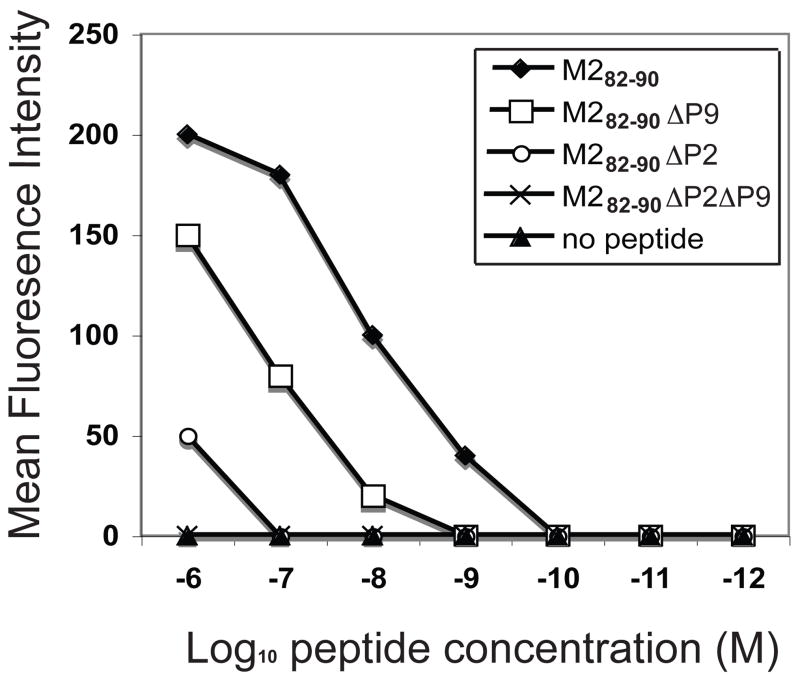
Nine amino acid long peptides were synthesized containing the wild-type M282–90 sequence (M282–90) or alternate sequences lacking either the canonical anchor residues at position 2 (M282–90ΔP2), position 9 (M282–90Δ P9) or both position 2 and 9 (M282–90ΔP2ΔP9) as shown in Table 1. We tested the relative affinity of each of the peptides for binding to the MHC class I molecule using the RMA-S cell MHC stabilization assay. A hierarchy of affinity was observed as evidenced by graded mean fluorescence intensities at varying intensities that differed for the various peptides. Cell surface MHC was not detected in the absence of peptide, as expected. The M282–90 peptide exhibited the highest affinity, the M282–90ΔP9 peptide showed about 10-fold reduced affinity, and the M282–90ΔP2 showed about 100-fold lower affinity to MHC class I molecules. Cell surface MHC class I binding was not detected for M282–90ΔP2ΔP9 peptide at any concentration tested (Figure 1).
Figure 1.
Binding of wild-type peptides or peptides mutated at H2-Kd anchor residues. RMA-S-Kd cells were incubated at 26 °C, and mixed with peptides at various concentrations. Binding of M282–90 (SYIGSINNI) or mutated peptides, M282–90ΔP2 (SRIGSINNI), M282–90ΔP9 (SYIGSINNT), and M282–90ΔP2ΔP9 (SRIGSINNT) to H2-Kd molecules was compared with that of the no-peptide control. The mean fluorescence intensity determined by FACS analysis is plotted against peptide concentration. The results shown represent the data from a single representative experiment, which was one of three with similar results.
T cell responses to mutated M2 genes in vivo
Next we constructed complete cDNA copies of the M2 gene with or without the immunodominant M282–90 epitope, as shown in Figure 2. The wild-type M2 gene was prepared as a DNA vaccine construct, as was a variant lacking both the canonical anchor residues at position 2 and 9, designated M2-mut. We immunized BALB/c mice with one of the DNA vaccine constructs two or three times and evaluated the induction of peptide-specific interferon-γ-secreting cells in the spleens of these vaccinated mice by ELISPOT. We previously showed that 15-mer peptides containing the immunodominant or subdominant epitopes worked better as reagents in ELISPOT or intracellular cytokine assays than the defined epitopes, which were of shorter length. Here, we used peptide M277–92 or peptide M281–96 as the dominant M282–90 epitope and peptide M2121–136 or peptide M2125–140 as the subdominant M2127–135 epitope in our ELISPOT assay. Both peptides from each individual M2 epitope gave comparable results in terms of the numbers of interferon-γ-secreting cells induced (Figure 3). Empty vector DNA immunization did not induce a significant response (Figure 3A and D). As expected, immunization with the M2-wt induced a robust response for the immunodominant M282–90 epitope, with over 200 spot forming cells per million splenocytes and a response to the subdominant epitope M2127–135 that was about four-fold lower (Figure 3B), as we have reported previously. Surprisingly, a T cell response was not detected in animals immunized with the M2-mut gene two weeks after vaccination (Figure 3C).
Figure 2.
Schematic diagram of the M2-wt or M2-mut plasmid DNAs. The M2-mut plasmid contains two substituted amino acids, Y83R and I90T.
Figure 3.
Frequencies of RSV M2-1 dominant and subdominant epitope-specific IFN-γ secreting mouse splenocytes after DNA immunization. BALB/c mice were immunized muscularly with 100 μg of empty vector (A and D) or DNA encoding either M2-wt protein (B and E) or M2-mut protein (C and F). Splenocytes were harvested 6 days after the second immunization (day14, B and C) or third immunization (day 21, E and F) and stimulated with peptides containing the dominant M282–90 epitope (peptide M277–92 and M281–96) or peptides containing the subdominant M127–135 epitope (peptide M2121–136 and M2125–140). Data are represented as mean ± SEM.
Following a third DNA immunization, responses to both immunodominant and subdominant epitopes were boosted, but the relative magnitudes remained comparable (Figure 3E). As expected, immunization with the M2-mut vaccine lacking the M282–90 epitope did not induce T cells to that epitope (Fig 3F). Remarkably, however, the M2-mut DNA vaccine induced a significantly enhanced T cell response to the subdominant epitope after three immunizations, with more than 400 spot forming cells per million cells detected with either of the peptides containing the subdominant epitope (Figure 3F). It should be noted that this magnitude of response was comparable to that mediated by the immunodominant epitope following M2-wt gene vaccination (Figure 3E).
Immunogenicity of mutated M2 genes measured by intracellular cytokine staining
There was the theoretical possibility that the interferon-γ-secreting cells induced by vaccination that were detected by ELISPOT could have been induced by cells other than class I MHC-restricted CD8+ T cells. Therefore, we also used an alternate technique to assess immunogenicity of the vaccine variants that defined the cell source of interferon-γ, namely flow cytometry-based intracellular cytokine assay (Figure 4). Splenocytes tested with a control peptide revealed that immunization with M2-wt or M2-mut vector was associated with a low background of non-specific interferon-γ-secreting CD8+ T cells (0.13 % in both cases). PMA/ionomycin stimulation was used as a maximal stimulus, yielding over 30% of cells expressing interferon-γ. Immunization with the M2-wt DNA vaccine induced a high percentage of CD8+ T cells reactive with the immunodominant epitope (2.6 – 2.8 %), while vaccination with the M2-mut construct lacking this epitope did not (0.1 – 0.31 %). The M2-wt vaccine induced lower responses to the subdominant epitope (0.53 – 0.69 %). The approximate 4:1 ratio of immunodominant to subdominant reactivity consistently seen with this technique was nearly identical to that detected by ELISPOT.
Figure 4.
Analysis of peptide-reactive CD8+ T lymphocytes 6 days after the third DNA immunization. Splenocytes were incubated with 1 μg/sample of an M282–90 containing peptide (peptide M277–92 or M281–96) or M2127–135 containing peptide (peptide M2121–136 or M2125–140) in an intracellular cytokine staining assay for IFN-γ. The dot plots represent the production of IFN-γ from one of the three representative experiments with similar results. Panels in the left column show results for splenocytes obtained from mice immunized with M2-mut DNA. Panels in the right column show results for splenocytes obtained from mice immunized with M2-mut DNA.
Frequency of M2-specific CD8+ T cells in M2-mut-immunized mice
We also assessed the number of M2-specific CD8+ T cells in mice immunized with M2-wt or M2-mut DNA using M282–90 and M2127–135 MHC tetramers. As expected, in mice vaccinated with M2-wt DNA, there was a higher frequency of CD8+ T cells specific for the M282–90 dominant epitope (1.2%) compared to the M2127–135 subdominant epitope (only 0.05%). In contrast, mice immunized with M2-mut DNA had a higher frequency of the subdominant epitope-specific CD8+ T cells (1.3%) versus the M282–90 specific CD8+ T cells (0.2%). Surprisingly, the frequency of M282–90-specfic T cells in M2-wt immunized mice was comparable to the frequency of M2127–135-specific T cells in M2-mut immunized animals (Figure 5).
Figure 5.
Direct enumeration of M282–90 or M2127–135 epitope-specific CD8+ T lymphocytes. BALB/c mice were immunized with M2-wt DNA or M2-mut DNA. Splenocytes were harvested 6 days following the third immunization and were analyzed unstained, stained with M282–90 tetramer or M2127–135 tetramer (B and D). Dot plots represent results for 20,000 CD3+/CD8+ T lymphocytes. Numbers shown in the rectangular gate correspond to the percentages of CD8+ lymphocytes that were tetramer-positive. Data shown are representative of three experiments.
Virus clearance in M2-mut DNA immunized mice
Given the change of epitope hierarchy in M2-mut DNA immunized animals, we were interested to see how this alteration affected viral replication in vivo. We challenged mice with RSV that were immunized previously with M2-wt or M2-mut DNA and measured viral titers in the lungs and nasal turbinates of these animals 4 days post-infection. Animals that were mock vaccinated with PBS or vaccinated with an empty vector exhibited high levels of viral titers in both the lungs (5.2–5.3 log10 PFU per gram of tissue) and nasal turbinates (4.7–4.8 log10 PFU/gram of tissue). Mice that were vaccinated with M2-wt DNA vaccine exhibited a 100-fold or 20-fold reduction in viral titer in the lungs or nasal turbinates respectively. A similar reduction was seen with mice vaccinated with the M2-mut DNA vaccine in the nasal turbinates, although there was only a 5-fold reduction in viral titer in the lungs (Table 2). Nonetheless, virus clearance was observed in both M2-wt and M2-mut DNA vaccinated group compared to control groups.
Table 2.
DNA immunization with a vector encoding RSV M2 protein lacking the immunodominant CTL epitope induces significant but reduced protection compared to immunization with the wild-type M2 genea
| Material used for immunization | Virus titer in the indicated tissue(log10 PFU/g tissue) | |
|---|---|---|
| Lung | Nasal turbinates | |
| PBS | 5.3 ± 0.1 | 4.8 ± 0.1 |
| pcDNA3.1 (empty vector) | 5.2 ± 0.04 b | 4.7 ± 0.09 b |
| M2-wt DNA vaccine | 3.2 ± 0.4 c | 3.4 ± 0.3 d |
| M2-Y83R_I90T DNA vaccine | 4.6 ± 0.1 c | 3.7 ± 0.4 d |
BALB/c mice (n=5) were immunized with one of the immunogens at day 0, 14 and 21, and challenged with live RSV on day 28 (day 7 after the third immunization, the expected peak day of CTL activity). Lung titers were determined on day 4 following challenge (the expected peak day of viral replication).
No significant difference from values obtained for mice immunized with PBS. (P≥0.05)
Significantly different from values obtained for mice immunized with PBS (P<0.01).
Significantly different from values obtained for mice immunized with PBS (P<0.02).
DISCUSSION
DNA vaccination has been proven to be a successful immunization scheme in animal models to induce CD8+ CTLs for protection. Most gene-based T-cell vaccines have focused on inducing responses to a few immunodominant epitopes [29–31]. We described previously a subdominant epitope in the RSV M2-1 protein (M2127–135) and showed that there is a tissue-specific regulation of the ratio of dominant epitope to subdominant epitope CTLs in various organs in mice during RSV infection. Other models have also shown that overall immunodominance patterns in antiviral CD8+ T cell responses can be changed by prolonged antigen stimulation [21, 32] or altering MHC alleles in transgenic mice [33]. In this study, we examined a DNA vaccine that lacked the M2-1 dominant epitope in vivo and showed that it could induce compensatory cellular responses from the subdominant epitope in the same viral protein.
MHC class I molecules display diverse peptides for T cell receptor recognition. Peptide binding onto allele-specific MHC class I molecules usually requires specific amino acid side-chain residues present at position 2 and 9 of the peptide. Binding of these residues into the MHC pocket stabilizes the MHC-peptide complex on the cell surface for T cell interaction. We mutated the amino acids at positions 2 and 9 of the immunodominant peptide M282–90 and measured the affinity of the peptide to Kd MHC-I molecules. Reduced peptide binding affinity was observed for peptides with a single mutation at either position 2 or 9. Direct binding could not be detected for a peptide with both of the anchor residues mutated, at any concentration tested. These data suggested that these mutations likely completely abolished loading of these peptides onto the MHC-I molecules on professional antigen presenting cells in vivo.
We have shown previously that the ratio of responses to the dominant epitope and the subdominant epitope in the M2-1 protein varies in different anatomic sites during RSV infection [27]. We investigated the responses of a DNA vaccine encoding both the dominant and subdominant epitopes in the RSV M2-1 protein (M2-wt). Response to the dominant epitope was seen after two DNA immunizations and the ratio of dominant to subdominant responses was maintained at a 3:1 ratio, as we have reported previously following RSV infection [27]. We also compared the responses in mice that were vaccinated with DNA lacking the M2 dominant epitope. We observed a delayed kinetics in the induction of M2127–135-specific T cells in M2-mut immunized mice when compared to the generation of M282–90-specific T cells in M2-wt vaccinated animals. Only after the third immunization did we see the induction of subdominant epitope-specific T cells. However, we do not yet understand the mechanism by which epitope dominance affects the kinetics of induction of T cells in vivo. We speculate that this interesting finding may in part be attributed to the 300-fold lower affinity of binding to MHC class I molecules for the M2127–135 peptide than for the M282–90 peptide, thus altering the MHC-I binding process and presentation. Although the responses were delayed, the numbers of M2127–135 tetramer-specific CD8+ T cells induced in M2-mut vaccinated mice were similar to the number of M282–90 tetramer-specific CD8+ T cells induced in M2-wt vaccinated animals. This finding may indicate a compensatory effect of T cells responding to the subdominant epitope when the dominant epitope is deleted.
In addition, we performed intracellular cytokine staining to determine responses from splenocytes in vaccinated animals. When splenocytes from M2-wt immunized mice were stimulated with M2 dominant or subdominant peptides, the frequency of M282–90-specific, interferon-γ secreting splenocytes was about three-fold higher than M2127–135-specific cells, consistent with the ELISPOT data as well as our previous observations [27]. In the case of M2-mut vaccinated animals, the frequency of M2127–135-specific T cells doubled while the frequency of M282–90 was markedly reduced. We showed that the induction of subdominant epitope-specific T cells was delayed in our vaccination model. An interesting feature of the data is that the frequency of M2127–135-specific T cells from M2-mut animals was slightly higher than the frequency of M282–90-specific T cells from M2-wt animals in this assay. It is possible that these CD8+ T cells responding to the subdominant epitope were functionally different than those responding to the dominant epitope in M2. It has been shown that subdominant epitopes from lymphocytic choriomeningitis virus (LCMV) induced protective CD8+ T cells populations with differing cytolytic activities and were found to be associated with the kinetics and epitope specificities of the T cells [34]. In addition, while this manuscript was in preparation, Krempl et al. reported a recombinant RSV infection approach to demonstrate that cytolytic activity of M2127–135-specific T cells was somewhat greater in mice infected with a recombinant RSV containing a mutation in the M282–90 epitope compared to those infected with wild-type virus when BAL cells were stimulated with M2127–135 peptide in vitro [35].
Viral replication also was assessed to determine if immunization with M2-mut DNA could reduce RSV titers in the lungs after wild-type virus challenge. Previously, it was reported that a recombinant vaccinia virus encoding M2 induced M282–90-specific cytotoxic T cells and those cells were the sole mediators of protection in immunized animals [36]. In our model, the viral titers in the lungs and the nasal turbinates were reduced in M2-mut immunized animals, but the extent of reduction was not as significant as those vaccinated with M2-wt. The lack of complete protection may be related to the immunization method or the route of immunization. Intranasal live virus infection or vaccination induces both systemic and mucosal immune responses, which could offer a significant advantage over intramuscular DNA immunization that induces principally a systemic immune response. In addition, live virus infection in general produces more antigen and more robust immune responses than DNA vaccination. Nonetheless, DNA immunization with RSV M2 plasmids is a useful tool in this study because the data reveal that immunization with M2 protein with deletion of an immunodominant epitope reduced viral titers in both the lungs and nasal turbinates of vaccinated animals.
In conclusion, this study demonstrated that a CTL epitope hierarchy could be manipulated by deletion of an immunodominant epitope in a single protein DNA vaccine. In the RSV model, the subdominant epitope, M2127–135, can serve as an important epitope in the absence of the otherwise immunodominant epitope, M282–90. However, the functionality and kinetics of T cells responding to a subdominant epitope may be different than those responding to a dominant epitope. Taken together, our findings have implications for understanding the immune response to RSV infection or vaccination and provide insight into methods for the rational design of vaccines that induce CD8+ T cells.
Acknowledgments
This work was supported in part by a grant from the NIAID, R01 AI-53222. We thank Dr. E. Pamer for providing RMA-S-Kd cells, and Dr. T. Hansen for providing RMA-S-Ld cells.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352(17):1749–59. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 2.Holzel A, Parker L, Patterson WH, White LL, Thompson KM, Tobin JO. The isolation of respiratory syncytial virus from children with acute respiratory disease. Lancet. 1963;1:295–8. doi: 10.1016/s0140-6736(63)92239-6. [DOI] [PubMed] [Google Scholar]
- 3.Chanock R, Finberg L. Recovery from infants with respiratory illness of a virus related to chimpanzee coryza agent (CCA). II Epidemiologic aspects of infection in infants and young children. Am J Hyg. 1957;66(3):291–300. doi: 10.1093/oxfordjournals.aje.a119902. [DOI] [PubMed] [Google Scholar]
- 4.Graham BS. Pathogenesis of respiratory syncytial virus vaccine-augmented pathology. Am J Respir Crit Care Med. 1995;152(4 Pt 2):S63–6. doi: 10.1164/ajrccm/152.4_Pt_2.S63. [DOI] [PubMed] [Google Scholar]
- 5.Murphy BR, Walsh EE. Formalin-inactivated respiratory syncytial virus vaccine induces antibodies to the fusion glycoprotein that are deficient in fusion-inhibiting activity. J Clin Microbiol. 1988;26(8):1595–7. doi: 10.1128/jcm.26.8.1595-1597.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy BR, Prince GA, Walsh EE, Kim HW, Parrott RH, Hemming VG, et al. Dissociation between serum neutralizing and glycoprotein antibody responses of infants and children who received inactivated respiratory syncytial virus vaccine. J Clin Microbiol. 1986;24(2):197–202. doi: 10.1128/jcm.24.2.197-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham BS, Bunton LA, Wright PF, Karzon DT. Role of T lymphocyte subsets in the pathogenesis of primary infection and rechallenge with respiratory syncytial virus in mice. J Clin Invest. 1991;88(3):1026–33. doi: 10.1172/JCI115362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cherrie AH, Anderson K, Wertz GW, Openshaw PJ. Human cytotoxic T cells stimulated by antigen on dendritic cells recognize the N, SH, F, M, 22K, and 1b proteins of respiratory syncytial virus. J Virol. 1992;66(4):2102–10. doi: 10.1128/jvi.66.4.2102-2110.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rock MT, Crowe JE., Jr Identification of a novel human leucocyte antigen-A*01-restricted cytotoxic T-lymphocyte epitope in the respiratory syncytial virus fusion protein. Immunology. 2003;108(4):474–80. doi: 10.1046/j.1365-2567.2003.01619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Venter M, Rock M, Puren AJ, Tiemessen CT, Crowe JE., Jr Respiratory syncytial virus nucleoprotein-specific cytotoxic T-cell epitopes in a South African population of diverse HLA types are conserved in circulating field strains. J Virol. 2003;77(13):7319–29. doi: 10.1128/JVI.77.13.7319-7329.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bangham CR, Openshaw PJ, Ball LA, King AM, Wertz GW, Askonas BA. Human and murine cytotoxic T cells specific to respiratory syncytial virus recognize the viral nucleoprotein (N), but not the major glycoprotein (G), expressed by vaccinia virus recombinants. J Immunol. 1986;137(12):3973–7. [PubMed] [Google Scholar]
- 12.Chang J, Srikiatkhachorn A, Braciale TJ. Visualization and characterization of respiratory syncytial virus F-specific CD8(+) T cells during experimental virus infection. J Immunol. 2001;167(8):4254–60. doi: 10.4049/jimmunol.167.8.4254. [DOI] [PubMed] [Google Scholar]
- 13.Jiang S, Borthwick NJ, Morrison P, Gao GF, Steward MW. Virus-specific CTL responses induced by an H-2K(d)-restricted, motif-negative 15-mer peptide from the fusion protein of respiratory syncytial virus. J Gen Virol. 2002;83(Pt 2):429–38. doi: 10.1099/0022-1317-83-2-429. [DOI] [PubMed] [Google Scholar]
- 14.Openshaw PJ, Anderson K, Wertz GW, Askonas BA. The 22,000-kilodalton protein of respiratory syncytial virus is a major target for Kd-restricted cytotoxic T lymphocytes from mice primed by infection. J Virol. 1990;64(4):1683–9. doi: 10.1128/jvi.64.4.1683-1689.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutigliano JA, Rock MT, Johnson AK, Crowe JE, Jr, Graham BS. Identification of an H-2D(b)-restricted CD8+ cytotoxic T lymphocyte epitope in the matrix protein of respiratory syncytial virus. Virology. 2005;337(2):335–43. doi: 10.1016/j.virol.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 16.Rutigliano JA, Ruckwardt TJ, Martin JE, Graham BS. Relative dominance of epitope-specific CD8+ T cell responses in an F1 hybrid mouse model of respiratory syncytial virus infection. Virology. 2007;362(2):314–9. doi: 10.1016/j.virol.2006.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terrosi C, Di Genova G, Savellini GG, Correale P, Blardi P, Cusi MG. Immunological characterization of respiratory syncytial virus N protein epitopes recognized by human cytotoxic T lymphocytes. Viral Immunol. 2007;20(3):399–406. doi: 10.1089/vim.2007.0041. [DOI] [PubMed] [Google Scholar]
- 18.Brehm MA, Pinto AK, Daniels KA, Schneck JP, Welsh RM, Selin LK. T cell immunodominance and maintenance of memory regulated by unexpectedly cross-reactive pathogens. Nat Immunol. 2002;3(7):627–34. doi: 10.1038/ni806. [DOI] [PubMed] [Google Scholar]
- 19.Busch DH, Pamer EG. MHC class I/peptide stability: implications for immunodominance, in vitro proliferation, and diversity of responding CTL. J Immunol. 1998;160(9):4441–8. [PubMed] [Google Scholar]
- 20.Probst HC, Tschannen K, Gallimore A, Martinic M, Basler M, Dumrese T, et al. Immunodominance of an antiviral cytotoxic T cell response is shaped by the kinetics of viral protein expression. J Immunol. 2003;171(10):5415–22. doi: 10.4049/jimmunol.171.10.5415. [DOI] [PubMed] [Google Scholar]
- 21.van der Most RG, Murali-Krishna K, Lanier JG, Wherry EJ, Puglielli MT, Blattman JN, et al. Changing immunodominance patterns in antiviral CD8 T-cell responses after loss of epitope presentation or chronic antigenic stimulation. Virology. 2003;315(1):93–102. doi: 10.1016/j.virol.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Yewdell JW, Bennink JR. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Annu Rev Immunol. 1999;17:51–88. doi: 10.1146/annurev.immunol.17.1.51. [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez F, Harkins S, Slifka MK, Whitton JL. Immunodominance in virus-induced CD8(+) T-cell responses is dramatically modified by DNA immunization and is regulated by gamma interferon. J Virol. 2002;76(9):4251–9. doi: 10.1128/JVI.76.9.4251-4259.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frahm N, Kiepiela P, Adams S, Linde CH, Hewitt HS, Sango K, et al. Control of human immunodeficiency virus replication by cytotoxic T lymphocytes targeting subdominant epitopes. Nat Immunol. 2006;7(2):173–8. doi: 10.1038/ni1281. [DOI] [PubMed] [Google Scholar]
- 25.Friedrich TC, Valentine LE, Yant LJ, Rakasz EG, Piaskowski SM, Furlott JR, et al. Subdominant CD8+ T-cell responses are involved in durable control of AIDS virus replication. J Virol. 2007;81(7):3465–76. doi: 10.1128/JVI.02392-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Subbramanian RA, Kuroda MJ, Charini WA, Barouch DH, Costantino C, Santra S, et al. Magnitude and diversity of cytotoxic-T-lymphocyte responses elicited by multiepitope DNA vaccination in rhesus monkeys. J Virol. 2003;77(18):10113–8. doi: 10.1128/JVI.77.18.10113-10118.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S, Miller SA, Wright DW, Rock MT, Crowe JE., Jr Tissue-specific regulation of CD8+ T-lymphocyte immunodominance in respiratory syncytial virus infection. J Virol. 2007;81(5):2349–58. doi: 10.1128/JVI.01910-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murali-Krishna K, Altman JD, Suresh M, Sourdive D, Zajac A, Ahmed R. In vivo dynamics of anti-viral CD8 T cell responses to different epitopes. An evaluation of bystander activation in primary and secondary responses to viral infection. Adv Exp Med Biol. 1998;452:123–42. doi: 10.1007/978-1-4615-5355-7_14. [DOI] [PubMed] [Google Scholar]
- 29.Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization. J Virol. 1997;71(4):2715–21. doi: 10.1128/jvi.71.4.2715-2721.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barouch DH, Craiu A, Santra S, Egan MA, Schmitz JE, Kuroda MJ, et al. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J Virol. 2001;75(5):2462–7. doi: 10.1128/JVI.75.5.2462-2467.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loirat D, Lemonnier FA, Michel ML. Multiepitopic HLA-A*0201-restricted immune response against hepatitis B surface antigen after DNA-based immunization. J Immunol. 2000;165(8):4748–55. doi: 10.4049/jimmunol.165.8.4748. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann CC, Altman JD, Hinton D, Stohlman SA. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J Immunol. 1999;163(6):3379–87. [PubMed] [Google Scholar]
- 33.Allan JE, Doherty PC. Consequences of a single Ir-gene defect for the pathogenesis of lymphocytic choriomeningitis. Immunogenetics. 1985;21(6):581–9. doi: 10.1007/BF00395882. [DOI] [PubMed] [Google Scholar]
- 34.Rodriguez F, Slifka MK, Harkins S, Whitton JL. Two overlapping subdominant epitopes identified by DNA immunization induce protective CD8(+) T-cell populations with differing cytolytic activities. J Virol. 2001;75(16):7399–409. doi: 10.1128/JVI.75.16.7399-7409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vallbracht S, Jessen B, Mrusek S, Enders A, Collins PL, Ehl S, et al. Influence of a single viral epitope on T cell response and disease after infection of mice with respiratory syncytial virus. J Immunol. 2007;179(12):8264–73. doi: 10.4049/jimmunol.179.12.8264. [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni AB, Morse HC, 3rd, Bennink JR, Yewdell JW, Murphy BR. Immunization of mice with vaccinia virus-M2 recombinant induces epitope-specific and cross-reactive Kd-restricted CD8+ cytotoxic T cells. J Virol. 1993;67(7):4086–92. doi: 10.1128/jvi.67.7.4086-4092.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]