Abstract
This study compared the behaviour profile of cases in the Australian Rett Syndrome Database (ARSD) with those in a British study using the Rett Syndrome Behaviour Questionnaire (RSBQ) then examined behavioural patterns as measured by the RSBQ by genetic status. There were 145 Australian cases meeting the criteria for the first arm of the study and 135 for the second arm. Comparison of the scores obtained from the British and Australian cohorts indicated that the RSBQ was a satisfactory measure for describing behaviours in Rett syndrome (RS). Overall, there were some differences amongst the behaviour patterns of cases with the well-known common mutations. Fear/anxiety was more commonly reported in those with R133C and R306C. Those with the R294X mutation were more likely to have mood difficulties and body rocking but less likely to have hand behaviours and to display repetitive face movements. In contrast, hand behaviours were more commonly reported in those with R270X or R255X. We found the RSBQ is an appropriate instrument for measuring behaviour in girls with RS. Some behaviours differ according to genetic mutation but there is both inter and intra mutation variation in behaviour and there is a need for larger studies involving international collaboration to improve statistical power.
Introduction
Rett syndrome (RS) is a neurodevelopmental disorder that affects 1 in 10 000 females (Leonard et al. 1997) and rarely occurs in males. Although studies have shown that some female cases may not have a period of normal early development (Leonard and Bower 1998; Leonard et al. in press; Nomura et al. 1984), this disorder is almost impossible to identify at birth, and there are usually no specific differentiating features until the onset of regression at 6-18 months of age. Clinical criteria (The Rett Syndrome Diagnostic Criteria Work Group 1988) and a four-stage progression model (Hagberg and Witt Engerstrom 1986) were developed to assist medical practitioners with the diagnosis of RS, but the stage to stage transition is not always clearly defined. There is considerable variability with respect to age of onset (Charman et al. 2002; Coleman et al. 1988; Goutieres and Aicardi 1986; Kerr et al. 1987), nature of the regression (Fiumara et al. 2002; Zappella 1992), clinical outcomes (Colvin et al. 2003), and expression of behaviours (Coleman et al. 1988; Mount et al. 2002a; Mount et al. 2001; 2002b; 2003; Sansom et al. 1993).
Genetic testing now plays an important role in RS diagnosis. In 1999, mutations within the MECP2 gene encoding X-linked methyl–CpG-binding protein 2 (MeCP2) were identified as the major cause of RS. While over 200 pathogenic mutations have been identified (Christodoulou and Grimm 2003), there are eight C>T transition mutations (T158M, R168X, R255X, R270X, R306C, R294X, R133C and R106W) accounting for 69% of mutation positive cases (Christodoulou and Grimm 2003). Variation in individual behaviours and the pattern of regression may be related to genotypic differences within RS (Mount et al. 2002a). There is evidence of a relationship between genotype and clinical severity, with some mutations having either a milder or a more severe clinical course (Colvin et al. 2003; Colvin et al. 2004; Fukuda et al. 2005; Huppke et al. 2002; Leonard et al. 2003; Schanen et al. 2004; Smeets et al. 2003).
Behavioural manifestations commonly seen in RS include pathognomonic hand stereotypies and breathing abnormalities (The Rett Syndrome Diagnostic Criteria Work Group, 1988). The diagnostic criteria for RS were recently revised and now include impaired sleep pattern from infancy and bruxism (Hagberg et al. 2002). Inappropriate vocalisation, night laughing and mouth grimacing, thought to be common in this disorder, are often associated with other causes of intellectual disability but should not be disregarded in RS research. Inappropriate vocalisation may restrict the ability of those families to interact with the community and sleep disturbances, and can be difficult to manage and burdensome for parents (Hunter 2002).
As more information becomes available about the genetic causes of intellectual disability (Poplawski 2003), there has been greater recognition of the association of syndrome specific behavioural symptomatology with genetic disorders (Dykens and Hodapp 2001). In an attempt to better delineate the behaviour profile of RS, Mount et al (2002) used the Developmental Behaviour Checklist (Einfeld and Tonge 1995) to compare the behaviour of girls with RS with a group of females with the same level of intellectual disability. They found that although girls with RS had a consistently higher score in the autistic related domain, there were qualitative differences between the behaviours seen in the two groups that could not be differentiated by the Developmental Behaviour Checklist (Mount et al. 2003). By reviewing published literature and consulting with parents and physicians about the behaviours found in RS in comparison with other intellectual disabilities, they devised the Rett Syndrome Behaviour Questionnaire (RSBQ). It contains 42 items designed to measure behaviour frequency and severity. The items are rated from 0-2 (Mount et al. 2002a) with a score of zero indicating the behaviour is not true for an individual; 1 meaning the behaviour is somewhat or sometimes true in the individual and 2 meaning that the behaviour is often or very true in the individual. The behaviours were organised, using factor analysis, into 8 domains: General Mood, Breathing Problems, Hand Behaviours, Repetitive Face Movements, Body Rocking and Expressionless Face, Night-time Behaviours, Fear/Anxiety, and Walking/Standing. The authors trialed the RSBQ on a cohort of females, identified through the UK Rett Syndrome Association and compared them with a group of girls of similar intellectual disability identified through local special needs schools.
The Australian Rett Syndrome Database (ARSD), which commenced in 1992, is an on-going population-based registry of Australian RS cases born since 1976. Cases are recruited from a variety of sources and ascertainment is considered almost complete (Leonard et al. 1997). Families and clinicians provide data on enrolment and follow up questionnaires, administered to parents and carers every two years (Colvin et al. 2003), seek information on various aspects of RS, including measures of current behaviour using the RSBQ and functional ability using a questionnaire version of the WeeFIM (the Functional Independence Measure for Children)(Ottenbacher et al. 1999). Genetic testing was initiated for the cohort in 2000 (Weaving et al. 2003).
We have only encounterd one instance of the RSBQ's research application (Charman et al. 2005) since the UK group published their initial work (Mount et al. 2002a). Our present study aimed to compare RSBQ scores in the ARSD with the UK cohort and to use the RSBQ scores to investigate behavioural differences associated with mutation effects in the Australian cohort.
Materials and Methods
This analysis is based on data on 246 verified female cases of RS included in the ARSD in 2003. A follow-up questionnaire had been completed on 187 of these in 2002, and on a further 14 only in 2000; therefore, current behaviour had been reported on a total of 201 (82%) at one of the follow-up times. The majority (179/201) had undergone molecular testing and of these 135 had a mutation identified in the MECP2 gene. This cohort of 135 subjects was used to investigate the association between mutation type and behaviour profile. To compare the Mount cohort and the ARSD with respect to frequency and severity of current behaviour we included the 145 cases who were aged less than 19 years and had had one follow-up questionnaire completed, irrespective of their mutation status.
Each domain of the RSBQ is calculated as the total score for all questions in that domain (Mount et al. 2002a). As we used an earlier version of the RSBQ (personal communication Rebecca Mount) one question Makes repetitive face movements involving fingers around the tongue was missing and the analysis had to be adjusted to account for this.
Mutation testing was performed in molecular genetics laboratories in Perth and Sydney using previously reported methods (Weaving et al. 2003). Commonly occurring mutations were coded individually. Mutations were deemed common when their frequency was greater than six, thus only the following seven mutations: T158M, R168X, R294X, R270X, R133C, R306C and R255X were coded as discrete entities. C-terminal deletions were coded as a separate group and the remaining mutations including R106W were allocated to the category “other mutations”.
Analysis of variance (ANOVA) was used to estimate the marginal mean RSBQ domain scores and 95% confidence intervals for each mutation type in the ASRD cohort. For comparing the proportions of individuals exhibiting behaviour in each question item between the ARSD registry and the Mount cohort, cross-tabulations were performed and chi-square statistics calculated. For investigating differences in severity of behaviour between the ARSD registry and the Mount cohort, one-sample t-tests were used to compare individual ARSD domain scores with mean domain scores from the Mount cohort. Individual domain scores from the Mount cohort were unavailable. Because of the large sample size (n=145), t-tests applied to mean scores do not violate distributional assumptions. Multiple Discriminant Analysis was used in determining to what extent individual mutations could be identified by discriminant function values comprising linear combinations of RSBQ domain scores. SPSS V11.0 was used to perform all the analyses.
Results
The age of females in the genetic analysis ranged from 2.8 to 27.4 years (mean=14.1; SD=6.2). Those older than 19 years were excluded from comparison with the Mount cohort.
Behaviours identified in the RSBQ were reported to occur at similar frequencies in the ARSD population and the Mount cohort in 38 of 42 (90%) questions (Table 1). In three items (Q14, 24 & 26, Table 1) the behaviour was reported to occur significantly more commonly in the ARSD population, and in one item (Q2, Table 1) significantly less commonly. Comparison of the two cohorts at the domain level, gave an indication of the difference in severity of behaviour between the two cohorts (Table 2). A higher domain score relates to more severe or frequent behaviour in the cohort. For the domains of hand behaviour, body rocking/expressionless face and face movement, the ARSD cohort had a higher domain score, suggesting this behaviour domain was more frequent or severe in the ARSD cohort than in the Mount cohort. There was no statistically significant meaningful difference between the two cohorts in the remaining five domains.
Table 1. Proportion of cases for which behavioural items were stated as ‘somewhat true’ or ‘always true’.
| Behavioural Characteristic | Proportion item true (%) | Diff (%) | |
|---|---|---|---|
| ARSD
(n=145) |
UK
(n=143) |
||
| Domain1: General Mood | |||
| 1. Abrupt changes in mood. | 75 | 77 | -2 |
| 2. Spells of screaming for no apparent reason during the day | 44 | 62 | -18 * |
| 3. There are times when she is miserable for no apparent reason. | 86 | 89 | -3 |
| 4. Spells of inconsolable crying for no apparent reason during the day. | 58 | 66 | -8 |
| 5. There are times when she is irritable for no apparent reason. | 80 | 85 | -5 |
| 6. Screams hysterically for long periods of time and cannot be consoled. | 37 | 48 | -11 |
| 7. There are certain days/periods where she performs much worse than usual. | 93 | 87 | +6 |
| 8. Vocalises for no apparent reason. | 78 | 78 | - |
| Domain 2: Breathing Problems | |||
| 9. There are times when breath is held. | 66 | 70 | -4 |
| 10. Swallows air. | 52 | 57 | -5 |
| 11. Abdomen fills with air and sometimes feels hard. | 56 | 59 | -3 |
| 12. There are times when breathing is deep and fast. | 66 | 76 | -10 |
| 13. Air or saliva is expelled from mouth with force. | 62 | 55 | +7 |
| Domain 3: Hand Behaviours | |||
| 14. Does not use hands for purposeful grasping. | 88 | 71 | +17 * |
| 15. Restricted repertoire of hand movements. | 87 | 81 | +6 |
| 16. Hand movements are uniform and monotonous. | 92 | 92 | - |
| 17. The amount of time spent looking at objects is longer than the time spent holding or manipulating them. | 86 | 80 | +6 |
| 18. Has difficulty breaking/stopping repetitive hand movements. | 87 | 90 | -3 |
| 19. Has frequent naps during the day. | 70 | 61 | -9 |
| Domain 4: Repetitive Face Movements | |||
| 20. Makes mouth grimaces. | 73 | 79 | -6 |
| 21. Makes repetitive tongue movements. | 56 | 56 | - |
| 22. Makes grimacing expressions with face. | 77 | 76 | +1 |
| Domain 5: Body Rocking and Expressionless Face | |||
| 23. Rocks body repeatedly | 52 | 43 | +9 |
| 24. Expressionless face. | 66 | 41 | +25 * |
| 25. Rocks self when hands are prevented from moving. | 51 | 44 | +7 |
| 26. Seems to look through people into the distance. | 86 | 73 | +13 * |
| 27. Uses eye gaze to convey feelings, needs and wishes. | 92 | 91 | +1 |
| 28. Tendency to bring hands together in front of chin or chest. | 75 | 70 | +5 |
| Domain 6: Night-time Behaviours | |||
| 29. Spells of screaming for no apparent reason during the night. | 42 | 46 | -4 |
| 30. Spells of inconsolable crying for no apparent reason during the night. | 34 | 43 | -9 |
| 31. Spells of laughter for no apparent reason during the night. | 68 | 64 | +4 |
| Domain 7: Fear/Anxiety | |||
| 32. Spells of apparent panic. | 63 | 73 | -10 |
| 33. Spells of apparent anxiety/fear in unfamiliar situations | 79 | 74 | +5 |
| 34. Seems frightened when there are sudden changes in own body positions. | 80 | 78 | +2 |
| 35. There are times when parts of the body are held rigid. | 96 | 93 | +3 |
| Domain 8: Walking Standing | |||
| 36. Walks with stiff legs. | 56 | 66 | -10 |
| 37. Although can stand independently tends to lean on objects or people. | 46 | 50 | -4 |
| Behaviours with no domain | |||
| 38. Spells of laughter for no apparent reason during the day. | 82 | 90 | -8 |
| 39. Shifts gaze with a slow horizontal turn of the head. | 78 | 69 | +9 |
| 40. Makes repetitive movements with hands apart. | 72 | 60 | +12 |
| 41. Appears isolated. | 71 | 62 | +9 |
| 42. Has wounds on hands as a result of repetitive hand movements. | 62 | 57 | +5 |
Difference in proportion is statistically significant (DF=1; α=0.05)
Table 2. ARSD cohort and UK group mean scores on the RSBQ.
| ARSD
(mean) |
UK
(mean) |
Difference in means | t-test | ||
|---|---|---|---|---|---|
| Domain | 95% CI of the difference in the means | p-value | |||
| General Mood | 7.80 | 8.34 | -0.54 | -1.17, 0.09 | 0.093 |
| Breathing problems | 4.74 | 4.99 | -0.25 | -0.75, 0.25 | 0.320 |
| Hand behaviours | 8.29 | 7.41 | 0.88 | 0.44, 1.32 | <0.001 |
| Face movements* | 4.15 | 3.54 | 0.61 | 0.35, 0.88 | <0.001 |
| Body rocking/expressionless face | 6.51 | 5.14 | 1.37 | 0.98, 1.76 | <0.001 |
| Night-time behaviours | 1.91 | 2.01 | -0.10 | -0.38, 0.18 | 0.476 |
| Anxiety/fear | 4.72 | 4.55 | 0.17 | -0.17, 0.50 | 0.322 |
| Walking/standing | 1.61 | 1.78 | -0.17 | -0.42, 0.07 | 0.154 |
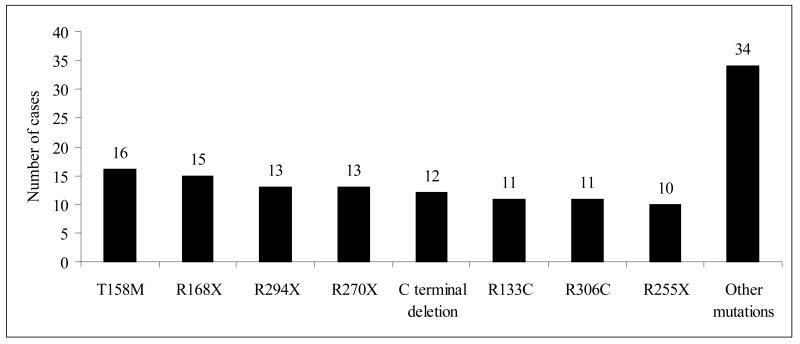
The distribution of mutations in the genetic cohort is shown in Figure 1. Ninety-three of the 135 females in this cohort had one of eight commonly recognised mutations. Thus, the common mutations made up 69% (66% excluding R106W) of all MECP2 mutations in this cohort. In 44 cases no MECP2 mutation was detected.
Figure 1.
Frequency of common mutations in the ARSD
The mean total RSBQ score varied by mutation but not significantly (p=0.52). The overall mutation effect did not account for a significant variation in any of the domain scores. However, the individual mutations that appeared most often to be different from the other mutations were R294X, R306C and R270X.
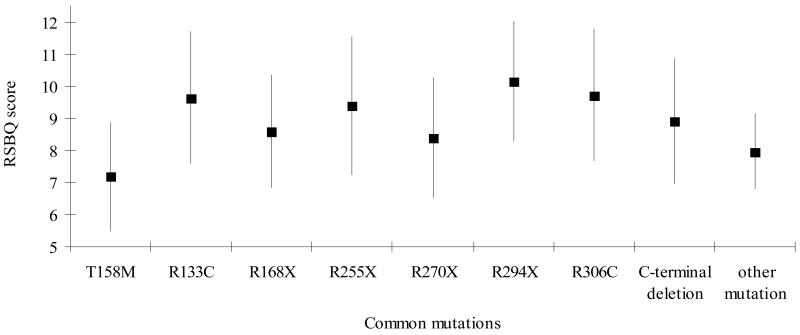
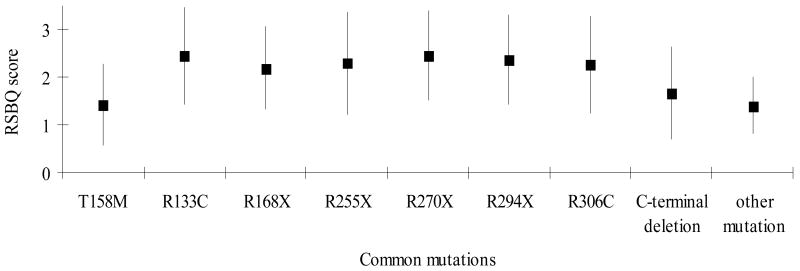
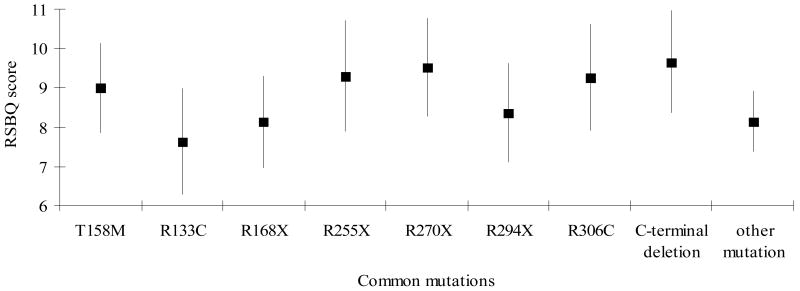
Cases with R294X were more likely to have mood difficulties (Figure 2.1), body rocking (results not shown) and night-time behaviours (Figure 2.2) but less likely to have hand behaviours (Figure 2.3) and to display repetitive face movements (results not shown). They also had a higher score for the walking or standing domain (results not shown). Similarly, those with R133C were more likely to have mood difficulties (Figure 2.1) and night-time behaviours (Figure 2.2) and less likely to have hand behaviours reported (Figure 2.3). They were also reported to experience more fear or anxiety (results not shown). The pattern with R306C was similar to R133C with respect to the mood difficulties (Figure 2.1) and fear or anxiety (results not shown) but not in relation to hand behaviours (Figure 2.3). These cases were also more likely to have body rocking and repetitive face movements reported (results not shown). Those with T158M were less likely to have mood disturbances (Figure 2.1) reported than the three previous mutations described whilst hand behaviours (Figure 2.3) were relatively commonly reported.
Figure 2.
Figure 2.1:Estimated marginal mean scores and 95% Confidence Interval for General Mood Score between common mutations
Figure 2.2:Estimated marginal mean scores and 95% Confidence Interval for Night-time Behaviours Score between common mutations
Figure 2.3:Estimated marginal mean scores and 95% Confidence Interval for Hand Behaviours Score between common mutations
Cases with R270X had the lowest score for the walking/standing domain (results not shown). They had more hand behaviours (Figure 2.3) and repetitive face movements as well as night-time behaviours (results not shown). Cases with R255X also had more hand behaviours reported (Figure 2.3). On the other hand cases with R168X were most likely to have repetitive face movements and less likely to have hand behaviours (Figure 2.3) or fear or anxiety reported. In contrast, the cases with C-terminal deletions were reported to have more severe hand behaviours (Figure 2.3) and body rocking but less repetitive face movements.
Discriminant analysis classified 26.7% of individuals into the correct mutation category based on combinations of RSBQ domain scores. This success rate fell to only 9.9% when cross-validation was used. The discriminant model performed best in identifying C-terminal deletion cases with 25.0% of these individuals correctly classified using cross-validation. Overall, the discriminant model was not significant (Wilks' Lambda Chi-Square (56df) = 49.7; p=0.71).
Discussion
We established that behaviours described by Mount et al (2002), in an opportunistic sample of 143 girls identified through the UK Rett syndrome parent association, were similar to those of the Australian population-based Rett syndrome cohort. When behaviours were grouped at the domain level, we found that the ARSD cohort differed significantly in 3/8 behaviours with higher scores, in the ARSD cohort in each case.
These differences in behaviour could be related to study design. Our study surveyed families of cases in the only ongoing population-based RS cohort where the process for diagnostic verification uses information from both clinicians and families and in most cases molecular data (Colvin et al. 2003; Leonard and Bower 1998; Leonard et al. 1997). On the other hand the Mount study (2002a) had less rigorous inclusion criteria, which could have resulted in a case group diluted by subjects without a clinically verified diagnosis of RS. The fact that in three of the eight domains behaviour occurred more frequently in the ARSD cohort than in the Mount cohort supports this hypothesis. Moreover, our questionnaire response fraction was considerably higher than in the UK sample (82% compared with 50%). It is, therefore, possible that the behaviour represented in our study may be more reflective of RS than the Mount study.
The main aim of this study was to determine whether the RSBQ could be helpful in capturing the behavioural phenotype associated with genetic variation in RS. Despite the small sample size, we consider that there was evidence of a pattern suggesting that cases with mutations R294X, R133C and R306C were more likely to exhibit behaviours relating either to mood, fear and anxiety or body rocking. Conversely, with one exception (R306C), these cases were less likely to exhibit hand behaviours. On the other hand, the cases with R270X and R255X mutations, both located in the nuclear localisation signal (NLS) region within the transcription repression domain (TRD), were more likely to manifest hand behaviours.
The RSBQ has eight behavioural domains (Mount et al. 2002a) representing behaviours both commonly recognised in RS as well as those identified much less frequently in the literature (Mount et al. 2001). The features reported in the general mood domain are not typically reported in RS other than during the regression period, following which there is said to be a period during which subjects are said to “wake up” and become more communicative. Two early studies did report screaming fits in 53/63 (Coleman et al. 1988) and 7/22 subjects (Naidu 1990). Our study and the UK study (Mount et al. 2002a) show that a substantial proportion of individuals have behavioural and mood difficulties which persist beyond regression.
The breathing problems domain, consisting principally of episodic hyperventilation and apnoeas during wakefulness, relates to one of the supportive criteria noted early in the history of RS. Recent research has comprehensively described the spectrum of breathing abnormalities and their relationship to autonomic dysfunction (Julu et al. 2001); however, information on clinical management is still lacking.
The hand behaviours domain included hand stereotypies and loss of purposeful hand function, which are hallmark features of the RS and necessary for diagnosis of the syndrome (Hagberg et al. 2002). Yet, research characterising the nature, course and mechanism of the hand stereotypies is limited (Mount et al. 2002a). The “repetitive face movements” which often include the tongue could also be considered to be part of the spectrum of stereotypical movements in RS or they could also be a manifestation of extrapyramidal features described early in RS research (Fitzgerald et al. 1990).
The Body rocking and expressionless face domain is somewhat heterogeneous in its inclusion of both eye gaze and expressionless face. Recently included in the supportive criteria for the atypical form (Hagberg et al. 2002) the eye gaze and use of ‘eye pointing’ to communicate are features which can help distinguish RS from other causes of severe intellectual disability. These behaviours are generally seen after the wake-up period before which RS may often be confused with autism (Percy et al. 1990; Percy et al. 1988). Rocking movements and expressionless face, which are less defined and overlap with features of autism, were recorded in 16% and 29% respectively of the reports including behavioural features reviewed by Mount (Mount et al. 2001).
The night-time behaviours domain included both laughing and the screaming, which are recognised features of RS (Hagberg et al. 2002) for which a pathophysiological mechanism has not been identified. Different methodologies have been used to examine sleep behaviour and have generally found night sleep to be disturbed, but compensated for, by an increase in day-time naps (Ellaway et al. 2001; Piazza et al. 1996). Research into the natural history of sleep disorders in RS over time is lacking.
Behaviours in the fear/anxiety domain such as “fear in unfamiliar situations” were reported in 46/63 cases in Coleman's study (Coleman et al. 1988) and anxiety in 81/107 cases in UK study (Sansom et al. 1993). It is difficult to surmise the underlying pathophysiology of these behaviours; however, they could be related to autonomic dysfunction.
The walking and stiff leg domain characterises the manifestation of the motor impairment and spasticity associated with this RS, which is recognised as necessary criteria (Hagberg B. et al. 2002). However, we found low functional ability (as measured by WeeFIM score) was associated with the absence of behaviours in the walking/standing domain, indicating cases with a higher score were more mobile (Robertson 2003). A higher score, such as in this example, will not necessarily be associated, as would be expected, with a more severe behavioural/functional outcome. Domain scores, when considered in relation to one another, can provide a behaviour profile that reflects the characteristics of cases with specific mutations.
One of the strengths of this study is that it is population-based (Colvin et al. 2003; Leonard and Bower 1998; Leonard et al. 1997) and therefore there should be minimal selection bias and the results should be generalisable. This study also has more cases than many other studies investigating the relationship between genotype and phenotype (Hoffbuhr et al. 2001; Huppke et al. 2002; Schanen et al. 2004). Nevertheless, the numbers of cases with individual mutations remain small. We only had 16 cases with a T158M mutation, the most commonly reported hot-spot mutation representing 12.1% of pathogenic mutations (Christodoulou and Grimm 2003). Thus, analytical power remains low and it is not surprising that the discriminant model was not statistically significant.
A number of scoring systems have already been developed and some further modified to quantify clinical severity (Amir and Zoghbi 2000; Hoffbuhr et al. 2001; Huppke et al. 2002; Leonard et al. 2003; Monros et al. 2001). Commenting that mildly affected girls could be more restless and aggressive; Huppke et al (2002) suggested that clinical severity may not necessarily reflect social severity in RS. This is consistent with the clinical observations of authors HL and CE. Nevertheless, our results support findings from previous analyses of the association between clinical variability and genetic characteristics. Three mutations having both a milder phenotype and more likely to be found in those surviving to adolescent/adulthood (Smeets et al. 2003) are the R133C (Leonard et al. 2003), R294X (Colvin et al. 2003) and R306C mutations (Fukuda et al. 2005; Kerr A. M. and Prescott in press; Schanen et al. 2004). We found cases with these mutations were more likely to exhibit behaviours relating to mood and body rocking and less likely to demonstrate hand behaviours. Cases with R306C and R133C showed more fear and anxiety. In contrast, cases with R270X appeared to show more hand behaviours and have a lower score on walking/standing, suggesting they were less mobile. This mutation has been found to have a more severe clinical phenotype (Colvin et al. 2004; Leonard et al. in press). Our interpretation of these findings is that individuals with R133C, R294X and R306C mutations have a milder phenotype and are functioning at a higher level so are more capable of externalising and demonstrating such behaviours than subjects with more severe mutations, such as the R270X mutation. It is possible that for family and carers, the burden of caring for subjects in the milder group is equally or more demanding than it is for those with more severe functionally incapacitating mutations.
In summary we have used the RSBQ to investigate the possibility of identifying variation in behavioural phenotypes associated with specific MECP2 mutations. We found that patterns of behaviour differ between groups of mutations previously shown to have either a mild or a severe phenotype but due to the rarity of RS the statistical power was limited. This work forms a useful basis for future international collaborative studies that could be carried out through InterRett, an international RS database where data collection is not limited to cases from one country (Moore et al. 2005).
Acknowledgments
The ARSD currently is funded by National Institute of Child Health and Human Development (1 R01 HD43100-01A1) and was previously funded by Financial Markets Foundation for Children and the Rett Syndrome Australian Research Fund. Helen Leonard is funded by NHMRC program grant 353514. We gratefully acknowledge Dr Rebecca Mount for the RSBQ, the families who participate in the ARSD, and the Australian Paediatric Surveillance Unit and the Rett Syndrome Association of Australia.
References
- Amir RE, Zoghbi HY. Rett syndrome: methyl-CpG-binding protein 2 mutations and phenotype-genotype correlations. Am J Med Genet. 2000;97:147–52. doi: 10.1002/1096-8628(200022)97:2<147::aid-ajmg6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Charman T, Cass H, Owen L, Wigram T, Slonims V, Weeks L, Wisbeach A, Reilly S. Regression in individuals with Rett syndrome. Brain Dev. 2002;24:281–3. doi: 10.1016/s0387-7604(02)00058-x. [DOI] [PubMed] [Google Scholar]
- Christodoulou J, Grimm A. RettBASE: IRSA MECP2 mutation database. 2003 doi: 10.1002/humu.10194. Westmead ed. [DOI] [PubMed] [Google Scholar]
- Coleman M, Brubaker J, Hunter K, Smith G. Rett syndrome: a survey of North American patients. J Ment Defic Res. 1988;32:117–124. doi: 10.1111/j.1365-2788.1988.tb01397.x. [DOI] [PubMed] [Google Scholar]
- Colvin L, Fyfe S, Leonard S, Schiavello T, Ellaway C, De Klerk N, Christodoulou J, Msall M, Leonard H. Describing the phenotype in Rett syndrome using a population database. Arch Dis Child. 2003;88:38–43. doi: 10.1136/adc.88.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin L, Leonard H, de Klerk N, Davis M, Weaving L, Williamson S, Christodoulou J. Refining the phenotype of common mutations in Rett syndrome. J Med Genet. 2004;41:25–30. doi: 10.1136/jmg.2003.011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykens E, Hodapp R. Research in mental retardation: toward an etiological approach. Journal of Child Psychology and Psychiatry. 2001;41:49–71. [PubMed] [Google Scholar]
- Einfeld SL, Tonge BJ. The Developmental Behavior Checklist: the development and validation of an instrument to assess behavioral and emotional disturbance in children and adolescents with mental retardation. J Autism Dev Disord. 1995;25:81–104. doi: 10.1007/BF02178498. [DOI] [PubMed] [Google Scholar]
- Ellaway C, Peat J, Leonard H, Christodoulou J. Sleep dysfunction in Rett syndrome: lack of age related decrease in sleep duration. Brain Dev. 2001;23:S101–103. doi: 10.1016/s0387-7604(01)00356-4. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PM, Jankovic J, Glaze DG, Schultz R, Percy AK. Extrapyramidal involvement in Rett's syndrome. Neurology. 1990;40:293–5. doi: 10.1212/wnl.40.2.293. [DOI] [PubMed] [Google Scholar]
- Fiumara A, Polizzi A, Mazzei R, Conforti L, Magariello A, Sorge G, Pavone L. Rett syndrome phenotype following infantile acute encephalopathy. J Child Neurol. 2002;17:700–702. doi: 10.1177/088307380201700910. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Yamashita Y, Nagamitsu S, Miyamoto K, Jin JJ, Ohmori I, Ohtsuka Y, Kuwajima K, Endo S, Iwai T, et al. Methyl-CpG binding protein 2 gene (MECP2) variations in Japanese patients with Rett syndrome: pathological mutations and polymorphisms. Brain Dev. 2005;27:211–7. doi: 10.1016/j.braindev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Goutieres F, Aicardi J. Atypical forms of Rett syndrome. Am J Med Genet. 1986;24:183–194. doi: 10.1002/ajmg.1320250521. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Hanefeld F, Percy A, Skjeldal O. An update on clinically applicable diagnostic criteria in Rett syndrome. Comments to Rett Syndrome Clinical Criteria Consensus Panel Satellite to European Paediatric Neurology Society Meeting, Baden Baden, Germany, 11 September 2001. European Journal of Paediatric Neurology. 2002;6:293–7. doi: 10.1053/ejpn.2002.0612. [DOI] [PubMed] [Google Scholar]
- Hagberg B, Witt Engerstrom I. Rett syndrome: a suggested staging system for describing impairment profile with increasing age toward adolescence. Am J Med Genet. 1986;1:47–59. doi: 10.1002/ajmg.1320250506. [DOI] [PubMed] [Google Scholar]
- Hoffbuhr K, Devaney J, LaFleur B, Sirianni N, Scacheri C, Giron J, Schuette D, Hoffman E, Naidu S. MeCP2 mutations in children with and without the phenotype of Rett syndrome. Neurology. 2001;56:1486–1495. doi: 10.1212/wnl.56.11.1486. [DOI] [PubMed] [Google Scholar]
- Hunter K. Looking from the inside out: a parent's perspective. Ment Retard Dev Disabil Res Rev. 2002;8:77–81. doi: 10.1002/mrdd.10019. [DOI] [PubMed] [Google Scholar]
- Huppke P, Held M, Hanefeld F, Laccone F. Influence of mutation type and location on phenotype in 123 patients with Rett syndrome. Neuropediatrics. 2002;33:63–68. doi: 10.1055/s-2002-32365. [DOI] [PubMed] [Google Scholar]
- Julu P, Kerr A, Apartopoulos F, Al-Rawas S, Witt Engerstrom I, Engerstrom L, Jamal G, Hansen S. Characterisation of breathing and associated central autonomic dysfunction in the Rett disorder. Arch Dis Child. 2001;85:29–37. doi: 10.1136/adc.85.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr A, Montague J, Stephenson J. The hands, and the mind, pre- and post-regression, in Rett syndrome. Brain Dev. 1987;9:487–490. doi: 10.1016/s0387-7604(87)80070-0. [DOI] [PubMed] [Google Scholar]
- Kerr AM, Prescott RJ. Predictive value of the early clinical signs in Rett Disorder. Brain Dev. doi: 10.1016/j.braindev.2004.10.007. In press. [DOI] [PubMed] [Google Scholar]
- Leonard H, Bower C. Is the girl with Rett syndrome normal at birth? Dev Med Child Neurol. 1998;40:115–121. [PubMed] [Google Scholar]
- Leonard H, Bower C, English D. The prevalence and incidence of Rett syndrome in Australia. Eur Child Adolesc Psychiatry. 1997;6:8–10. [PubMed] [Google Scholar]
- Leonard H, Colvin L, Christodoulou J, Schiavello T, Williamson S, Davis M, Ravine D, Fyfe S, de Klerk N, Matsuishi T, et al. Patients with the R133C mutation: is their phenotype different from patients with Rett syndrome with other mutations? J Med Genet. 2003;40:e52. doi: 10.1136/jmg.40.5.e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard H, Moore H, Carey M, Fyfe S, Hall S, Robertson L, Wu R, Bao X, Hong P, Christodoulou J, et al. Genotype and early development in Rett syndrome: the value of international data. Brain Dev. doi: 10.1016/j.braindev.2005.03.023. In press. [DOI] [PubMed] [Google Scholar]
- Monros E, Armstrong J, Aibar E, Poo P, Canos I, Pineda M. Rett syndrome in Spain: mutation analysis and clinical correlations. Brain Dev. 2001;23:S251–253. doi: 10.1016/s0387-7604(01)00374-6. [DOI] [PubMed] [Google Scholar]
- Moore H, Leonard H, Fyfe S, de Klerk N, Leonard N. InterRett – the application of bioinformatics to international Rett syndrome research. Ann Hum Biol. 2005;32:228–236. doi: 10.1080/03014460500075068. [DOI] [PubMed] [Google Scholar]
- Mount RH, Charman T, Hastings RP, Reilly S, Cass H. The Rett Syndrome Behaviour Questionnaire (RSBQ): refining the behavioural phenotype of Rett syndrome. J Child Psychol Psychiatry. 2002a;43:1099–110. doi: 10.1111/1469-7610.00236. [DOI] [PubMed] [Google Scholar]
- Mount RH, Hastings RP, Reilly S, Cass H, Charman T. Behavioural and emotional features in Rett syndrome. Disabil Rehabil. 2001;23:129–38. doi: 10.1080/09638280150504207. [DOI] [PubMed] [Google Scholar]
- Mount RH, Hastings RP, Reilly S, Cass H, Charman T. Behaviour problems in adult women with Rett syndrome. J Intellect Disabil Res. 2002b;46:619–24. doi: 10.1046/j.1365-2788.2002.00442.x. [DOI] [PubMed] [Google Scholar]
- Mount RH, Hastings RP, Reilly S, Cass H, Charman T. Towards a behavioral phenotype for Rett syndrome. Am J Ment Retard. 2003;108:1–12. doi: 10.1352/0895-8017(2003)108<0001:TABPFR>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Segawa M, Hasegawa M. Rett syndrome--clinical studies and pathophysiological consideration. Brain Dev. 1984;6:475–86. doi: 10.1016/s0387-7604(84)80030-3. [DOI] [PubMed] [Google Scholar]
- Ottenbacher KJ, Msall ME, Lyon N, Duffy LC, Granger CV, Braun S. Measuring developmental and functional status in children with disabilities. Dev Med Child Neurol. 1999;41:186–94. doi: 10.1017/s0012162299000377. [DOI] [PubMed] [Google Scholar]
- Percy A, Gillberg C, Hagberg B, Witt-Engerstrom I. Rett syndrome and the autistic disorders. Neurol Clin. 1990;8:659–76. [PubMed] [Google Scholar]
- Percy AK, Zoghbi HY, Lewis KR, Jankovic J. Rett syndrome: qualitative and quantitative differentiation from autism. J Child Neurol. 1988 3:S65–7. doi: 10.1177/0883073888003001s12. [DOI] [PubMed] [Google Scholar]
- Piazza C, Fisher W, Kiesewetter K, Bowman L, Moser H. Aberrant sleep patterns in children with Rett syndrome. Brain Dev. 1996;12:488–493. doi: 10.1016/s0387-7604(12)80213-0. [DOI] [PubMed] [Google Scholar]
- Poplawski NK. Investigating intellectual disability: a genetic perspective. J Paediatr Child Health. 2003;39:492–506. doi: 10.1046/j.1440-1754.2003.00201.x. [DOI] [PubMed] [Google Scholar]
- Robertson L. Associations between behaviour and genetic characteristics of Rett syndrome [Honours] Perth: University of Western Australia; 2003. p. 99. [Google Scholar]
- Sansom D, Krishnan V, Corbett J, Kerr A. Emotional and behavioural aspects of Rett syndrome. Dev Med Child Neurol. 1993;35:340–345. doi: 10.1111/j.1469-8749.1993.tb11646.x. [DOI] [PubMed] [Google Scholar]
- Schanen C, Houwink EJ, Dorrani N, Lane J, Everett R, Feng A, Cantor RM, Percy A. Phenotypic manifestations of MECP2 mutations in classical and atypical Rett syndrome. Am J Med Genet. 2004;126:129–40. doi: 10.1002/ajmg.a.20571. [DOI] [PubMed] [Google Scholar]
- Smeets E, Schollen E, Moog U, Matthijs G, Herbergs J, Smeets H, Curfs L, Schrander-Stumpel C, Fryns JP. Rett syndrome in adolescent and adult females: clinical and molecular genetic findings. Am J Med Genet. 2003;122:227–33. doi: 10.1002/ajmg.a.20321. [DOI] [PubMed] [Google Scholar]
- The Rett Syndrome Diagnostic Criteria Work Group. Diagnostic criteria for Rett syndrome. The Rett Syndrome Diagnostic Criteria Work Group. Ann Neurol. 1988;23:425–8. doi: 10.1002/ana.410230432. [DOI] [PubMed] [Google Scholar]
- Weaving LS, Williamson SL, Bennetts B, Davis M, Ellaway CJ, Leonard H, Thong MK, Delatycki M, Thompson EM, Laing N, et al. Effects of MECP2 mutation type, location and X-inactivation in modulating Rett syndrome phenotype. Am J Med Genet. 2003;118:103–14. doi: 10.1002/ajmg.a.10053. [DOI] [PubMed] [Google Scholar]
- Zappella M. The Rett girls with preserved speech. Brain Dev. 1992;14:98–101. doi: 10.1016/s0387-7604(12)80094-5. [DOI] [PubMed] [Google Scholar]