Abstract
Human Glioblastoma Multiforme (GBM) is the most malignant form of human brain tumors. A characteristic of GBM is the marked presence of tumor infiltrated microglia/macrophages and lymphocytes. The goal of this study was directed toward understanding the role of the chemokine system CX3CL1 and its receptor CX3CR1 in the GL261 murine model of malignant glioma. In situ hybridization analysis identified CX3CL1 and CX3CR1 expression in GL261 tumors. The impact of CX3CR1 deletion on the growth of intracranial GL261 gliomas and associated immune cell infiltration was evaluated in CX3CR1 gene-disrupted C57BL/6 mice. A slight increase in the tumor growth rate in CX3CR1−/− mice was evident with similar numbers of microglia and CD4+, CD8+, FoxP3+, or Ly49G2+ lymphocytes within tumors established in CX3CR1+/− and −/− mice. These data indicate that CX3CR1 has little or no effects on either gliomagenesis or the migration of microglia and lymphocytes into GL261 tumors.
Keywords: chemokine, GL261, glioblastoma multiforme, tumor immunology
1. Introduction
Human glioblastoma multiforme (GBM), a grade IV astrocytoma, is the most common and malignant form of human primary brain tumors. GBM patients have a less than two percent five year survival rate. Current standard treatment of GBM is surgical resection of the tumor mass, followed by adjuvant radiotherapy and chemotherapy. However, these treatments are not very successful and have only a modest impact on the survival rate of GBM patients. Due to the relative ineffectiveness of these traditional treatments, other methods, such as immunotherapy, are being evaluated (Kawakami et al., 2008). The marked presence of glioma infiltrating microglia and lymphocytes supports the concept of targeting the immune system to treat GBM. Studies indicate that murine microglia express multiple Toll like receptors (Olson and Miller, 2004) and are able to activate CD4+ helper T cells (McMahon et al., 2005). However, successful immunotherapy of GBM will involve overcoming the highly immunosuppressive environment created by the tumor, that includes immunosuppressive cytokines such as TGFβ and Interleukin 10 (Roussel et al., 1996) and immune cells such as the regulatory T cell (Fecci et al., 2006). A greater understanding of the immune response during glioma formation will help in the development of improved therapeutics.
Chemokines are small proteins that induce chemotaxis of responsive cells and are attractive molecules to mediate the migration of immune cells into the tumor. In addition to these chemoattractive functions, chemokines also exert direct effects on tumor growth, angiogenesis, and metastasis. For example, inhibition of CXCR4/CXCL12 signaling pathway decreased metastasis of osteosarcoma and melanoma (Kim et al., 2007) as well as the growth of medulloblastoma and glioblastoma (Rubin et al., 2003). The chemokine receptor, CXCR7, has been shown to promote breast and lung cancer growth (Miao et al., 2007). While chemokines can facilitate tumor growth, they also exhibit antitumor activity. The Duffy antigen receptor for chemokine (DARC) inhibits murine melanoma growth that is accompanied with higher leukocyte infiltration and reduced angiogenesis (Horton et al., 2007). Despite the complexity of their functions in tumorigenesis, chemokines and their receptors are potential targets for cancer therapy and worthy of further evaluation.
The chemokine receptor system CX3CR1 and its ligand CX3CL1 are known to be involved in immune responses that underlie various human diseases and their corresponding animal models. For instance, CX3CR1 is responsible for recruiting dendritic cells and a subset of monocytes in models of atherosclerosis (Liu et al., 2007, Tacke et al., 2007). CX3CR1 deficiency results in impaired microglia migration in a mouse model of age-related macular degeneration (Combadiere et al., 2007). Enhanced neuronal cell loss is also evident in CX3CR1 deficient mice after systemic lipopolysaccharide injection, in toxin-induced Parkinsonism, and the SOD1G93A transgenic mouse model of motor neuron disease (Cardona et al., 2006). A role for CX3CL1/CX3CR1 system in tumorigenesis has also been established. CX3CL1 has been shown to mediate both natural killer cell-dependent and T cell-dependent antitumor activity (Lavergne et al., 2003, Yu et al., 2007, Xin et al., 2005). Mice lacking CX3CR1 have an impairment in postischemic neovascularization (Waeckel et al., 2005), suggesting that this chemokine system may be similarly involved in tumor angiogenesis. Thus CX3CL1/CX3CR1 might be a suitable target in the development of novel therapies to treat cancer.
The specific functions of CX3CL1/CX3CR1 in gliomagenesis have not been established. In this study, we sought to determine a role of CX3CR1 in glioma formation and the associated recruitment of microglia and lymphocytes, using the GL261 murine model of glioma (Ausman et al., 1970, Szatmari et al., 2006). CX3CL1 and CX3CR1 expression were determined in GL261 tumors established in its syngeneic host, the C57BL/6 mouse. The role of this chemokine system was then characterized in CX3CR1 deficient C57BL/6 mice. The results indicate that CX3CR1 has little to no effect on glioma growth. Moreover the migration of microglia and CD4+, CD8+, Foxp3+, and Ly49G2+ lymphocytes into the tumor tissue was not impacted by the lack of CX3CR1.
2. Materials and Methods
2.1 Mice and GL261 cell line
CX3CR1-deficient (−/−) mice, backcrossed to the C57BL/6 background for greater than 10 generations, were obtained from JAX Laboratories. The generation of these mice have been previously described (Jung et al., 2000). The protein coding sequence of the CX3CR1 gene was exchanged with GFP in heterozygous (one allele replaced) and homozygous (both alleles replaced) mice. In these mice, all cells normally expressing CX3CR1 express GFP. Colonies of CX3CR1 (−/−) and (+/−) mice were maintained at the University of Florida. All mice used in studies presented herein were derived from breeding CX3CR1 (+/−) and (−/−) mice; hence all comparisons were made between littermates. All procedures involving mice were carried out in accordance with the guidelines of the University of Florida Institutional Animal Care and Use Committee (IACUC). The GL261 glioma cell line was maintained in RPMI-1640 medium (Gibco BRL), supplemented with 10% FBS, 1% Penicillin-Streptomycin, 4mM L-Glutamine and grown at 37°C with 5% CO2.
2.2. Histology and Kaplain-Meier analysis for survival
GL261 glioma cells (2 × 105) in a total volume not exceeding 3μl were injected 3 mm deep into the right cerebral hemisphere (1 mm posterior and 2 mm lateral from Bregma) of CX3CR1 +/− and −/− mice. For determining tumor growth, glioma-bearing mice (3 weeks after GL261 cell injection) were euthanized using sodium pentobarbital (32 mg/kg) and subsequently perfused with 0.9% saline followed by buffered 4% paraformaldehyde (PFA). Brains were surgically removed and post-fixed with 4% PFA. After fixation, tissues were incubated in 30% sucrose solution at 4°C overnight followed by liquid nitrogen freezing. Frozen brains were then sectioned and subjected to either hematoxilin and eosin (H&E) staining, in situ hybridization, or immunohistochemistry. For Kaplan-Meier survival rate analysis, percentages of surviving mice in the two groups of animals were recorded daily after GL261 glioma implantation. The endpoint was defined by a lack of physical activity and a body weight reduction of greater than 15%. The data were subjected to Log-rank test in order to determine if significant differences existed in survival between the experimental groups.
2.3. In situ hybridization and Immunohistochemistry
In situ hybridization was performed as described previously (Harrison et al., 2003). For immunohistochemistry, brain sections were permeablized with 0.5% of Triton X-100 in phosphate-buffered saline (PBS) for 15 min at room temperature followed by blocking with 10% goat serum in PBS for 30 min. In some cases (anti-Foxp3 immunohistochemistry), sections underwent an antigen retrieval treatment. In brief, slides were first permeablized with 0.5% Triton X-100 followed by heating slides (immersed in a boiling water bath for 25 minutes) in a buffer containing 10 mM Sodium Citrate, 0.05% Tween 20, pH 6.0. Slides were then cooled to room temperature for 20 minutes, washed with PBS three times, and finally subjected to standard immunohistochemistry procedures. The sections were incubated in primary antibodies at 4 °C overnight. The following antibodies were used: rat anti-CD4 (dilution 1:50, BD Pharmingen), rat anti-CD8 (dilution 1:50, Serotec), rat anti-Foxp3 (dilution 1:50, eBioscience), and rat anti-Ly49G2 (dilution 1:50, BD Pharmingen). The following day, sections were washed three times with PBS and incubated subsequently in goat anti-rat Alexa 594 (dilution 1:1000, BD Pharmingen). The sections were then washed three times with PBS and finally counterstained with DAPI. For quantification of CD4+, CD8+, CD11b+, Foxp3+, and Ly49G2+ cells, the number of cells per high-powered field in several sections from multiple animals were determined and the mean and S.E.M.s calculated. The data were subjected to statistical analysis (one-tail T-test).
3. Results
3.1 CX3CL1 and CX3CR1 expression in GL261 tumors
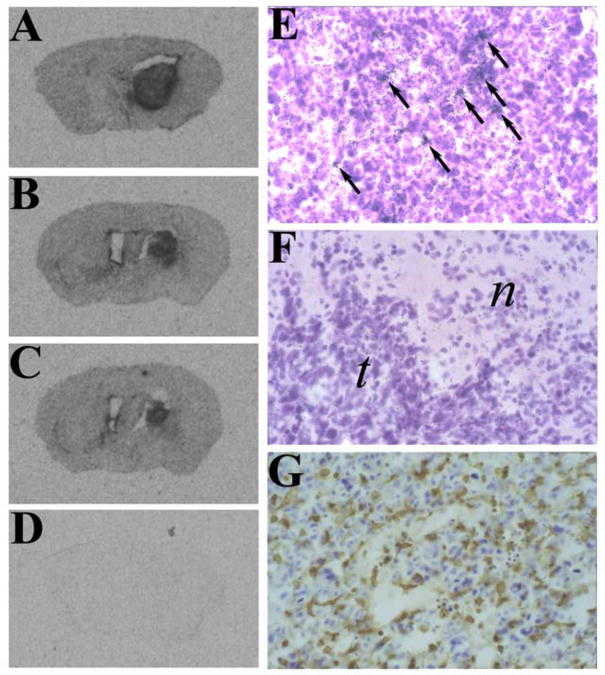
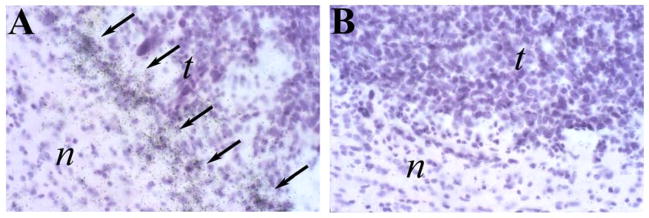
CX3CR1 and CX3CL1 expression in GL261 glioma in vivo was established using the technique of in situ hybridization analysis. The results indicated that both CX3CR1 and CX3CL1 were expressed in GL261 gliomas analyzed from wild-type C57BL/6 mice. Strong hybridization signals for CX3CR1 were evident throughout the tumor mass (Figure 1, panels A–C) and indicated that levels of CX3CR1 mRNA are elevated within the tumor as compared to the surrounding normal brain tissue. Higher resolution analysis showed that these tumor infiltrated CX3CR1-expressing cells were relatively abundant (Figure 1, panel E) and similar in number to CD11b+ cells (Figure 1, panel G). These correlative results suggested that tumor infiltrating microglia are the primary source of CX3CR1. In contrast, CX3CL1 hybridization signals were less prevalent. When present, CX3CL1-expressing cells were found near the perimeter of the tumor mass (Figure 2). These CX3CL1-expressing cells were therefore hypothesized to be important for directing CX3CR1-expressing microglia into the tumor from the brain parenchyma.
Figure 1. CX3CR1 is expressed in GL261 tumors.
A–F) In situ hybridization (ISH) analysis of GL261 tumors using anti-sense (A–C, E) and sense (D, F) CX3CR1 riboprobes. Panels A–C are representative autoradiographs from three different tumor-bearing mice, implanted with either 200,000 (A) or 100,000 (B,C) GL261 cells. Panels E and F depict representative fields from developed emulsion dipped slides. Arrows in panel E identify some of the specific hybridization signals. Normal (n) and tumor (t) tissues are indicated in the sense riboprobed section (panel F). G) A representative tumor section stained with anti-CD11b.
Figure 2. CX3CL1 is expressed in GL261 tumors.
ISH analysis of GL261 tumors using anti-sense (A) and sense (B) CX3CL1/FKN riboprobes. Arrows show hybridization signals. Normal (n) and tumor (t) tissues are depicted in each figure panel.
3.2 Tumor growth and animal survival rates are not affected by CX3CR1 deficiency
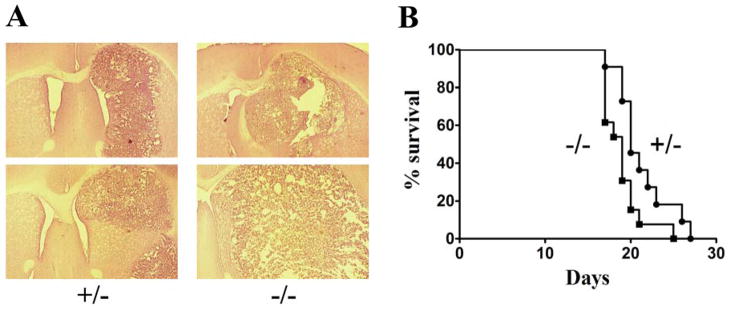
The effects of CX3CR1-deficiency on GL261 glioma formation in vivo was then determined by characterizing tumor growth and animal survival in CX3CR1 deficient C57BL/6 mice. Tumor sections from GL261 bearing CX3CR1+/− and −/− mice, obtained 3 weeks after GL261 cell implantation, indicated that tumor size was slightly larger in homozygous (−/−) animals as compared to the heterozygous mice (Figure 3A). This result suggested that GL261 tumor growth rate was slightly faster in CX3CR1 −/− mice. Consistent with the histological examination, Kaplan-Meier analysis of tumor bearing mice indicated a slightly shorter life span of glioma-bearing CX3CR1 −/− mice than the life span of CX3CR1 +/− mice (Figure 3B). The median survival time of CX3CR1 −/− mice after GL261 cell implantation was 19 days, while that of CX3CR1 +/− mice was 20 days (p = 0.0332). These survival times are also similar to what is observed in glioma-bearing wild type C57BL/6 mice (data not shown).
Figure 3. Tumorigenesis in CX3CR1+/− and −/− mice.
A) Representative H&E stained sections from GL261 tumor bearing CX3CR1+/− and −/− animals. Two representative sections from at least eight different animals in each group are shown. B) Kaplan-Meier survival analysis of GL261 tumor bearing CX3CR1+/− and −/− animals. The median survival of the tumor bearing +/− (N = 11) and −/− (N = 13) mice were 20 and 19 days, respectively. Log-Rank analysis determined that the two curves were different (p = 0.0332).
3.3 CX3CR1 does not mediate microglia migration into glioma tissue
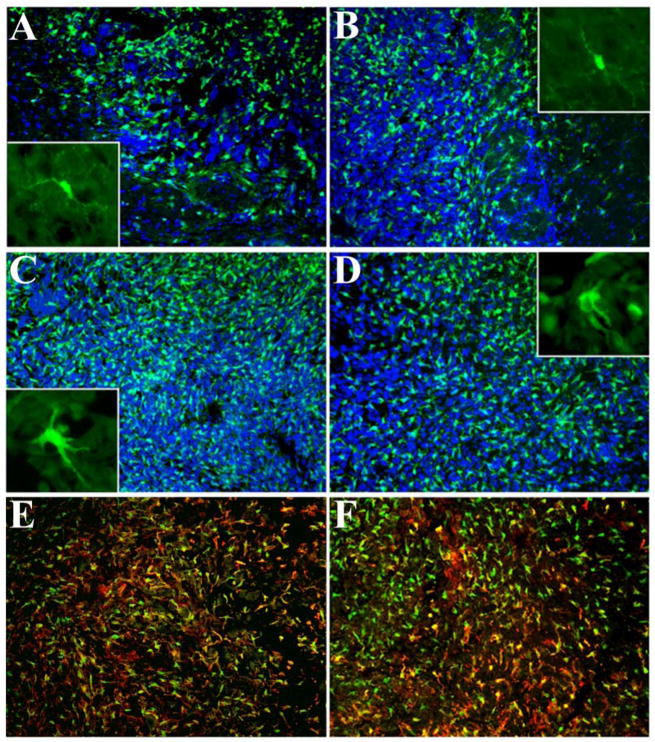
Microglia are the major CX3CR1-expressing cells in the brain (Harrison et al., 1998, Nishiyori et al., 1998). To track the tumor infiltrating CX3CR1-expressing microglia we visualized GFP-expressing cells using fluorescence microscopy. Figure 4 shows abundant CX3CR1-expressing cells were found inside the tumors from both CX3CR1 +/− and −/− mice. The CX3CR1 −/− mice showed similar numbers of microglia within the tumors as compared to tumors from heterozygous (+/−) mice. Moreover, the microglia in the normal brain parenchyma from both +/− and −/− mice exhibited a comparable ramified morphology, while microglia inside the tumors from both groups of mice displayed similar morphological characteristics consistent with an activated phenotype (insets to Figure 4, panels A–D). In both CX3CR1 +/− and −/− mice, most GFP-expressing cells also expressed CD11b (Figure 4, panels E and F); no obvious differences in the CD11b expression pattern were observed between the two groups of mice. Quantitative analysis of both CD11b+ and GFP+ cells in the two animal groups indicated that numbers of these cells did not significantly differ between CX3CR1 +/− and −/− mice (Table I). These collective observations suggested that CX3CR1 deficiency had no substantial effects on the recruitment, morphology, and level of expression of CD11b by CX3CR1-expressing microglia.
Figure 4. Infiltration of CX3CR1+/Cd11b+ cells into GL261 gliomas.
GFP-expressing cells at the perimeter of the tumor in +/− (A) and −/− (B) mice. Panels C and D show GFP-expressing cells inside the tumor in +/− and −/− mice, respectively. Insets of panels A–D show higher magnifications of GFP-expressing microglia in normal brain parenchyma adjacent to the tumor (panels A and B) and GFP-expressing cells within the tumors (panels C and D). Sections depicted in panels A–D were counterstained with DAPI and the final pictures are a result of the merged images. Panels E and F depict CD11b expression by intratumoral GFP-expressing cells in +/− (E) and −/− (F) mice.
Table I.
Numbers of tumor infiltrated GFP+ and CD11B+ cells
| Animal Genotypes | GFP+ | CD11B+ |
|---|---|---|
| CX3CR1 +/− | 544 ± 79 (6) | 384 ± 28 (6) |
| CX3CR1 −/− | 561 ± 54 (6) | 396 ± 62 (6) |
| P value | 0.42 | 0.5 |
Shown are mean (±S.E.M.) numbers of tumor infiltrated cells expressing either GFP or CD11B. Animal numbers for each group are indicated in the brackets.
3.4 CX3CR1 is not necessary for lymphocyte infiltration into GL261 gliomas
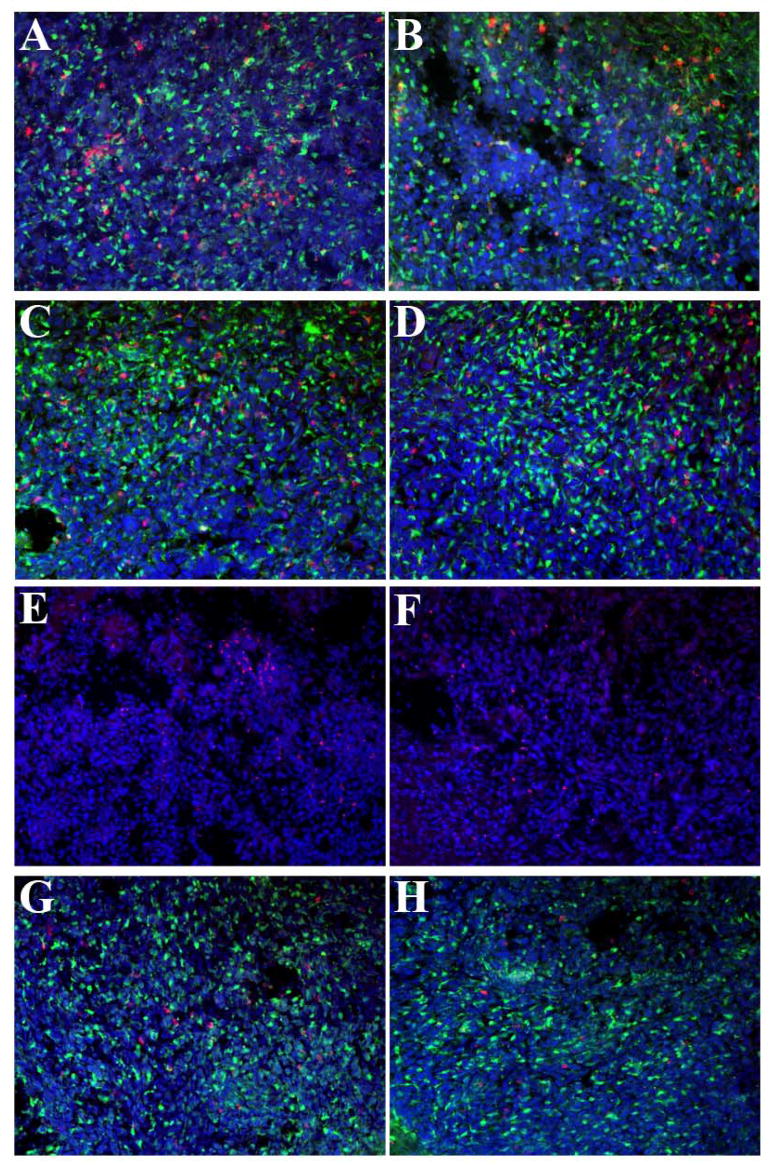
To address the lymphocyte response to the glioma in CX3CR1 deficient mice, immunohistochemical analysis using several T lymphocyte markers was performed and the numbers of these cells were quantified in CX3CR1 +/− and −/− mice. Figure 5 depicts a series of representative sections subjected to immunohistochemistry from the two groups of animals while Table II summarizes the quantitative analysis of several sections from multiple animals. CD4+, CD8+, FoxP3+, and Ly49G2+ cells were all present within GL261 tumors in both types of mice (Figure 5). CD4+, CD8+, and Ly49G2+ cells were all GFP negative and indicated that these tumor infiltrating lymphocyte populations do not express CX3CR1. The regulatory T cell (Treg) subpopulation of CD4+ T cells, identified by staining sections with the anti-Foxp3 antibody, comprised about half of the numbers of CD4+ cells. These data are consistent with two previous reports on the presence of the Treg population in the GL261 model (El Andaloussi et al., 2006, Grauer et al., 2007). While there were no significant differences in the numbers of the specific tumor-infiltrated CD4+, CD8+, FoxP3+, and Ly49G2+ cells between CX3CR1 +/− and −/− glioma bearing mice (Table I), quantitative analysis of each of these cells indicated that tumors from CX3CR1 −/− mice exhibited a tendency toward fewer CD4+, CD8+, FoxP3+, and Ly49G2+ cells inside the tumors compared with numbers found in tumors from +/− mice. Nonetheless, CX3CR1 does not appear to be necessary for the recruitment of these lymphocyte subsets into GL261 gliomas.
Figure 5. CD4+, CD8+, FoxP3+, and Ly49G2+ cells are present in GL261 tumors from CX3CR1+/− and/− mice.
Representative fluorescence micrographs depicting tumor infiltration of lymphocytes from +/− (A, C, E, G) and −/− (B,D, F, H) mice. A, B) CD4+ cells; C, D) CD8+ cells; E, F) Foxp3+ cells; G, H) Ly49G2+ cells. Lymphocyte markers are defined by red fluorescence. Sections were counterstained with DAPI. The final pictures represent merged images
Table II.
Numbers of tumor infiltrated lymphocytes
| Animal Genotype | lymphocytes
|
|||
|---|---|---|---|---|
| CD4 | CD8 | Foxp3 | Ly49G2 | |
| CX3CR1 +/− | 112 ± 13 (13) | 72.7 ± 11.6 (11) | 58.4 ± 5.5 (12) | 26.5 ± 4.6 (10) |
| CX3CR1 −/− | 102 ± 14 (11) | 53.9 ± 8.8 (11) | 48.3 ± 6.5 (12) | 23.8 ± 4.9 (12) |
| P value | 0.618 | 0.214 | 0.319 | 0.692 |
Shown are mean (±S.E.M.) numbers of tumor infiltrated lymphocytes. Animal numbers for each group are indicated in the brackets.
4. Discussion
In this study we have shown that CX3CR1 deficiency resulted in a slightly shorter life span of tumor bearing mice with no significant differences in numbers of tumor infiltrated microglia and lymphocytes. These results favor a lack of effects of CX3CR1 signaling on antiglioma activity as well as in the intratumoral recruitment of microglia and lymphocytes. Previous studies done in CX3CR1 deficient mice have pointed out that CX3CL1 and CX3CR1 are important for migration of macrophages, microglia and lymphocytes in vivo. For example, CX3CR1-deficient mice show an aberrant accumulation of microglia at subretinal areas that might contribute to age-related macular degeneration (Combadiere et al., 2007, Tuo et al., 2007). In addition, a reduction in the lesion size and accumulation of immune cells during atherosclerosis were found in CX3CR1−/− mice (Teupser et al., 2004, Combadiere et al., 2003, Liu et al., 2007). Moreover, CX3CR1 deficient animals showed impaired recruitment of NK cells in both EAE and in tumorigenesis (Yu et al., 2007, Huang et al., 2006). Thus, it was somewhat surprising to find that CX3CR1 deficiency did not impact glioma infiltration of CX3CR1-expressing microglia in vivo. Equally unanticipated was the lack of morphological alterations in tumor-associated microglia in CX3CR1−/− mice given the results of Cardona et al (Cardona et al., 2006) in which microglia from CX3CR1 deficient animals display a greater extent of activation in various models of neurotoxicity.
While Log-rank statistical analysis indicated that the two survival curves were significantly different, suggesting that CX3CR1 deficiency may actually favor glioma growth, the difference in median survival time of one day indicates that CX3CR1 plays a small role in GL261 tumorigenesis. The four types of lymphocytes we examined here, though not significantly different between the two groups of mice, all showed slightly fewer numbers in −/− mice than in +/− mice. This raises the possibility that CX3CR1 deficiency might have a global impact on the host’s immune system and its ability to suppress glioma growth.
One reasonable explanation for our results is that the function of CX3CR1 is masked by a highly immunosuppressive environment created by the GL261 glioma. TGFβ is one of the major immunosuppressive cytokines produced by tumors and contributes to immune tolerance of malignant cells (Teicher, 2007). Inhibition of TGFβ prevented the growth of EL-4 thymoma, B16F10 melamoma (Gorelik and Flavell, 2001), and SMA-560 glioma (Tran et al., 2007) in vivo. TGFβ is known to be expressed in human and rodent gliomas (Samuels et al., 1989, Bodmer et al., 1989, Kiefer et al., 1994) and we have determined that TGFβ is present in GL261 gliomas in vivo (data not shown). Previous published results from our lab have shown that TGFβ upregulates CX3CR1 expression in rat microglia although the signaling efficiency of this receptor was markedly inhibited after CX3CL1 stimulation (Chen et al., 2002). Therefore, CX3CR1 signaling in tumor infiltrated microglia from wild type or CX3CR1 +/− mice might be blocked by the high levels of TGFβ present within the GL261 tumors. If CX3CR1 function is inhibited under these conditions, a lack of a microglial cell phenotype in CX3CR1 deficient GL261 tumor bearing mice might be expected. Studies have shown that microglia under an immunosuppressive environment can still mediate phagocytosis and non MHC restricted cytotoxicity but lack the ability to secrete IL-1β, IL-6, and TNF-α (Hussain et al., 2006b, Hussain et al., 2006a). Given studies by Zujovic et al. (Zujovic et al., 2000) and Cardona et al. (Cardona et al., 2006) that CX3CR1 can regulate proinflammatory cytokine secretion, the relationship between CX3CR1 signaling blockade and cytokine secretion by glioma infiltrating microglia should be further studied. It is possible that microglia from CX3CR1 deficient animals secrete higher levels of factors that might facilitate glioma growth and invasiveness more directly, e.g. tumor growth factors and extracellular proteases.
Acknowledgments
The authors acknowledge Ms. Ellen Espenschied for technical assistance. This work was supported by grants from National Institute of Health/NIAID (AI058256) and the University of Florida Shands Cancer Center to J.K.H.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AUSMAN JI, SHAPIRO WR, RALL DP. Studies on the chemotherapy of experimental brain tumors: development of an experimental model. Cancer Res. 1970;30:2394–400. [PubMed] [Google Scholar]
- BODMER S, STROMMER K, FREI K, SIEPL C, DE TRIBOLET N, HEID I, FONTANA A. Immunosuppression and transforming growth factor-beta in glioblastoma. Preferential production of transforming growth factor-beta 2. J Immunol. 1989;143:3222–9. [PubMed] [Google Scholar]
- CARDONA AE, PIORO EP, SASSE ME, KOSTENKO V, CARDONA SM, DIJKSTRA IM, HUANG D, KIDD G, DOMBROWSKI S, DUTTA R, LEE JC, COOK DN, JUNG S, LIRA SA, LITTMAN DR, RANSOHOFF RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–24. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- CHEN S, LUO D, STREIT WJ, HARRISON JK. TGF-beta1 upregulates CX3CR1 expression and inhibits fractalkine-stimulated signaling in rat microglia. J Neuroimmunol. 2002;133:46–55. doi: 10.1016/s0165-5728(02)00354-5. [DOI] [PubMed] [Google Scholar]
- COMBADIERE C, FEUMI C, RAOUL W, KELLER N, RODERO M, PEZARD A, LAVALETTE S, HOUSSIER M, JONET L, PICARD E, DEBRE P, SIRINYAN M, DETERRE P, FERROUKHI T, COHEN SY, CHAUVAUD D, JEANNY JC, CHEMTOB S, BEHAR-COHEN F, SENNLAUB F. CX3CR1-dependent subretinal microglia cell accumulation is associated with cardinal features of age-related macular degeneration. J Clin Invest. 2007;117:2920–8. doi: 10.1172/JCI31692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMBADIERE C, POTTEAUX S, GAO JL, ESPOSITO B, CASANOVA S, LEE EJ, DEBRE P, TEDGUI A, MURPHY PM, MALLAT Z. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–16. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- EL ANDALOUSSI A, HAN Y, LESNIAK MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105:430–7. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- FECCI PE, MITCHELL DA, WHITESIDES JF, XIE W, FRIEDMAN AH, ARCHER GE, HERNDON JE, 2ND, BIGNER DD, DRANOFF G, SAMPSON JH. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66:3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- GORELIK L, FLAVELL RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nat Med. 2001;7:1118–22. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- GRAUER OM, NIERKENS S, BENNINK E, TOONEN LW, BOON L, WESSELING P, SUTMULLER RP, ADEMA GJ. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121:95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- HARRISON JK, JIANG Y, CHEN S, XIA Y, MACIEJEWSKI D, MCNAMARA RK, STREIT WJ, SALAFRANCA MN, ADHIKARI S, THOMPSON DA, BOTTI P, BACON KB, FENG L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARRISON JK, LUO D, STREIT WJ. In situ hybridization analysis of chemokines and chemokine receptors in the central nervous system. Methods. 2003;29:312–8. doi: 10.1016/s1046-2023(02)00354-7. [DOI] [PubMed] [Google Scholar]
- HORTON LW, YU Y, ZAJA-MILATOVIC S, STRIETER RM, RICHMOND A. Opposing roles of murine duffy antigen receptor for chemokine and murine CXC chemokine receptor-2 receptors in murine melanoma tumor growth. Cancer Res. 2007;67:9791–9. doi: 10.1158/0008-5472.CAN-07-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG D, SHI FD, JUNG S, PIEN GC, WANG J, SALAZAR-MATHER TP, HE TT, WEAVER JT, LJUNGGREN HG, BIRON CA, LITTMAN DR, RANSOHOFF RM. The neuronal chemokine CX3CL1/fractalkine selectively recruits NK cells that modify experimental autoimmune encephalomyelitis within the central nervous system. Faseb J. 2006;20:896–905. doi: 10.1096/fj.05-5465com. [DOI] [PubMed] [Google Scholar]
- HUSSAIN SF, YANG D, SUKI D, ALDAPE K, GRIMM E, HEIMBERGER AB. The role of human glioma-infiltrating microglia/macrophages in mediating antitumor immune responses. Neuro Oncol. 2006a;8:261–79. doi: 10.1215/15228517-2006-008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSSAIN SF, YANG D, SUKI D, GRIMM E, HEIMBERGER AB. Innate immune functions of microglia isolated from human glioma patients. J Transl Med. 2006b;4:15. doi: 10.1186/1479-5876-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUNG S, ALIBERTI J, GRAEMMEL P, SUNSHINE MJ, KREUTZBERG GW, SHER A, LITTMAN DR. Analysis of fractalkine receptor CX(3)CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol Cell Biol. 2000;20:4106–14. doi: 10.1128/mcb.20.11.4106-4114.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAWAKAMI Y, FUJITA T, KUDO C, SAKURAI T, UDAGAWA M, YAGUCHI T, HASEGAWA G, HAYASHI E, UEDA Y, IWATA T, WANG Q, OKADA S, TSUKAMOTO N, MATSUZAKI Y, SUMIMOTO H. Dendritic cell based personalized immunotherapy based on cancer antigen research. Front Biosci. 2008;13:1952–8. doi: 10.2741/2814. [DOI] [PubMed] [Google Scholar]
- KIEFER R, SUPLER ML, TOYKA KV, STREIT WJ. In situ detection of transforming growth factor-beta mRNA in experimental rat glioma and reactive glial cells. Neurosci Lett. 1994;166:161–4. doi: 10.1016/0304-3940(94)90475-8. [DOI] [PubMed] [Google Scholar]
- KIM SY, LEE CH, MIDURA BV, YEUNG C, MENDOZA A, HONG SH, REN L, WONG D, KORZ W, MERZOUK A, SALARI H, ZHANG H, HWANG ST, KHANNA C, HELMAN LJ. Inhibition of the CXCR4/CXCL12 chemokine pathway reduces the development of murine pulmonary metastases. Clin Exp Metastasis. 2007 doi: 10.1007/s10585-007-9133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAVERGNE E, COMBADIERE B, BONDUELLE O, IGA M, GAO JL, MAHO M, BOISSONNAS A, MURPHY PM, DEBRE P, COMBADIERE C. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003;63:7468–74. [PubMed] [Google Scholar]
- LIU P, YU YR, SPENCER JA, JOHNSON AE, VALLANAT CT, FONG AM, PATTERSON C, PATEL DD. CX3CR1 Deficiency Impairs Dendritic Cell Accumulation in Arterial Intima and Reduces Atherosclerotic Burden. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.158675. [DOI] [PubMed] [Google Scholar]
- MCMAHON EJ, BAILEY SL, CASTENADA CV, WALDNER H, MILLER SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–9. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- MIAO Z, LUKER KE, SUMMERS BC, BERAHOVICH R, BHOJANI MS, REHEMTULLA A, KLEER CG, ESSNER JJ, NASEVICIUS A, LUKER GD, HOWARD MC, SCHALL TJ. CXCR7 (RDC1) promotes breast and lung tumor growth in vivo and is expressed on tumor-associated vasculature. Proc Natl Acad Sci U S A. 2007;104:15735–40. doi: 10.1073/pnas.0610444104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NISHIYORI A, MINAMI M, OHTANI Y, TAKAMI S, YAMAMOTO J, KAWAGUCHI N, KUME T, AKAIKE A, SATOH M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–72. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- OLSON JK, MILLER SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–24. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- ROUSSEL E, GINGRAS MC, GRIMM EA, BRUNER JM, MOSER RP. Predominance of a type 2 intratumoural immune response in fresh tumour-infiltrating lymphocytes from human gliomas. Clin Exp Immunol. 1996;105:344–52. doi: 10.1046/j.1365-2249.1996.d01-753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN JB, KUNG AL, KLEIN RS, CHAN JA, SUN Y, SCHMIDT K, KIERAN MW, LUSTER AD, SEGAL RA. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci U S A. 2003;100:13513–8. doi: 10.1073/pnas.2235846100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMUELS V, BARRETT JM, BOCKMAN S, PANTAZIS CG, ALLEN MB., JR Immunocytochemical study of transforming growth factor expression in benign and malignant gliomas. Am J Pathol. 1989;134:894–902. [PMC free article] [PubMed] [Google Scholar]
- SZATMARI T, LUMNICZKY K, DESAKNAI S, TRAJCEVSKI S, HIDVEGI EJ, HAMADA H, SAFRANY G. Detailed characterization of the mouse glioma 261 tumor model for experimental glioblastoma therapy. Cancer Sci. 2006;97:546–53. doi: 10.1111/j.1349-7006.2006.00208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TACKE F, ALVAREZ D, KAPLAN TJ, JAKUBZICK C, SPANBROEK R, LLODRA J, GARIN A, LIU J, MACK M, VAN ROOIJEN N, LIRA SA, HABENICHT AJ, RANDOLPH GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEICHER BA. Transforming growth factor-beta and the immune response to malignant disease. Clin Cancer Res. 2007;13:6247–51. doi: 10.1158/1078-0432.CCR-07-1654. [DOI] [PubMed] [Google Scholar]
- TEUPSER D, PAVLIDES S, TAN M, GUTIERREZ-RAMOS JC, KOLBECK R, BRESLOW JL. Major reduction of atherosclerosis in fractalkine (CX3CL1)-deficient mice is at the brachiocephalic artery, not the aortic root. Proc Natl Acad Sci U S A. 2004;101:17795–800. doi: 10.1073/pnas.0408096101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAN TT, UHL M, MA JY, JANSSEN L, SRIRAM V, AULWURM S, KERR I, LAM A, WEBB HK, KAPOUN AM, KIZER DE, MCENROE G, HART B, AXON J, MURPHY A, CHAKRAVARTY S, DUGAR S, PROTTER AA, HIGGINS LS, WICK W, WELLER M, WONG DH. Inhibiting TGF-beta signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 2007;9:259–70. doi: 10.1215/15228517-2007-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUO J, BOJANOWSKI CM, ZHOU M, SHEN D, ROSS RJ, ROSENBERG KI, CAMERON DJ, YIN C, KOWALAK JA, ZHUANG Z, ZHANG K, CHAN CC. Murine ccl2/cx3cr1 deficiency results in retinal lesions mimicking human age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48:3827–36. doi: 10.1167/iovs.07-0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAECKEL L, MALLAT Z, POTTEAUX S, COMBADIERE C, CLERGUE M, DURIEZ M, BAO L, GERARD C, ROLLINS BJ, TEDGUI A, LEVY BI, SILVESTRE JS. Impairment in postischemic neovascularization in mice lacking the CXC chemokine receptor 3. Circ Res. 2005;96:576–82. doi: 10.1161/01.RES.0000159389.55544.20. [DOI] [PubMed] [Google Scholar]
- XIN H, KIKUCHI T, ANDARINI S, OHKOUCHI S, SUZUKI T, NUKIWA T, HUQUN HAGIWARA K, HONJO T, SAIJO Y. Antitumor immune response by CX3CL1 fractalkine gene transfer depends on both NK and T cells. Eur J Immunol. 2005;35:1371–80. doi: 10.1002/eji.200526042. [DOI] [PubMed] [Google Scholar]
- YU YR, FONG AM, COMBADIERE C, GAO JL, MURPHY PM, PATEL DD. Defective antitumor responses in CX3CR1-deficient mice. Int J Cancer. 2007;121:316–22. doi: 10.1002/ijc.22660. [DOI] [PubMed] [Google Scholar]
- ZUJOVIC V, BENAVIDES J, VIGE X, CARTER C, TAUPIN V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–15. [PubMed] [Google Scholar]