Abstract
Brevenal is a nontoxic short-chain trans-syn polyether that competes with brevetoxin (PbTx) for the active site on voltage-sensitive sodium channels. The PbTxs are highly potent polyether toxins produced during blooms of several species of marine dinoflagellates, most notably Karenia brevis. Blooms of K. brevis have been associated with massive fish kills, marine mammal poisoning, and are potentially responsible for adverse human health effects such as respiratory irritation and airway constriction in beach-goers. Additionally, the consumption of shellfish contaminated with PbTxs results in neurotoxic shellfish poisoning (NSP). The purpose of the present study was to determine whether PbTx could induce DNA damage in a human cell type, the lymphocyte, and if so, whether the damage could be antagonized or ameliorated by brevenal, a brevetoxin antagonist. The DNA damage may occur through both endogenous and exogenous physiological and pathophysiological processes. Unrepaired or erroneously repaired DNA damage may result in gene mutation, chromosome aberration, and modulation of gene regulation, which have been associated with immunotoxicity and carcinogenesis. A single-cell gel electrophoresis assay, or comet assay, was used to determine and compare DNA damage following various treatments. The data were expressed as tail moments, which is the percentage of DNA in the tail multiplied by the length between the center of the head and center of the tail (in arbitrary units). The negative control tail moment was 29.2 (SE=±0.9), whereas the positive control (hydrogen peroxide) was 72.1 (1.5) and solvent (ethanol) was 24.2 (2.1). The PbTx-2 (from Sigma, St. Louis, MO, USA), 10−8 M was 41.3 (3.6), PbTx-9 (Sigma), 10−8 M was 57.0 (5.3), PbTx-2 (from University of North Carolina at Wilmington, UNCW), 10−8 M was 49.4 (9.9), and PbTx-3 (UNCW), 10−8 M was 64.0 (6.4). 1.0 μg/ml brevenal applied 1 h before the PbTxs protected the lymphocytes from DNA damage; PbTx-2 (Sigma), 31.3 (2.1); PbTx-9 (Sigma), 35.5 (2.9); PbTx-2 (UNCW), 33.9 (1.4); PbTx-3 (UNCW), 34.9 (1.25). The tail moment for 1.0 μg/ml brevenal alone was 30.8 (2.6). The results indicate that extensive genotoxic damage is induced by PbTx-2 and 9 (Sigma), and PbTx-2 and 3 (UNCW) in normal human lymphocytes, which is fully antagonized by brevenal. This suggests that the immune systems of individuals exposed to PbTx during harmful algal bloom (HAB) events may be at risk.
Keywords: Brevetoxin, Brevenal, Lymphocyte, Gel electrophoresis, Tail moment
Introduction
Brevetoxins (PbTx) are cyclic polyether compounds produced by several types of marine dinoflagellates (including Karenia brevis and Chatonella antiqua) during red tide events in the Gulf of Mexico, off the coasts of Florida and North Carolina, and other coastal areas around the world, resulting in millions of dollars in damage. At present, ten PbTx isoforms (collectively PbTx 1–10) have been discovered. The neurotoxic compounds produced by these organisms are a serious public health concern and heavily impact the commercial fishing industry by causing massive fish kills. On average an estimated $18 million dollars is lost each year in the US fishing industry due to red tides (Anderson et al 2000). In addition, there have been catastrophic mortalities of other threatened marine species, including manatees, bottlenose dolphins and sea turtles. Other animal species, such as birds that reside in coastal areas, are also negatively affected during red tides (Fairey et al 2001). A study conducted by Kimm-Brinson and Ramsdell (2001) found PbTx-1 induced developmental abnormalities and embryo toxicity in Medaka fish embryos.
Humans are exposed to PbTxs in several ways. Neurotoxic shellfish poisoning (NSP) occurs when shellfish accumulate PbTxs by filter feeding (Gordon et al 2001; LePage et al 2002). The shellfish are then harvested, sold at a market, and eaten. The average annual public health impact was estimated at $1 million dollars per year from shellfish poisoning due to harmful algal blooms (HABs) during 1987–1992 (Anderson et al 2000). Additionally, coastal residents and visitors to coastal areas become exposed to PbTxs in aerosolized form as coastal spray. Like nearly all marine toxins, PbTxs are tasteless, odorless, lipid soluble, and heat and acid stable. Upon exposure by ingestion, neurological and gastrointestinal effects occur, lasting several days. These include paresthesia, myalgia, vertigo, ataxia, thermal dysthesia, bradycardia, pupil dilation, diarrhea, and mild to severe headache. In aerosolized form, PbTxs also cause airway irritation (Music et al 1973), induce asthma attacks, and cause airway constriction (Abraham et al 2004).
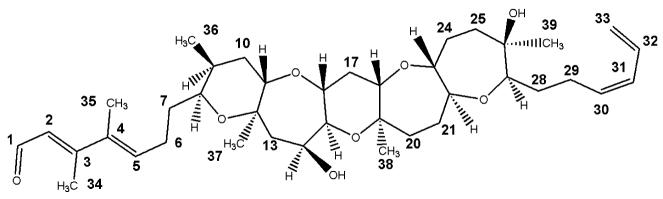
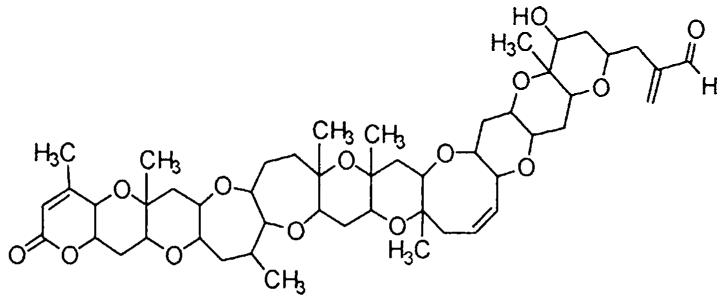
PbTxs (Fig. 1) act on subunit five of the voltage-gated Na+ channel at nanomolar concentrations (Jeglitsch et al 1997; Kulagina et al 2004; Lombet et al 1987; Trainer and Baden 1999), causing the channel to remain in the open state and thus prolonging the duration of sodium transport across the membrane, similar to the effect of a depolarizing neuromuscular blocking agent. Brevenal (Fig. 2), a naturally produced PbTx antagonist, antagonizes PbTx at the active site on the Na+ channel (Bourdelais et al 2004). It is theorized that brevenal competitively displaces PbTx at the active site, thus negating the effect of the toxin. The purpose of the present study was to determine whether PbTx could induce DNA damage in human lymphocytes, and if damage were to occur, whether it could be antagonized by brevenal.
Fig. 1.
Structure of PbTx-2
Fig. 2.
Structure of brevenal
Materials and methods
The PbTx-2 (FW 895.1 lot#082K1237) and PbTx-9 (FW 899.1 lot#102K1347), both from the marine dinoflagellate K. brevis, were purchased from Sigma (St. Louis, MO, USA). The Center for Marine Science at The University of North Carolina at Wilmington (UNCW) provided PbTx-2, PbTx-3, and brevenal. Heparinized blood samples were obtained by veni-puncture from a healthy young female volunteer (ECU/IRB#03-0290). Lymphocytes were extracted using the Hypaque-Ficoll method of lymphocyte isolation from blood (Boyum 1968; Collins et al 1997). Lymphocytes were collected and counted using a coulter counter (Beckman Coulter Z2 Cell and Particle Counter, part number: 6605700). About 500 μg of Sigma and UNCW PbTxs were dissolved in 556 ll 80% ethanol (EtOH) and diluted to make 10−8 and 10−12 M concentrations using Hanks Balanced Salt Solution (HBSS) without calcium and magnesium (IGN lot#1810486). About 0.468 mg of brevenal was dissolved in 0.468 ml 80% ethanol and diluted to 100 and 1.0 μg/ml concentrations using HBSS. Frosted slides used in the assay were dipped into a mixture containing 500 mg normal melting point agarose dissolved in 50 ml one time Dulbecco's Phosphate Buffered Saline (Gibco lot#21600-010).
Treatment
Human lymphocytes (approximately 500 lymphocytes were used per slide per group) were exposed to either Sigma or UNCW PbTx at 10−8 or 10−12 M, a negative control (HBSS buffer), a positive control (2.75% hydrogen peroxide), and solvent (80% EtOH) in HBSS for 1 h. Lymphocytes were allowed to incubate in 1.0 μg/ml brevenal at 4 °C for 1 h and then exposed to 10−8 M Sigma or UNCW PbTxs, or hydrogen peroxide at 4 °C for 2 h. Likewise, human lymphocytes were allowed to incubate in 10−8 M Sigma or UNCW PbTxs or hydrogen peroxide at 4 °C for 1 h, and exposed to 1.0 μg/ml brevenal at 4 °C for 2 h. Lymphocytes were also treated with the DNA polymerase inhibitor aphidicolin. Lymphocytes were exposed to 10−9 M aphidicolin, 10−9 M aphidicolin and 10−8 M PbTx, or 10−9 M aphidicolin and 1.0 μg/ml brevenal for 1 h followed by 10−8 M PbTx for 2 h.
Gel electrophoresis
Following the treatment period, the lymphocytes were removed from chemical exposure by centrifugation of the sample followed by aspiration of the supernatant, mixing with low melting point agarose, and spreading onto pre-frosted slides. Once the agarose/lymphocyte layer was firmly affixed to the slide, the entire slide was immersed into a lysis solution containing salts and other detergents for a minimum of 2 h to lyse the cell membrane from the lymphocytes (Tice and Strauss 1995). The slides were then submersed into an alkalinizing solution for 1 h to unwind the lymphocyte DNA. Next the slides were put into an alkaline electrophoresis solution with pH>14 in a gel electrophoresis box where lymphocyte DNA undergoes separation (Singh et al 1998). If DNA was damaged during the chemical treatment period there were many sections of fragmented DNA in the lymphocyte nucleus. If there was no damage to the DNA there were no DNA fragments. In the gel box an electric current was initiated (25 V, 300 mA, 40 min). The more fragments of DNA present in the lymphocyte nucleus, the greater the number of small fragments attracted to the negative electrode of the gel box due to the inherent charge on DNA, resulting in DNA separation. Following a 40-minute period in the gel box, the slides were removed and neutralized using a tris-base solution and allowed to dry overnight (Charles et al 2002; Rojas et al 1999).
Fluorescent microscopy
A fluorescent stain (SYBR green, Trevigen, Lot Number: 4250-050-05) was applied to the slide where the lymphocytes were located. The slide was then viewed under a fluorescence microscope and the comet size, head length, tail length, and amount of DNA present in the head and tail (measured by the intensity of SYBR green fluorescence) were measured using imaging analysis software (Northern Eclipse Imaging Analysis). The imaging software was able to detect SYBR green bound to DNA. The intensity of the fluorescence was assigned a numerical value where the greater the fluorescent intensity (an indicator of amount of DNA present), the greater the value. About 50 comets per slide were measured and the experiment was performed in triplicate or more. The tail moment, which is defined as the percentage of DNA in the tail multiplied by the length between the center of the head and center of the tail as determined from the software (Lee et al 2004) was calculated and reported.
Statistical analysis
Control and treated groups were compared using a oneway analysis of variance and the least significant difference test (LSD test), p<0.05. Means and standard errors were calculated (Enterprise Guide, SAS Statistical Software, Cary, NC, USA).
Results
The effect of various concentrations of different PbTxs are shown in Table 1. The results are expressed as the “tail moment”, which is defined as the percentage of DNA in the tail multiplied by the length between the center of the head and the center of the tail.
Table 1.
Tail moments of human lymphocytes exposed to different treatments
| Treatment | N | Tail moment Mean±SE |
|---|---|---|
| Negative control | 30 | 29.2+0.9 |
| H2O2 (2.75%) | 30 | 72.1+1.5* |
| Solvent (EtOH) | 3 | 24.2+2.1 |
| PbTx-2 10−8 M (Sigma) | 6 | 41.3+3.6* |
| PbTx-2 10−12 M (Sigma) | 3 | 46.85+1.5* |
| PbTx-2 10−8 M (UNCW) | 3 | 49.4+9.9* |
| PbTx-2 10−12 M (UNCW) | 3 | 41.8+8.1* |
| PbTx-3 10−8 M (UNCW) | 6 | 64.0+6.4* |
| PbTx-3 10−12 M (UNCW) | 3 | 41.4+5.2* |
| PbTx-9 10−8 M (Sigma) | 3 | 57.0+5.3* |
| PbTx-9 10−12 M (Sigma) | 3 | 41.0+2.9* |
Significantly different from the negative control, p<0.05.
Clearly PbTxs in concentrations of 10−8 and 10−12 M cause DNA damage in human lymphocytes. Human lymphocytes exposed to a 2-h PbTx-2 at 10−8 M treatment period were also investigated (Table 2). DNA damage of a magnitude similar to a 1-h exposure was found following a 2-h treatment period. The solvent, EtOH, did not damage the lymphocyte DNA.
Table 2.
Tail moments of human lymphocytes treated with different sources of brevetoxins for 2 h
| Treatment | N | Tail moment Mean±SE |
|---|---|---|
| Negative control | 30 | 29.2+0.9 |
| H2O2 (2.75%) | 30 | 72.1+1.5* |
| 2 h. PbTx-2 10−8 M (Sigma) | 3 | 50.1+2.0* |
| 2 h. PbTx-2 10−8 M (UNCW) | 3 | 51.0+2.4* |
| 2 h. PbTx-3 10−8 M (UNCW) | 3 | 49.1+0.8* |
| 2 h. PbTx-9 10−8 M (Sigma) | 3 | 50.1+1.8* |
Significantly different from the negative control, p<0.05.
Experiments conducted with 1-h brevenal pretreatment followed by PbTx treatment for 2 h resulted in undamaged lymphocytes (Table 3). Lymphocytes were also pretreated with 10−8 M PbTx for 1 h and then treated with 1.0 μg/ml brevenal for 2 h. Brevenal at 1 and 100 μg/ml fully antagonized the DNA damage elicited by the PbTxs, whether given before or after the toxins, in nearly all instances. Brevenal alone caused no measurable DNA damage in human lymphocytes.
Table 3.
The effect of brevenal of human lymphocytes exposed to brevetoxins
| Treatment | N | Tail moment Mean±SE |
|---|---|---|
| Negative control | 30 | 29.2+0.9 |
| H2O2 (2.75%) | 30 | 72.1+1.5* |
| Brevenal 1.0 μg/ml | 9 | 30.8+2.6 |
| Brevenal 100 μg/ml | 3 | 36.3+1.7 |
| Brevenal 1.0 μg/ml 1 h before PbTx-2 10−8 M (Sigma) | 3 | 31.3+2.1 |
| PbTx-2 10−8 M (Sigma) 1 h before Brevenal 1.0 μg/ml | 3 | 33.5+0.3 |
| Brevenal 1.0 μg/ml 1 h before PbTx-2 10−8 M (UNCW) | 3 | 33.9+1.4 |
| PbTx-2 10−8 M (UNCW) 1 h before Brevenal 1.0 μg/ml | 3 | 40.3+1.9 |
| Brevenal 1.0 μg/ml 1 h before PbTx-9 10−8 M (Sigma) | 3 | 35.5+2.9 |
| PbTx-9 10−8 M Sigma 1 h before Brevenal 1.0 μg/ml | 3 | 40.1+2.4 |
| Brevenal 1.0 μg/ml 1 h before PbTx-3 10−8 M (UNCW) | 6 | 34.9+1.25 |
| PbTx-3 10−8 M (UNCW) 1 h before Brevenal 1.0 μg/ml | 3 | 42.4+0.75** |
| Brevenal 1.0 μg/ml simultaneously with PbTx-2 10−8 M (Sigma) | 3 | 27.4+1.2 |
| Brevenal 1.0 μg/ml simultaneously with PbTx-2 10−8 M (UNCW) | 3 | 49.2+7.1 |
Significantly different from the negative control, p<0.05.
Significantly different from brevenal 1.0 μg/ml, p<0.05.
Additionally, brevenal does not prevent hydrogen peroxide-induced DNA damage (Table 4) regardless of the treatment order, and the extent of DNA damage is not significantly different to that from hydrogen peroxide alone.
Table 4.
The effect of brevenal on hydrogen peroxide
| Treatment | N | Tail moment Mean ± SE |
|---|---|---|
| Negative control | 30 | 29.2+0.9 |
| H2O2 (2.75%) | 30 | 72.1+1.5* |
| Brevenal 1.0 μg/ml 1 h before H2O2 | 3 | 72.1+3.7** |
| H2O2 1 h before Brevenal 1.0 μg/ml | 3 | 74.5+4.3** |
Significantly different from the negative control, p<0.05.
Significantly different from H2O2 alone, p<0.05.
Figure 3a–f depicts several images of human lymphocyte nuclei as seen under the fluorescent microscope.
Fig. 3a–f.
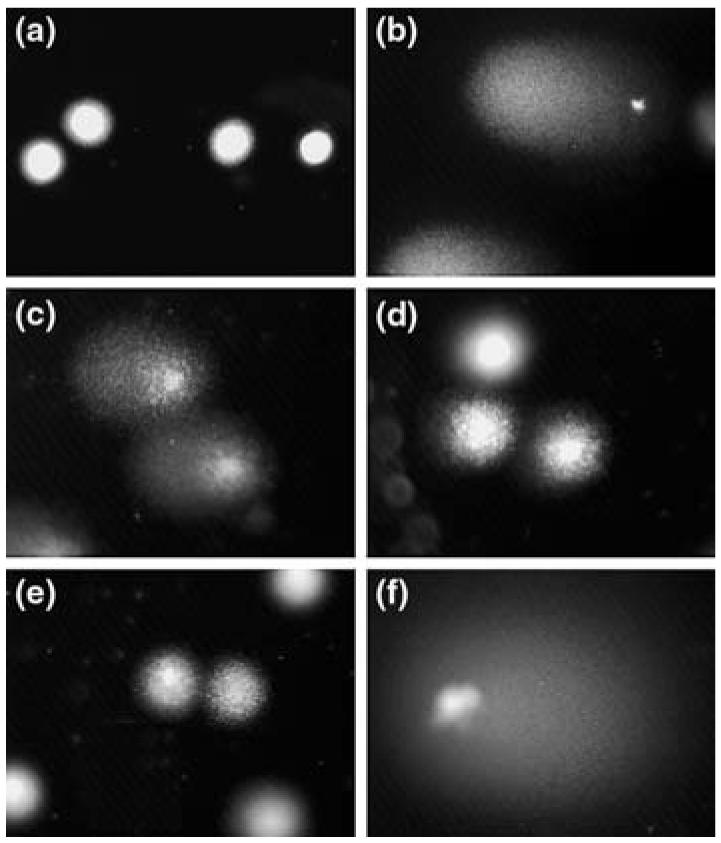
Human lymphocytes were left untreated or exposed to hydrogen peroxide, brevetoxin, or brevenal for 1 h. a A human lymphocyte that has not been exposed to any genotoxic damaging chemical. There is no DNA damage. b Human lymphocytes treated with hydrogen peroxide (positive control). A large amount of DNA damage is present. c Human lymphocytes treated with 10−8 M brevetoxin. Both sources of brevetoxin used in this experiment at a concentration of 10−8 M resulted in a similar degree of DNA damage. d,e Human lymphocytes first treated with brevenal for 1 h prior to treatment with 10−8 M brevetoxin for 2 h (d) or first treated with 10−8 M brevetoxin for 1 h prior to treatment with brevenal for 2 h (e). Upon brevenal pretreatment (1.0 μg/ml) the lymphocytes are protected from the damaging effects caused by brevetoxin. f Human lymphocytes after treatment with the DNA polymerase inhibitor aphidicolin. Lymphocytes were treated first with 10−9 M aphidicolin and 1.0 μg/ml brevenal for 1 h, followed by 10−8 M PbTx for 2 h
To test the possibility that brevenal was antagonizing the effects of PbTx on DNA repair processes, an inhibitor of DNA polymerase was examined for its effect on the brevenal antagonistic effect. Aphidicolin was the inhibitor of DNA polymerase used in this study. Treatments with PbTx alone induced DNA damage that was alleviated by the addition of brevenal. By inhibiting the DNA repair mechanism via aphidicolin, the extent of DNA damage seen with aphidicolin, PbTx, and brevenal was found to be similar to PbTx alone (Table 5).
Table 5.
The effect of aphidicolin on lymphocytes exposed to brevetoxin or brevetoxin and brevenal
| Treatment | N | Tail moment Mean ± SE |
|---|---|---|
| Negative control | 30 | 29.2+0.9 |
| H2O2 (2.75%) | 30 | 72.1+1.5* |
| Brevenal 1.0 μg/ml | 9 | 30.8+2.6 |
| PbTx-3 10−8 M (UNCW) | 6 | 64.0+6.4* |
| Brevenal 1.0 μg/ml 1 h before PbTx-3 10−8 M (UNCW) | 6 | 34.9+1.25 |
| Aphidicolin 10−9 M | 3 | 46.2+2.8** |
| Aphidicolin 10−9 M and PbTx-3 10−8 M (UNCW) | 3 | 63.6+4.1** |
| Aphidicolin 10−9 M and brevenal 1.0 μg/ml 1 h before PbTx-3 10−8 M (UNCW) | 3 | 64.5+1.3** |
Significantly different from the negative control, p<0.05.
Significantly different from brevenal 1.0 μg/ml, p<0.05.
Discussion
Human T and B-lymphocytes play an important role in immune function. These special types of white blood cells have unique cell surface receptors that are highly selective for foreign antigens capable of triggering the immune response. Less selective response elements, such as granulocytes (neutrophils and eosinophils) are also involved in the inflammatory process. These cells recognize foreign material in the body but do not recognize specific antigens. Granulocytes destroy the invading entity via phagocytosis or by another cytotoxic response. When a foreign antigen binds to a T lymphocyte, cytokines are produced. Cytokines, such as interleukins 2, 4, 5, 10, and 13, directly stimulate Blymphocytes, monocytes, and neutrophils. Upon activation, B cells produce antibodies to attack and destroy the foreign antigen. During a secondary exposure to a previously encountered antigen, memory T lymphocytes are able to detect the previously encountered antigen and raise an immune response more rapidly compared to the first exposure.
The PbTxs trigger airway constriction and may induce asthma attacks (Abraham et al 2004; Asai et al 1982). Asthma is a hypersensitivity to an allergen or foreign substance that can trigger the immune response (Schuster et al 2000). In addition to being neurotoxic and gastrointestinally toxic, brevetoxins cause airway irritation, airway constriction, and bronchoconstriction (Backer et al 2003). The harmful effects of PbTx on the respiratory system trigger an immune response that may be highly sensitive in certain individuals (in asthmatics, individuals with chronic obstructive pulmonary disease, and other types of pulmonary diseases).
In this study, the DNA damage measured by the comet assay determines the extent of single and double DNA strand breaks upon exposure to PbTx. The DNA damage was apparent upon PbTx exposure and each PbTx subtype tested caused human lymphocyte DNA damage of similar magnitude. As expected, the 10−8 M PbTx caused greater damage than the smaller concentration of PbTx 10−12 M. However, 10−12 M PbTx, a 10,000 fold more dilute concentration than 10−8 M, still caused significant DNA damage compared to controls. Damaging the DNA of a lymphocyte will likely impair the function of the lymphocyte. Among the effects of DNA damage are the potential for mutagenic response and/or disruption of cell signaling processes such as those signaling pathways involved with cell cycling, proliferation, migration, differentiation, plasticity, inflammation, immunomodulation, apoptosis, cell survival, and developmental processes (Clayson et al 1994).
PbTxs at 10−8 and 10−12 M concentrations are potent inducers of DNA damage in normal human lymphocytes and the damage induced was fully antagonized by brevenal. Different sources of PbTx cause DNA damage greater than that of the controls, but not greater than hydrogen peroxide-exposed lymphocytes. Lymphocytes not treated with any genotoxic chemicals depicted a typical undamaged circular-shaped lymphocyte nucleus. Hydrogen peroxide treatment caused the greatest extent of DNA damage of all the treated groups in this experiment. Lymphocytes exposed to PbTxs for an additional 2 h induced significant damage compared to control lymphocytes.
The PbTx antagonist, brevenal, both prevents and reverses the effect of PbTx. Brevenal is a naturally-occurring short-chain polyether that competes with PbTx for the active site of the Na+ channel, thus negating this effect of the toxin. As a result, PbTx is competitively displaced from the active site of the Na+ channel. In our studies, the incubation of lymphocytes with brevenal prior to the addition of PbTx completely alleviated DNA damage caused by PbTx alone. More importantly, the addition of brevenal after PbTx incubation reversed PbTx-induced DNA damage.
To further investigate the role of brevenal in DNA repair, aphidicolin was used to ascertain the role of DNA repair in the mechanism of brevenal antagonism (Speit et al 2004). By inhibiting a main mechanism of DNA repair via aphidicolin, it was possible to demonstrate that brevenal allows DNA repair processes to restore PbTx-induced DNA damage. The present results indicate that by inhibiting DNA polymerase, there is a statistically significant amount of DNA damage, even in the presence of brevenal.
It has been previously shown that brevenal competitively displaces titrated PbTx in a synaptosome receptor-binding assay, one that considers specific sodium channel receptor site binding for natural PbTxs. In that assay, however, it was clear that the concentration of brevenal needed to compete for PbTx occupied sites was over two orders of magnitude greater than for PbTx. Similarly, PbTx-induced DNA damage at 10−8 M in human lymphocytes was antagonized by 1.0 μg/ml (1.5×10−6 M) of brevenal, a difference of two orders of magnitude. However, in the current study, lower concentrations of brevenal were not tested and it is possible that brevenal antagonism would be measurable at lesser amounts.
Other organisms involved in HABs produce a variety of toxins including: ciguatoxins, maitotoxins, saxitoxin, scaritoxins, okadoic acid, and palytoxins (in addition to other toxins), often in high amounts. Many of these toxins bind to ion channels in eukaryotic cells that are located on either cellular or nuclear membranes, causing ion transport disruptions (Ito et al 2003; Rein and Barrone 1999). The PbTxs are known to bind to voltage-gated sodium channels on voltage-gated cells but their activity on non-voltage-gated cells (lymphocytes) and nuclear membranes is unknown. The PbTx has also been shown to induce a concentration-dependent increase in cytosolic Ca2+ in cerebellar neurons (LePage et al 2002). It is possible that brevetoxin and brevenal may bind to nuclear receptors. Binding of PbTxs to the nuclear membrane receptors in the lymphocytes could lead to ion concentration disturbances (especially Ca2+) in the nucleus, resulting in damage to the nuclear DNA through Ca2+ activation of endonucleases or alterations in the tertiary structure of the DNA. Brevenal may work to inhibit brevetoxin-induced DNA damage by blocking the effect of brevetoxin on nuclear ion concentrations.
In a multitude of cell types, activation of a variety of receptors (such as G-protein coupled receptors, ion channels, receptor tyrosine kinases, and cytokine receptors) initiate the MAP kinase cascades, which serve as central downstream integrators for modulating the cellular response to external stimuli. It is possible that the effects of PbTx may be measured on diverse upstream targets that can lead to changes in a common downstream effector, MAP kinase, and provide a basis for evaluating MAP kinase signaling as a common mode of action. It is clear that MAP kinase signaling is an important mediator of critical cellular functions, including cell proliferation, cell differentiation, and cell death.
Thus, alterations in MAP kinase signaling might be expected to be part of the “mode of action” that leads to changes in critical cellular functions that are ultimately expressed as the adverse effects seen in the different tissues affected by PbTxs. In particular, human inflammatory cytokines and receptor genes may be disrupted. Cytokines play an important role in mediating immune cascade reactions during the inflammatory response. The information obtained in this study indicates that PbTx damages human lymphocyte DNA and may negatively impact the immune system.
Acknowledgements
Supported by the North Carolina Agromedicine Center and USDA/CSREES; The PbTxs and the brevenal were provided under NIEHS grant P01 ES10594. The experiments conducted are in accordance with IRB regulations (UMCIRB#03-0290).
References
- Abraham WM, Bourdelais AJ, Sabater JR, Ahmed A, Lee TA, Serebriakov I, Baden DG. Airway responses to aerosolized brevetoxins in an animal model of asthma. Am J Respir Crit Care Med. 2004;10 doi: 10.1164/rccm.200406-735OC. 1164/rccm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Kaoru Y, White AW. Technical Report. Woods Hole Oceanographic Institution; Woods Hole, MA, USA: 2000. Estimated annual economic impacts from harmful algal blooms (HABs) in the United States. [Google Scholar]
- Asai S, Krzanowski JJ, Anderson WH, Martin DF, Polson JB, Lockey RF, Bukantz SC, Szentivanyi A. Effects of toxin of red tide, Ptychodiscus brevis, on canine tracheal smooth muscle: a possible new asthma-triggering mechanism. J Allergy Clin Immunol. 1982;69:418–428. doi: 10.1016/0091-6749(82)90116-6. [DOI] [PubMed] [Google Scholar]
- Backer LC, Fleming LE, Rowan A, Cheng YS, Benson J, Pierce RH, Zaias J, Bean J, Bossart GD, Johnson D, Quimbo R, Baden DG. Recreational exposure to aerosolized breve-toxins during Florida red tide events. Harmful Algae. 2003;2:19–28. [Google Scholar]
- Bourdelais AJ, Campbell S, Jacocks H, Naar J, Wright JL, Carsi J, Baden DG. Brevenal is a natural inhibitor of brevetoxin action in sodium channel receptor binding assays. Cell Mol Neurobiol. 2004;24:553–563. doi: 10.1023/B:CEMN.0000023629.81595.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Invest Suppl. 1968;21:77–89. [PubMed] [Google Scholar]
- Charles GD, Linscombe VA, Tornesi B, Mattsson JL, Gollapudi BB. An in vitro screening paradigm for extracts of whole foods for detection of potential toxicants. Food Chem Toxicol. 2002;40:1391–1402. doi: 10.1016/s0278-6915(02)00085-6. [DOI] [PubMed] [Google Scholar]
- Clayson DB, Mehta R, Iverson F. International commission for protection against environmental mutagens and carcinogens: Oxidative DNA damage—the effects of certain genotoxic and operationally non-genotoxic carcinogens. Mutat Res. 1994;317:25–42. doi: 10.1016/0165-1110(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Collins AR, Dobson VL, Dusinska M, Kennedy G, Stetina R. The comet assay: what can it really tell us? Mutat Res. 1997;375:183–193. doi: 10.1016/s0027-5107(97)00013-4. [DOI] [PubMed] [Google Scholar]
- Fairey ER, Shuart NG, Busman M, Moeller PDR, Ramsdell JS. Biomonitoring brevetoxin exposure in mammals using blood collection cards. Environ Health Perspect. 2001;109:717–720. doi: 10.1289/ehp.01109717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CJ, Kimm-Brinson KL, Padnos B, Ramsdell JS. Acute and delayed thermoregulatory response of mice exposed to brevetoxin. Toxicon. 2001;39:1367–1374. doi: 10.1016/s0041-0101(01)00092-7. [DOI] [PubMed] [Google Scholar]
- Ito K, Toyoda I, Higashiyama M, Uemura D, Sato MH, Yoshimura SH, Ishii T, Takeyasu K. Channel induction by palytoxin in yeast cells expressing Na+, K+-ATPase or its chimera with sarco/endoplasmic reticulum Ca2+-ATPase. FEBS Lett. 2003;543:108–112. doi: 10.1016/s0014-5793(03)00418-6. [DOI] [PubMed] [Google Scholar]
- Jeglitsch G, Rein K, Baden DG, Adams DJ. Brevetoxin-3 (PbTx-3) and its derivatives modulate single tetrodotoxin-sensitive sodium channels in rat sensory neurons. J Pharmacol Exp Ther. 1997;284:516–525. [PubMed] [Google Scholar]
- Kimm-Brinson KL, Ramsdell JS. The red tide toxin, brevetoxin, induces embryo toxicity and developmental abnormalities. Environ Health Perspect. 2001;109:377–381. doi: 10.1289/ehp.01109377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulagina NV, O'Shaughnessy TJ, Ma W, Ramsdell JS, Pancrazio JJ. Pharmacological effects of the marine toxins, brevetoxin and saxitoxin, on murine frontal cortex neuronal networks. Toxicon. 2004;44:669–676. doi: 10.1016/j.toxicon.2004.07.023. [DOI] [PubMed] [Google Scholar]
- Lee E, Oh E, Lee J, Sul D, Lee J. Use of the tail moment of lymphocytes to evaluate DNA damage in human biomonitoring studies. Toxicol Sci. 2004;81:121–132. doi: 10.1093/toxsci/kfh184. [DOI] [PubMed] [Google Scholar]
- LePage KT, Baden DG, Murray TF. Brevetoxin derivatives act as partial agonists at neurotoxin site 5 on the voltage gated Na+ channel. Brain Res. 2002;959:120–127. doi: 10.1016/s0006-8993(02)03737-x. [DOI] [PubMed] [Google Scholar]
- Lombet A, Bidard JN, Lazdunski M. Ciguatoxin and brevetoxins share a common receptor site on the neuronal voltage-dependent Na+ channel. FEBS Lett. 1987;219:355–359. doi: 10.1016/0014-5793(87)80252-1. [DOI] [PubMed] [Google Scholar]
- Music SI, Howell JT, Brumback CL. Red tide, its public health implications. JFMA. 1973;60:27–29. [PubMed] [Google Scholar]
- Rein KS, Barrone J. Polyketides form dinoflagellates: origins, pharmacology and biosynthesis. Comp Biochem Physio B. 1999;124:117–131. doi: 10.1016/s0305-0491(99)00107-8. [DOI] [PubMed] [Google Scholar]
- Rojas E, Lopez MC, Valverde M. Single cell gel electrophoresis assay: methodology and applications. J Chromatogr B Biomed Sci Appl. 1999;722:225–254. doi: 10.1016/s0378-4347(98)00313-2. [DOI] [PubMed] [Google Scholar]
- Schuster M, Tschernig T, Krug N, Pabst R. Lymphocytes migrate from the blood into the bronchoalveolar lavage and lung parenchyma in the asthma model of the brown Norway rat. Am J Respir Crit Care Med. 2000;161:558–566. doi: 10.1164/ajrccm.161.2.9812021. [DOI] [PubMed] [Google Scholar]
- Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1998;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- Speit G, Schutz P, Hoffmann H. Enhancement of genotoxic effects in the comet assay with human blood samples by aphidicolin. Toxicol Lett. 2004;153:303–310. doi: 10.1016/j.toxlet.2004.04.047. [DOI] [PubMed] [Google Scholar]
- Tice RR, Strauss GH. The single cell gel electrophoresis/comet assay: a potential tool for detecting radiation-induced DNA damage in humans. Stem Cells. 1995;1:207–214. [PubMed] [Google Scholar]
- Trainer VL, Baden DG. High affinity binding of red tide neurotoxins to marine mammal brain. Aqua Toxicol. 1999;46:139–148. [Google Scholar]