Abstract
Extrinsic denervation contributes to enteric motor dysfunction after small bowel transplantation (SBT). Our aim was to determine changes in nonadrenergic, noncholinergic innervation with vasoactive intestinal polypeptide (VIP) and substance P (Sub P) in rat jejunal circular muscle after SBT. Muscle strips were studied in tissue chambers from six groups of rats (n ≥ 6 per group): naïve controls (NC), animals 1 week after anaesthesia/sham celiotomy (SC-1), and 1 and 8 weeks after jejunal and ileal transection/reanastomosis (TA-1, TA-8) and after syngeneic, orthotopic SBT (SBT-1, SBT-8). Response to exogenous VIP and Sub P and their endogenous release during electrical field stimulation (EFS) were studied. Exogenous VIP and Sub P caused a dose-dependent inhibition and stimulation of mechanical activity in all groups respectively (P < 0.05). The responses to VIP and Sub P were decreased (compared to NC) in all groups at 1 and 8 weeks postoperatively. The VIP antagonist ([D-p-Cl-Phe6,Leu17]-VIP) did not prevent the inhibition by exogenous VIP in any group, while the Sub P antagonist ([D-Pro2,D-Trp7,9]-Sub P) prevented the effect of exogenous Sub P in NC, TA-8 and SBT-8 (P < 0.05). Responses to exogenous VIP were unaffected by the nitric oxide synthase inhibitor l-NG-nitro arginine and precontraction of muscle strips with Sub P. Endogenous release of VIP and Sub P during EFS was preserved after SBT. In circular muscle of rat jejunum, changes in neuromuscular transmission with VIP and Sub P during the first 8 weeks after SBT are not mediated by extrinsic denervation.
Keywords: extrinsic denervation, small bowel transplantation, small intestinal motility, substance P, vasoactive intestinal polypeptide
INTRODUCTION
Small bowel transplantation (SBT) has become an alternative to chronic total parenteral nutrition in selected patients with intestinal failure.1–4 Many challenges remain, however, in the postoperative management of these patients of which enteric motor dysfunction with high stomal output/diarrhoea is one.1,5–7 The extrinsic denervation obligated by SBT appears to play a role in the pathophysiology of enteric dysfunction after SBT; indeed, after autotransplantation of the jejunoileum in which no immunosuppressants are necessary and immune phenomena are avoided, dogs develop a clinical picture of enteric motor dysfunction with a profuse, watery diarrhoea that, like the clinical situation, resolves over time as the gut adapts.8
Our laboratory has studied changes in adrenergic, cholinergic and nitrergic innervation in longitudinal and circular muscle of jejunum and ileum after syngeneic SBT in rats.9–14 In contrast, alterations in nonadrenergic, noncholinergic (NANC) innervation after SBT are less well-understood, especially with the inhibitory neuropeptide vasoactive intestinal polypeptide (VIP) and the excitatory neuropeptide and tachikinin substance P (Sub P), which play important roles in enteric motor innervation.15 Previous studies in jejunal longitudinal muscle demonstrated decreased sensitivity to exogenous Sub P and a decrease in endogenous release of VIP during electrical field stimulation (EFS).16,17 In jejunal circular muscle, response to exogenous Sub P was suppressed 1 year after SBT, while innervation mediated by VIP was unaffected.18 Changes in innervation of circular muscle mediated by VIP and Sub P early after SBT have not been studied.
Our aim was to determine changes in response of jejunal circular muscle to exogenous VIP and Sub P and endogenous release of these neurotransmitters 1 and 8 weeks after SBT. To control for nonspecific, confounding effects of anaesthesia and celiotomy, as well as for disruption of myoneural continuity by intestinal transection, multiple control groups were studied. Our hypothesis was that SBT causes functional changes in response to VIP and Sub P, as well as in their endogenous release, and that these changes contribute to enteric motor dysfunction after SBT.
MATERIALS AND METHODS
Preparation of animals
Procedures and animal care were approved and performed according to guidelines of the Institutional Animal Care and Use Committee (IACUC) of the Mayo Foundation in accordance with the National Institutes of Health and Public Health Service Policy of the Humane Use and Care of Laboratory Animals.
Experimental groups
To avoid confounding effects of immune phenomena or pharmacological immunosuppression, syngeneic, male Lewis rats (Harlan, Indianapolis, IN, USA) were used. Anaesthesia was induced by inhalation of 2% isoflurane (Abbott Laboratories, North Chicago, IL, USA) and maintained by intraperitoneal sodium pentobarbital (30–50 mg kg−1; AmproPharmacy, Arcadia, CA, USA). Although experimental groups were identical to a prior study,17 different rats were used. To accomplish jejunoileal extrinsic denervation, orthotopic small bowel isotransplantation was performed in 16 rats as described previously.10 The jejunoileum was removed with a segment of donor aorta after flushing the intestinal lumen and graft vasculature with chilled, 154 mmol L−1 NaCl; NaCl to flush the vasculature was heparinized. Donor aorta was anastomosed to recipient aorta (end-to-side) and donor portal vein to recipient inferior vena cava (end-to- side). After resecting recipient’s jejunoileum, intestinal continuity was re-established by end-to-end jejunojejunostomy and ileoileostomy. In 16 other rats, the proximal jejunum and distal ileum were transected and re-anastomosed by end-to-end anastomosis (TA) to control for disruption of myoneural continuity. Rats after SBT and TA were studied 1 and 8 weeks postoperatively (SBT-1, SBT-8, TA-1, TA-8; n = 8 per group).
Combined effects of anaesthesia and celiotomy were studied in six rats which underwent celiotomy and standardized bowel manipulation. The jejunoileum was exteriorized and manipulated with two cotton applicators from duodenojejunal junction to ileocecal junction. These sham-operated animals were studied 1 week postoperatively (SC-1; n = 6).
All rats were allowed access to water and 5% dextrose solution (Baxter, Deerfield, IL, USA) immediately postoperatively. Acetaminophen (100–300 mg kg−1; Goldline Laboratories Inc., Miami, FL, USA) was added to drinking water for 2 days prior to and 2 days after operation; buprenorphine hydrochloride (0.05–0.1 mg kg−1 s.c.; Reckitt Benckiser Healthcare Ltd, Hull, East Yorkshire, UK) was administered once postoperatively. Dextrose solution was replaced by normal rat chow 2 days postoperatively. Rats were studied at 1 week (SC-1, TA-1, SBT-1) and 8 weeks (TA-8, SBT-8) after operation.
Six rats not undergoing operation served as NC (n = 6).
Recording mechanical activity
A jejunal segment 10 cm distal to either duodenojejunal junction or the jejunojejunostomy (after SBT and TA) was harvested and kept in chilled, modified Krebs–Ringer’s bicarbonate solution (concentrations in mmol L−1: NaCl 116.4, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 23.8, calcium disodium edetate 0.26 and glucose 11.1) pre-oxygenated with 95% O2/5% CO2 (Praxair, Burr Ridge, IL, USA). After opening along its mesenteric border, eight full-thickness muscle strips (8 × 2 mm) were cut transversely in the direction of the circular muscle and suspended in 10-mL tissue chambers containing 37.5 °C modified Krebs–Ringer’s bicarbonate solution bubbled continuously with 95% O2/5% CO2. The muscle strips were attached to a fixed hook and a noncompliant force transducer (Kulite Semiconductors Products, Inc., Leonia, NJ, USA) to measure isometric force.
Mechanical activity was monitored in real time on a Grass 7D polygraph (Grass Instrument Co, Quincy, MA, USA) and saved digitally on a computer using dedicated software (MP-100A-CE and AcqKnowledge; Biopac Systems, Inc., Goleta, CA, USA) for subsequent detailed analysis.
Experimental design
After equilibration for 90 min with intervening washouts of bath solution every 15 min, muscle strips were stretched at 10-min intervals until reaching optimal length (LO) beyond which further stretching did not increase contractile amplitude or frequency.10 All subsequent experiments were performed under NANC conditions established by atropine (10−7 mol L−1), phentolamine (10−5 mol L−1) and propranolol (5 × 10−6 mol L−1) in the bath solution. Strips without spontaneous mechanical activity were excluded (<2%). At least two muscle strips per rat were studied under each experimental condition.
Cumulative dose–responses to VIP (3 × 10−9–3 × 10−7 mol L−1) were studied. After washout of the bath solution, effects of the VIP antagonist ([D-p-Cl-Phe6,Leu17]-VIP; 10−6 mol L−1) alone on baseline mechanical activity were studied, then cumulative dose–responses to VIP were repeated in the presence of the VIP antagonist. Thereafter, effects of the VIP antagonist, nitric oxide (NO) synthase inhibitor l-NG-nitro arginine (l-NNA; 10−4 mol L−1), and combination of both drugs on responses to single doses of VIP (3 × 10−7 mol L−1) were studied.
Cumulative dose–responses to Sub P (3 × 10−9–3 × 10−7 mol L−1) were also studied. After determining effects of the Sub P antagonist ([D-Pro2,D-Trp7,9]-Sub P; 10−5 mol L−1) on baseline mechanical activity, cumulative dose–responses to Sub P were repeated in the presence of the Sub P antagonist. Afterwards, muscle strips were precontracted with Sub P (10−7 mol L−1) for 90 s, and a submaximal dose of VIP (10−7 mol L−1) was studied without and with the VIP antagonist (10−6 mol L−1) and l-NNA (10−4 mol L−1).
In four other strips per rat, EFS was studied with constant voltage (20 V), pulse width (4 ms), and duration of stimulation (10 s) at 6 Hz to examine EFS-induced inhibitory effects and at 20 Hz to study EFS-induced excitatory effects. Preliminary experiments in naïve rats had established appropriate parameters for our experiments.14,18,19 Ten minutes were allowed for spontaneous mechanical activity to recover before the next EFS; bath solution was exchanged after each series of stimulations. EFS responses were studied without and with the VIP antagonist, l-NNA, combination of both drugs and in absence and presence of the Sub P antagonist.
Muscle strips were blotted on filter paper and weighed at the conclusion of each experiment allowing normalization of motility data per milligram tissue wet weight.
Data analysis
Baseline mechanical activity was measured as area under the contractile curve for 5 min (g × 5 min mg−1 tissue) for each strip at LO. Effects of cumulative doses of VIP and Sub P on mechanical activity were quantitated for a 5-min interval after each increase in concentration and were compared to a 5-min interval of baseline activity directly before the first dose was administered. When the response to a dose of VIP, Sub P, or their antagonists was studied, effects on mechanical activity in a 5-min interval were compared to baseline activity during the 5 min before drug administration. When muscle strips were precontracted with Sub P, the VIP effect on stimulated mechanical activity was measured for 5 min and compared to the last 60 s of the 90-s precontraction interval directly before administering VIP. Drug responses are presented as % change from baseline activity (defined as 0%), which represents mechanical activity 5 min or 60 s before drug administration. Positive values represent increases and negative values decreases in mechanical activity.
Analysis during EFS was more complicated. We showed previously that alterations in neurotransmission with VIP persist during the first postoperative week in longitudinal muscle.17 Because we were interested in alterations in EFS response due to extrinsic denervation and wanted to eliminate confounding effects of anaesthesia/celiotomy, EFS responses compared to NC are shown separately for groups at 1 week postoperatively (SC-1, TA-1, SBT-1) and for groups at 8 weeks postoperatively (TA-8 and SBT-8). We studied exclusively the 10 s of stimulation and not the ‘off contraction’ after stopping EFS. In previous studies, the EFS response at low frequencies and effects of antagonists on EFS responses differed between the first 4 s and last 6 s of EFS.16–18 Therefore, we quantitated effects of EFS at 6 Hz separately for the entire 10 s, first 4 s and last 6 s of EFS. Mechanical activity was expressed as per cent change from baseline activity during an equally long interval measured during 40 s immediately before EFS.
Statistical comparisons
Data are summarized as mean ± SEM. Analysis of variance (anova) was used to determine overall effects of different drugs or EFS. Repeated measurements within the same rat were treated as repeated factors (e.g. dose–responses to VIP or Sub P or responses during EFS under different conditions) and were analysed by repeated measures anova. The six groups of rats were independent factors. When the overall effect was significant when analysed by anova, post hoc pairwise comparisons were performed using paired Student’s t-tests (repeated factors) or two-sample t-tests (independent group factors). Additional t-tests were used for single comparisons (e.g. vs baseline mechanical activity). Bonferroni correction was applied when evaluating statistical significance of multiple t-tests and anova on Ranks when data were not distributed normally.
Drugs
Atropine sulphate, phentolamine hydrochloride, dl-propranolol hydrochloride, l-NNA, Sub P, [D-Pro2,D-Trp7,9]-Sub P, VIP, and [D-p-Cl-Phe6,Leu17]-VIP were purchased from Sigma-Aldrich (St Louis, MO, USA).
RESULTS
Weight
All animals remained healthy throughout the study. Groups studied 1 week postoperatively (SC-1, TA-1, SBT-1) neither gained nor lost weight; groups 8 weeks postoperatively (TA-8, SBT-8) gained weight normally (x̄ = 100–150 g).
Spontaneous mechanical activity
When compared across all six groups, there were only minimal differences in spontaneous mechanical activity. Several differences were, however, noted. Spontaneous activity in the group 1 week after SBT (SBT-1; 10.7 ± 1.1 g × 5 min mg−1 tissue), although not different from sham control group at 1 week postoperatively (SC-1; 8.4 ± 1.8 g × 5 min mg−1 tissue, P = 0.27), was greater than in the other four groups (NC: 4.6 ± 0.5; TA-1: 5.8 ± 1.2; TA-8: 5.3 ± 0.8; and SBT-8: 6.5 ± 0.7 g × 5 min mg−1 tissue; each P < 0.05; anova).
Response to exogenous VIP
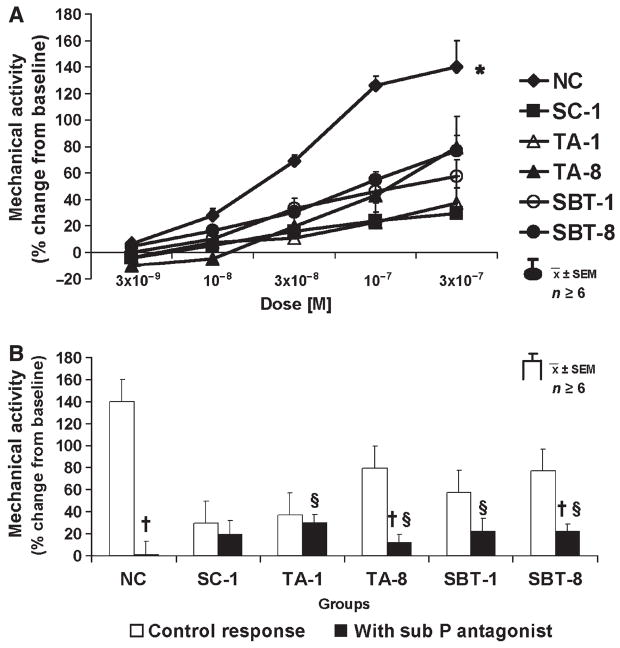
Spontaneous mechanical activity was inhibited by VIP (3 × 10−9–3 × 10−7 mol L−1) in dose-dependent fashion in all groups (Fig. 1). Inhibition of activity was more pronounced in NC compared to other groups (P < 0.05). The VIP antagonist (10−6 mol L−1) had no effect on spontaneous activity in any group (0 ± 1% in SC-1 to 10 ± 3% in TA-8; P = ns). In addition, the VIP antagonist had no apparent effect on VIP-induced inhibition in any group (data not shown).
Figure 1.
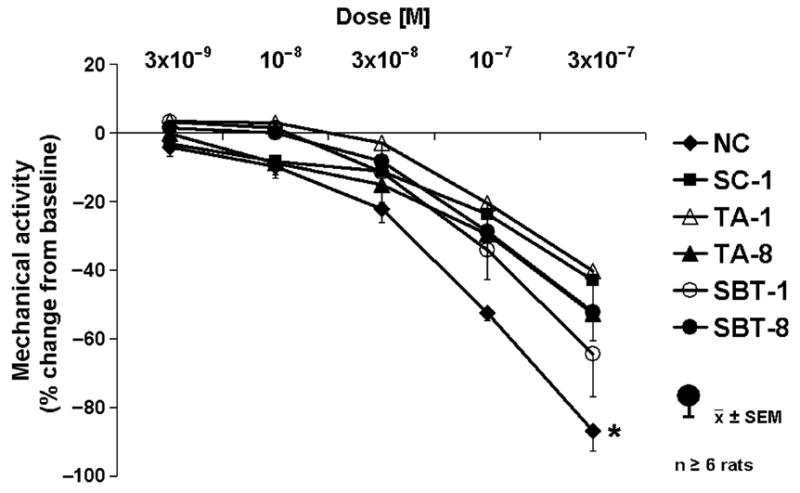
Dose–response to exogenous VIP. P < 0.05 for dose-dependent inhibition of spontaneous contractile activity in all groups (anova). *P < 0.05 compared to dose–response to VIP in all other groups (anova).
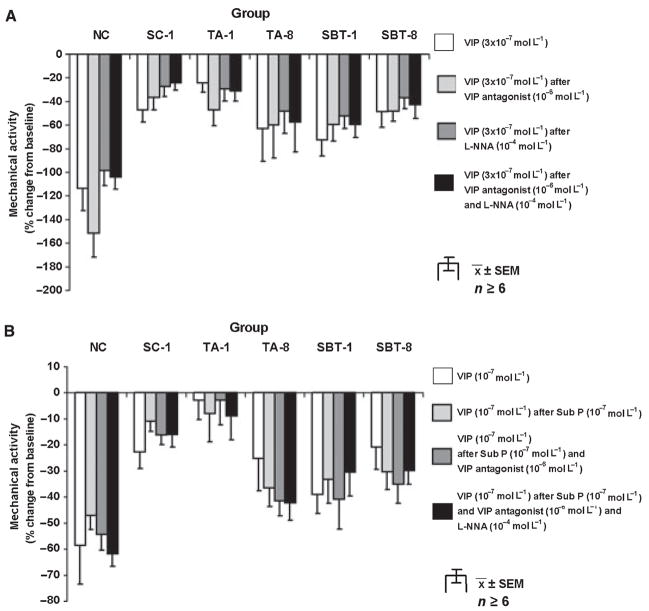
When administered as a single dose (3 × 10−7 mol L−1), the inhibitory effect of VIP was unaffected by either the VIP antagonist (10−6 mol L−1), l-NNA (10−4 mol L−1), or their combination (Fig. 2A). Precontraction with a submaximal dose of Sub P (10−7 mol L−1) did not alter the relative inhibitory potential of a submaximal dose of VIP (10−7 mol L−1; Fig. 2B). The VIP antagonist (10−6 mol L−1) alone and combined with l-NNA (10−4 mol L−1) had no effect on response to VIP (10−7 mol L−1) after precontraction with Sub P.
Figure 2.
Spontaneous mechanical activity. (A) Response to single dose of VIP (3 × 10−7 mol L−1) under control conditions with VIP antagonist, l-NNA, and combination of both; *P < 0.05 compared to response to VIP with l-NNA alone in NC. (B) Effect of submaximal dose of VIP under control conditions and after precontraction with Sub P (10−7 mol L−1) without and with VIP antagonist (10−6 mol L−1) alone and combined with l-NNA (10−4 mol L−1).
Response to exogenous Sub P
Sub P (3 × 10−9–3 × 10−7 mol L−1) increased mechanical activity dose-dependently in all groups (Fig. 3A); responses to Sub P were greater in NC compared to other groups (P < 0.05). Changes in mechanical activity after administering the Sub P antagonist (10−5 mol L−1) ranged from 2 ± 4% in TA-1 to 52 ± 23% in TA-8 but were not significant after Bonferroni correction (P > 0.01, paired t-test). The Sub P antagonist (10−5 mol L−1) abolished effects of Sub P in NC (140 ± 19% vs 0 ± 13%; P < 0.05) and decreased pro-contractile responses to Sub P in other groups as well but to a lesser extent (Fig. 3B).
Figure 3.
Response to Sub P. (A) Dose–response; P < 0.05 for dose-dependent increase of spontaneous mechanical activity in all groups, *P < 0.05 compared to dose–response to Sub P in all other groups. (B) Effect of Sub P antagonist (10−5 mol L−1) on response to exogenous Sub P (3 × 10−7 mol L−1); §P < 0.05 compared to baseline mechanical activity. §P < 0.05 compared to response to Sub P (3 × 10−7 mol L−1) without Sub P antagonist.
Response to EFS
Electrical field stimulation at low frequency (6 Hz) changed the contractile pattern from regular contractions to a pattern of initial inhibition (~4 s) followed by a more tonic contractile response. Response to EFS differed between groups 1 week postoperatively (SC-1, TA-1, SBT-1) and 8 weeks postoperatively (TA-8, SBT-8); therefore, they are presented separately (Figs 4–6). Results of EFS at 6 Hz are shown in Fig. 4 for the 1-week groups compared to NC. In the NC group, although the pattern of contractions was altered, there was no overall effect of EFS in NC for the entire 10 s when quantitated as area under the curve (Fig. 4A), while net inhibition was evident during the first 4 s of EFS (Fig. 4B). In contrast, net inhibition was not present in SC-1, TA-1 and SBT-1 (Fig. 4B). The VIP antagonist, l-NNA, or their combination had no effect on net inhibition in NC during the first 4 s of EFS. In contrast, when the 10 s of EFS as well as the last 6 s of EFS was evaluated in NC (Fig. 4A,C), although the VIP antagonist had no effect, l-NNA alone and combined with the VIP antagonist caused a net increase in activity. A comparable but less pronounced effect occurred in SC-1. In contrast, in TA-1 and SBT-1, the response to EFS at 6 Hz for the 10 s and last 6 s in absence of any antagonist was a net increase in activity (Fig. 4A,C); the VIP antagonist without or with l-NNA had no effect.
Figure 4.
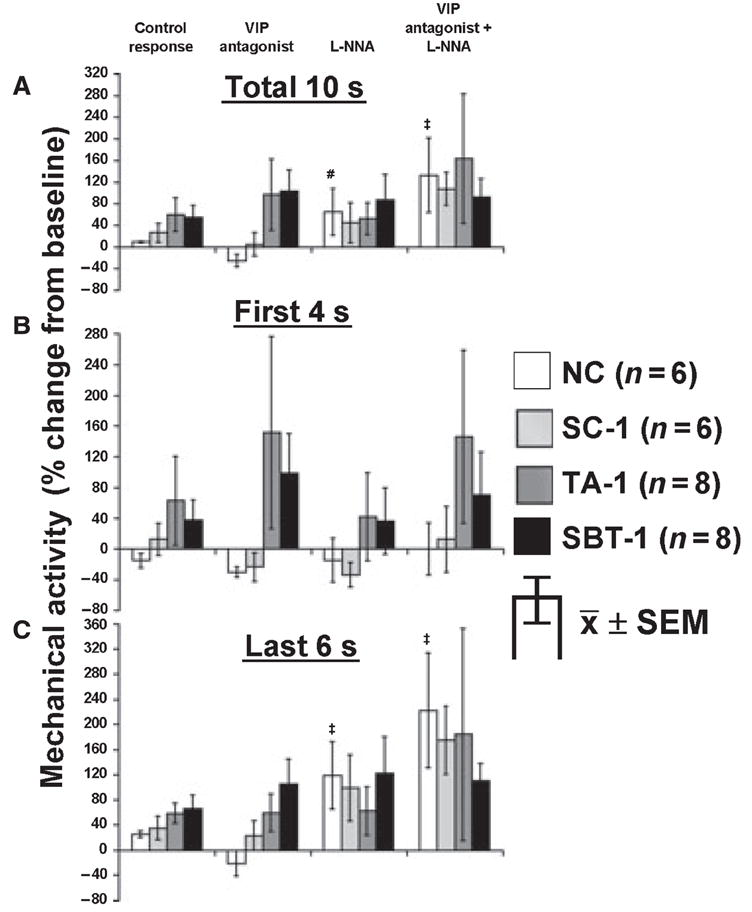
Response to EFS at 6 Hz in NC, SC-1, TA-1 and SBT-1. Effect of VIP antagonist (10−6 mol L−1) and l-NNA (10−4 mol L−1) on EFS response at 6 Hz for (A) entire 10-s interval, (B) first 4 s and (C) last 6 s of EFS. #P < 0.05 compared to response with VIP antagonist alone. ‡P < 0.05 compared to responses under all other conditions and baseline mechanical activity (paired t-test).
Figure 6.
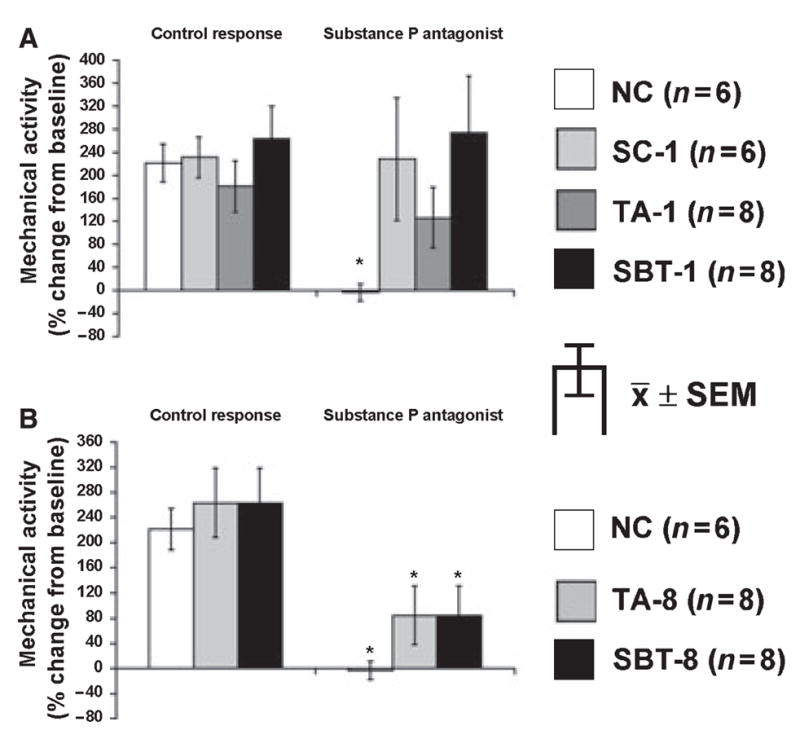
Effect of Sub P antagonist (10−5 mol L−1) on excitatory EFS response at 20 Hz for entire 10-s interval in (A) NC and groups 1 week postoperatively (SC-1, TA-1 and TA-1) and (B) NC and groups 8 weeks postoperatively (TA-8, SBT-8). *P < 0.05 compared to control response.
When 8-week groups were compared to NC, similar effects of EFS in the absence or presence of inhibitors were evident. A net inhibition occurred during the first 4 s in all groups that was unaffected by the VIP antagonist, l-NNA, or their combination except in TA-8 where the VIP antagonist combined with l-NNA increased the EFS response (Fig. 5B). Over the entire 10 s and last 6 s of EFS (Fig. 5A,C), the VIP antagonist had no effect on contractile response; in contrast, l-NNA alone and especially in combination with the VIP antagonist caused a net increase in mechanical activity to EFS at 6 Hz in NC and both 8-week groups.
Figure 5.
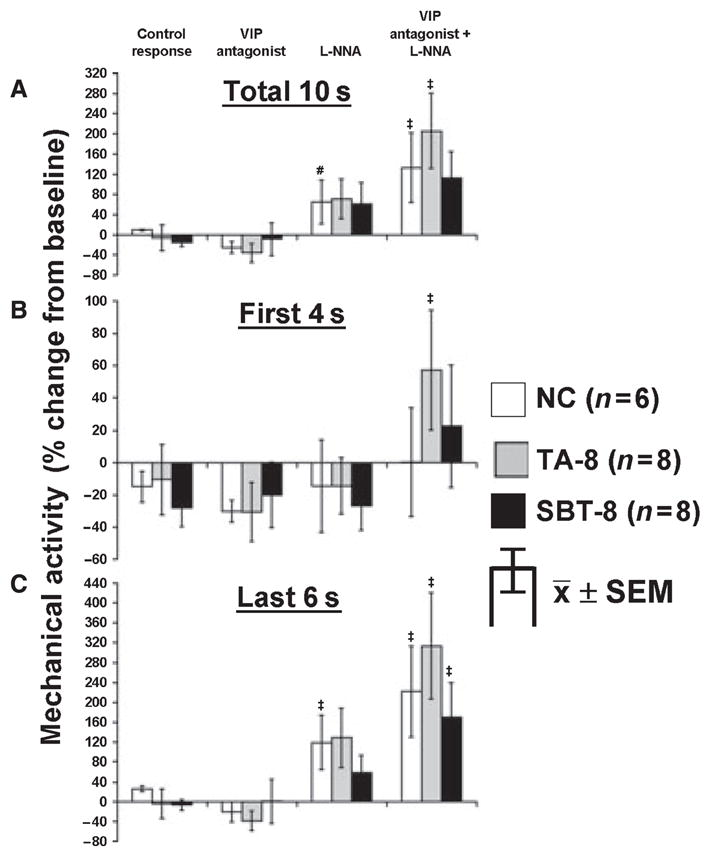
Response to EFS at 6 Hz in NC, TA-8 and SBT-8. Effect of VIP antagonist (10−6 mol L−1) and l-NNA (10−4 mol L−1) on EFS response at 6 Hz for (A) entire 10-s interval, (B) first 4 s and (C) last 6 s of EFS. #P < 0.05 compared to response with VIP antagonist alone. ‡P < 0.05 compared to responses under all other conditions and baseline mechanical activity (paired t-test).
Electrical field stimulation at 20 Hz induced a net increase in mechanical activity in all groups, but the EFS response in presence of the Sub P antagonist differed in 1- (Fig. 6A) and 8-week groups (Fig. 6B). In NC, the Sub P antagonist prevented the increase in mechanical activity. For all 1-week groups (SC-1, TA-1, SBT-1), the Sub P antagonist had no inhibitory effect, while in 8-week groups, the net increase in mechanical activity was inhibited partially, although not as great as in NC.
DISCUSSION
Our aim was to determine the effects of extrinsic denervation obligated by SBT on functional NANC innervation with the neuropeptides VIP and Sub P in circular smooth muscle of rat jejunum to compliment our work in longitudinal muscle.17,18 We hypothesized that changes in function and/or release of these two transmitters that occur after extrinsic denervation might contribute to enteric motor dysfunction observed after SBT.1,5–8 Studying effects of exogenous VIP and Sub P, as well as their endogenous release in response to EFS, enabled us to explore indirectly receptor expression and downstream signalling, as well as endogenous release of VIP and Sub P. In neurally intact (naïve) controls, VIP and Sub P caused inhibition and excitation of mechanical activity respectively. These responses were diminished profoundly 1 and 8 weeks after TA and SBT. Inhibition of NO production did not affect the response to exogenous VIP, and precontraction of muscle strips with Sub P did not increase the inhibitory potential of VIP as occurs in rat jejunal longitudinal muscle.17,18 Endogenous release of VIP and Sub P during EFS was preserved 8 weeks after SBT. Overall, changes in response to exogenous VIP and Sub P or their endogenous release during EFS could not be attributed to extrinsic denervation but are likely secondary to intestinal transection accompanying SBT.
The increase in spontaneous mechanical activity under NANC conditions 1 week after SBT suggests alterations in neuronal NANC output from the enteric nervous system (ENS) or changes at the level of the smooth muscle cell. These alterations appear to be temporary, because spontaneous activity returned to control levels 8 weeks after SBT, suggesting that adaptive mechanisms occur in the interplay between ENS and smooth muscle after extrinsic denervation or that these changes resolve over time. Increased spontaneous mechanical activity noted 1 week after SBT might explain a functional counterpart of enteric dysfunction mediated by NANC mechanisms.
These observations with VIP-mediated effects compliment our previous work.16–18 Although VIP is a well-known, important, inhibitory NANC neurotransmitter in the ENS, its functions remain incompletely understood.20 Effects of VIP are complex and vary between species, anatomic regions, and even within muscle layers in the same anatomic region. VIP also interacts with release of NO. Our results in rat jejunal circular muscle contrast with previous observations in rat longitudinal muscle.16,17 Although VIP has little effect on spontaneous contractions in rat longitudinal muscle,17,21,22 our study and others show that VIP exerts profound inhibitory effects on jejunal circular muscle.18,23,24 At 1 week postoperatively, responses to VIP were blunted in all groups, suggesting nonspecific effects of anaesthesia/celiotomy, possibly related to impaired contractile responses demonstrated by Kalff et al.25 for up to 1 week postoperatively. Persistent decreased sensitivity to VIP 8 weeks postoperatively in TA and SBT groups confirms an effect related to intestinal transection and not extrinsic denervation that accompanied only the SBT group, findings supported by Tomita et al.24 at 18 weeks post-SBT. Interestingly, our previous work in rat jejunal longitudinal muscle showed a nonspecific, increased inhibitory response to VIP at 1 week postoperatively that reverted to normal at 8 weeks postoperatively17 and changed into a decreased inhibitory response to VIP at 1 year after SBT;16 we interpreted this temporal effect to a combination of adaptation to chronic denervation and potentially a result of reinnervation. Our current experiments at 1 and 8 weeks should not reflect reinnervation based on our study and others using immunohistochemical markers of reinnervation.17,26–28 These differences in contractile response between different muscle layers within jejunum highlight the complex neuromuscular interplay regulating mechanical activity and different responses to VIP in circular and longitudinal muscle.
Vasoactive intestinal polypeptide induces inhibitory effects through induction of cAMP production binding to VPAC1 and VPAC2 receptors on the smooth muscle cell and in some tissues via neural release of NO.20,29,30 We used the VIP antagonist [D-p-Cl-Phe6-Leu17]-VIP, because it blocks VPAC1 and 2 receptors competitively, 29,30 albeit with variable efficiency. Surprisingly, we found no inhibition of the effects of exogenous VIP by the VIP antagonist alone, in combination with NO synthase inhibitor l-NNA, or after precontraction with Sub P which is consistent with our previous study in rat circular muscle after chronic denervation;18 however, this same inhibitor blocked, in part, effects of VIP on rat longitudinal muscle and was even more effective after precontraction with Sub P.16,17 This lack of effect remains unexplained, especially because this VIP antagonist appears to block effects of endogenously released VIP by EFS in this study (see below) and elsewhere.16,17 This differential inhibitory effect of the VIP antagonist suggests that VPAC1 and 2 receptors may not be the sole receptors for VIP. Indeed, Murthy et al.31 suggested that VIP induces production of NO within smooth muscle by binding to the natriuretic peptide clearance receptor. While this suggestion remains possible, lack of effect of l-NNA questions this possible pathway in rat jejunal circular muscle. Overall, differing effects of VIP in circular and longitudinal muscle of rat jejunum reinforces the complex variation of motor control within different muscle layers of the bowel.
We used low frequency EFS to explore the role of VIP in neural modulation of mechanical activity. At 6 Hz, a net inhibitory response was noted in the first 4 s in NC followed by a change from phasic to more tonic contractions in the last 6 s. This same response did not occur in any operated group 1 week postoperatively, probably secondary to nonspecific suppression of neuronal excitability early postoperatively as discussed above.25 In addition, because both the VIP antagonist and l-NNA had no effect on the EFS response 1 week postoperatively, the endogenous release of VIP and NO and/or their effect appears to be diminished in the early postoperative period. By 8 weeks postoperatively, inhibitory effects of 6 Hz during the first 4 s of EFS were restored and did not differ from controls.
We used EFS at 6 Hz to study the effects of intestinal transection (disruption of intrinsic neural continuity) and extrinsic denervation on endogenous release of VIP and NO. Neither VIP antagonist, NO synthase inhibitor, nor both together had consistent effects on inhibition during the first 4 s of EFS, consistent with our other studies,16–18 suggesting involvement of other NANC inhibitory transmitters in this early EFS inhibitory response. In contrast, the VIP antagonist and l-NNA uncovered VIPergic and nitrergic responses during the last 6 s and entire 10 s of EFS. Although the VIP antagonist given alone had no effect, inhibition mediated by endogenous release of VIP by EFS was uncovered when the dominant inhibitory effect of NO release was prevented by l-NNA, an observation we noted in rat longitudinal and circular jejunal muscle.16–18 This observation suggests that, although the response to exogenous VIP is decreased, endogenous release of VIP with neural stimulation is preserved after SBT.
We also studied the excitatory neurotransmitter Sub P. The response to exogenous Sub P was depressed in all groups 1 week postoperatively. This depressed effect persisted in TA and SBT groups at 8 weeks postoperatively, again suggesting an effect related to intestinal transection and not extrinsic denervation, confirming our observations 1 year after SBT in circular muscle18 and 8 weeks and 1 year after SBT in longitudinal muscle.16,17 In this study, we were unable to confirm the increase in sensitivity to Sub P reported by Tomita et al.24
The Sub P antagonist inhibited responses to exogenous Sub P in all groups and especially so in NC. This observation lead us to study whether endogenous release of Sub P during EFS at 20 Hz is preserved in these models of denervation. Different responses were noted in 1-week vs 8-week groups. Inability of the Sub P antagonist to inhibit responses to Sub P 1 week postoperatively in all groups, yet to block effects of EFS at 8 weeks after denervation, again demonstrates ongoing alterations in endogenous release of Sub P early postoperatively. Nevertheless, our experiments show endogenous neural release of Sub P is preserved 8 weeks after SBT, consistent with observations 1 year after SBT.18
Interestingly, although the effect of exogenous VIP and Sub P was decreased in all groups 1 and 8 weeks postoperatively, the effect of endogenously released neurotransmitters was preserved 8 weeks after transection and SBT. This observation suggests that adaptive mechanisms are taking place over time to restore normal neuromuscular function, for example possibly by an increase of the amount of neurotransmitters released from the ENS.
We conclude that VIP and Sub P are important neurotransmitters in rat circular muscle of jejunum, even after SBT. Abdominal operations altered nonspecifically contractile responses to these neurotransmitters administered exogenously or released endogenously by neural stimulation, effects which may be important in postoperative ileus and which persist for at least 1 week postoperatively. Small bowel transplantation does affect sensitivity to exogenous and endogenously released VIP and Sub P, but the effect appears related to intestinal transection rather than extrinsic denervation. Our hypothesis that extrinsic denervation accompanying SBT mediates abnormalities in enteric function of circular muscle of rat jejunum secondary to the changes in VIP or Sub P pathways seems unlikely.
Acknowledgments
We want to thank Judith A. Duenes for her expert technical help in the performance of these experiments and Deborah I. Frank for her expert assistance in the preparation of this manuscript.
FUNDING
This work was supported by grant DK 39337 from the National Institutes of Health, United States Public Health Services (M.G.S.) and grant KA 2329/1-1 from the Deutsche Forschungsgemeinschaft, Germany (M.S.K.).
Abbreviations
- ANOVA
analysis of variance
- EFS
electrical field stimulation
- ENS
enteric nervous system
- IACUC
Institutional Animal Care and Use Committee
- LO
optimal length
- l-NNA
l-NG-nitro arginine
- NANC
nonadrenergic, noncholinergic
- NC
naïve controls
- NO
nitric oxide
- SBT
small bowel transplantation
- SBT-1/-8
groups 1 and 8 weeks after small bowel transplantation
- SC-1
sham controls 1 week postoperatively
- Sub P
substance P
- TA-1/-8
groups 1 and 8 weeks after jejunal and ileal transection/reanastomosis
- VIP
vasoactive intestinal polypeptide
Footnotes
Parts of this work were presented at the third Academic Surgical Congress in Huntington Beach, CA, February 13–15, 2008.
COMPETING INTERESTS
The authors have no competing interests.
References
- 1.Asfar S, Atkison P, Gent C, et al. Small bowel transplantation. A life-saving option for selected patients with intestinal failure. Dig Dis Sci. 1996;41:875–83. doi: 10.1007/BF02091526. [DOI] [PubMed] [Google Scholar]
- 2.Rovera GM, DiMartini A, Graham TO, et al. Quality of life after intestinal transplantation and on total parenteral nutrition. Transplant Proc. 1998;30:2513–4. doi: 10.1016/s0041-1345(98)00704-0. [DOI] [PubMed] [Google Scholar]
- 3.O’Keefe SJ, Emerling M, Koritsky D, et al. Nutrition and quality of life following small intestinal transplantation. Am J Gastroenterol. 2007;102:1–8. doi: 10.1111/j.1572-0241.2007.01125.x. [DOI] [PubMed] [Google Scholar]
- 4.O’Keefe SJ, Matarese L. Small bowel transplantation. Curr Gastroenterol Rep. 2006;8:360–6. doi: 10.1007/s11894-006-0020-x. [DOI] [PubMed] [Google Scholar]
- 5.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270–80. doi: 10.1097/00000658-199509000-00006. Discussion 80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rovera G, Furukawa H, Reyes J, Todo S, Hutson W. The use of clonidine for the treatment of high intestinal output following small bowel transplantation. Transplant Proc. 1997;29:1853–4. doi: 10.1016/s0041-1345(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 7.Silver HJ, Castellanos VH. Nutritional complications and management of intestinal transplant. J Am Diet Assoc. 2000;100:680–4. 7–9. doi: 10.1016/S0002-8223(00)00197-8. Quiz 5–6. [DOI] [PubMed] [Google Scholar]
- 8.Sarr MG, Duenes JA, Walters AM. Jejunal and ileal absorptive function after a model of canine jejunoileal autotransplantation. J Surg Res. 1991;51:233–9. doi: 10.1016/0022-4804(91)90100-z. [DOI] [PubMed] [Google Scholar]
- 9.Balsiger BM, Ohtani N, Anding WJ, Duenes JA, Sarr MG. Chronic extrinsic denervation after small bowel transplantation in rat jejunum: Effects and adaptation in nitrergic and non-nitrergic neuromuscular inhibitory mechanisms. Surgery. 2001;129:478–89. doi: 10.1067/msy.2001.112070. [DOI] [PubMed] [Google Scholar]
- 10.Murr MM, Miller VM, Sarr MG. Contractile properties of enteric smooth muscle after small bowel transplantation in rats. Am J Surg. 1996;171:212–7. doi: 10.1016/S0002-9610(99)80102-0. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 11.Ohtani N, Balsiger BM, Anding WJ, Duenes JA, Sarr MG. Small bowel transplantation induces adrenergic hypersensitivity in ileal longitudinal smooth muscle in rats. J Gastrointest Surg. 2000;4:77–85. doi: 10.1016/s1091-255x(00)80036-0. [DOI] [PubMed] [Google Scholar]
- 12.Shibata C, Balsiger BM, Anding WJ, Duenes JA, Miller VM, Sarr MG. Functional changes in nonadrenergic, noncholinergic inhibitory neurons in ileal circular smooth muscle after small bowel transplantation in rats. Dig Dis Sci. 1998;43:2446–54. doi: 10.1023/a:1026630115009. [DOI] [PubMed] [Google Scholar]
- 13.Shibata C, Murr MM, Balsiger B, Anding WJ, Sarr MG. Contractile activity of circular smooth muscle in rats one year after small bowel transplantation: differing adaptive response of the jejunum and ileum to denervation. J Gastrointest Surg. 1998;2:463–72. doi: 10.1016/s1091-255x(98)80038-3. [DOI] [PubMed] [Google Scholar]
- 14.Balsiger BM, Duenes JA, Ohtani N, et al. Nitric oxide pathways in circular muscle of the rat jejunum before and after small bowel transplantation. J Gastrointest Surg. 2000;4:86–92. doi: 10.1016/s1091-255x(00)80037-2. [DOI] [PubMed] [Google Scholar]
- 15.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 16.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Effect of chronic, extrinsic denervation on functional NANC innervation with vasoactive intestinal polypeptide and substance P in longitudinal muscle of rat jejunum. Neurogastroenterol Motil. 2007 doi: 10.1111/j.1365-2982.2007.01021.x. (Epub ahead of print Oct 17) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Role of VIP and substance P in NANC innervation in the longitudinal smooth muscle of the rat jejunum - influence of extrinsic denervation. J Surg Res. 2007;141:22–30. doi: 10.1016/j.jss.2007.01.021. Epub 2007 May 18. [DOI] [PubMed] [Google Scholar]
- 18.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Long-Term Effects of Extrinsic Denervation on VIP and Substance P Innervation in Circular Muscle of Rat Jejunum. J Gastrointest Surg. 2007;11:1339–50. doi: 10.1007/s11605-007-0212-1. [DOI] [PubMed] [Google Scholar]
- 19.Murr MM, Sarr MG. Small bowel transplantation: effects on function of nonadrenergic, noncholinergic nerves. J Gastrointest Surg. 1997;1:439–45. doi: 10.1016/s1091-255x(97)80131-x. [DOI] [PubMed] [Google Scholar]
- 20.Van Geldre LA, Lefebvre RA. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr Pharm Des. 2004;10:2483–97. doi: 10.2174/1381612043383890. [DOI] [PubMed] [Google Scholar]
- 21.Niioka S, Takeuchi T, Kishi M, et al. Nonadrenergic, noncholinergic relaxation in longitudinal muscle of rat jejunum. Jpn J Pharmacol. 1997;73:155–61. doi: 10.1254/jjp.73.155. [DOI] [PubMed] [Google Scholar]
- 22.Okishio Y, Niioka S, Yamaji M, et al. Mediators of non-adrenergic, noncholinergic relaxation in Sprague Dawley rat intestine: comparison with the mediators of other strains. J Vet Med Sci. 2000;62:821–8. doi: 10.1292/jvms.62.821. [DOI] [PubMed] [Google Scholar]
- 23.Vanneste G, Robberecht P, Lefebvre RA. Inhibitory pathways in the circular muscle of rat jejunum. Br J Pharmacol. 2004;143:107–18. doi: 10.1038/sj.bjp.0705918. Epub 2004 Aug 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tomita R, Fujisaki S, Park E, Ikeda T, Koshinaga T. Substance P and vasoactive intestinal peptide in rat smallbowel isografts. Am J Surg. 2005;189:63–70. doi: 10.1016/j.amjsurg.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Kalff JC, Buchholz BM, Eskandari MK, et al. Biphasic response to gut manipulation and temporal correlation of cellular infiltrates and muscle dysfunction in rat. Surgery. 1999;126:498–509. [PubMed] [Google Scholar]
- 26.Hirose R, Taguchi T, Hirata Y, Yamada T, Nada O, Suita S. Immunohistochemical demonstration of enteric nervous distribution after syngeneic small bowel transplantation in rats. Surgery. 1995;117:560–9. doi: 10.1016/s0039-6060(05)80256-9. [DOI] [PubMed] [Google Scholar]
- 27.Pernthaler H, Pfurtscheller G, Klima G, et al. Regeneration of sympathetic activities in small bowel transplants. Eur Surg Res. 1993;25:316–20. doi: 10.1159/000129295. [DOI] [PubMed] [Google Scholar]
- 28.Kiyochi H, Ono A, Yamamoto N, Ohnishi K, Shimahara Y, Kobayashi N. Extrinsic sympathetic reinnervation after intestinal transplantation in rats. Transplantation. 1995;59:328–33. [PubMed] [Google Scholar]
- 29.Fizanne L, Sigaudo-Roussel D, Saumet JL, Fromy B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J Physiol. 2004;554:519–28. doi: 10.1113/jphysiol.2003.053835. Epub 2003 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rekik M, Delvaux M, Frexinos J, Bueno L. Role of vasoactive intestinal polypeptide in the adaptation of intestinal smooth muscle cells to mechanical distension. J Pharmacol Exp Ther. 1998;287:832–8. [PubMed] [Google Scholar]
- 31.Murthy KS, Teng B, Jin J, Makhlouf GM. G protein-dependent activation of smooth muscle eNOS via natriuretic peptide clearance receptor. Am J Physiol. 1998;275:C1409–16. doi: 10.1152/ajpcell.1998.275.6.C1409. [DOI] [PubMed] [Google Scholar]