Abstract
Intestinal denervation contributes to enteric motor dysfunction after intestinal transplantation [small bowel transplantation (SBT)]. Our aim was to determine long-term effects of extrinsic denervation on functional non-adrenergic, non-cholinergic innervation with vasoactive intestinal polypeptide (VIP) and substance P. Contractile activity of jejunal longitudinal muscle from six age-matched, naïve control rats (NC) and eight rats 1 year after syngeneic SBT were studied in tissue chambers. Spontaneous contractile activity did not differ between groups. Exogenous VIP inhibited contractile activity dose-dependently in both groups, greater in NC than in SBT. The VIP antagonist ([D-p-Cl-Phe6,Leu17]-VIP) and the nitric oxide synthase inhibitor L-NG-nitro arginine prevented inhibition by exogenous VIP and electrical field stimulation (EFS) in both groups. Exogenous substance P increased contractile activity dose-dependently, greater in NC than in SBT. The substance P antagonist ([D-Pro2,D-Trp7,9]-substance P) inhibited effects of exogenous substance P and increased the EFS-induced inhibitory response. Immunohistofluorescence showed staining for tyrosine hydroxylase in the jejunoileum 1 year after SBT suggesting sympathetic reinnervation. In rat jejunal longitudinal muscle after chronic denervation, response to exogenous VIP and substance P is decreased, while endogenous release of both neurotransmitters is preserved. These alterations in excitatory and inhibitory pathways occur despite extrinsic reinnervation and might contribute to enteric motor dysfunction after SBT.
Keywords: enteric nervous system, extrinsic denervation, motility, small intestine, substance P, vasoactive intestinal polypeptide
INTRODUCTION
Due to technical improvements and new immunosuppressive medications, small bowel transplantation (SBT) has become clinical reality over the last decade.1 SBT offers an alternative to chronic total parenteral nutrition, can be a lifesaving procedure for patients with chronic intestinal failure, and has the potential to improve quality of life.1,2 Many challenges in the postoperative management of these patients remain, however, including enteric motor dysfunction and high stomal output/diarrhoea.1,3,4 A similar enteric dysfunction is also observed in dog models of auto-transplantation of the small bowel, in which no immunosuppressants are used and immune phenomena are avoided; these observations have lead some investigators to suggest that factors such as the obligate extrinsic denervation by SBT are believed to contribute to the enteric dysfunction after SBT.5
In a model of syngeneic SBT in rats, our group has studied previously the effects of SBT on cholinergic, adrenergic, and nitrergic innervation of longitudinal and circular smooth muscle of the jejunum and ileum.6–13 Alterations in other non-adrenergic, non-cholinergic (NANC) neurotransmitters, such as the inhibitory neurotransmitter vasoactive intestinal polypeptide (VIP) and the excitatory neurotransmitter substance P, are less well-studied after SBT. Both neuropeptides play an important role in enteric motor innervation, such as the peristaltic reflex, and might therefore contribute to the enteric motor dysfunction after SBT.14 A recent study from our laboratory showed a decrease in the sensitivity to exogenous substance P and a decrease of the endogenous release of VIP 8 weeks after SBT in the longitudinal muscle of rat jejunum.15 The study of long-term effects of extrinsic denervation, including adaptation and possible reinnervation after SBT, is also of interest in terms of the myoneural function of the transplanted intestine. 16–18
Therefore, the aim of the current study was to determine long-term changes (after 1 year) in enteric innervation with the two NANC neuropeptides VIP and substance P after SBT. We studied the effect of exogenous application as well as the effect of endogenous release of these neurotransmitters from the enteric nervous system triggered by electrical field stimulation (EFS). By using age-matched control rats, we controlled for any confounding effects of ageing on our findings. In addition, we evaluated sympathetic reinnervation by immunohistochemistry. Our hypothesis was that SBT is associated with long-term changes in the functional innervation with VIP and substance P, which contribute to the enteric motor dysfunction after SBT.
MATERIALS AND METHODS
Preparation of animals
The study was approved by the Institutional Animal Care and Use Committee (IACUC) of the Mayo Foundation. Procedures and animal care were performed according to the guidelines of the IACUC of the Mayo Foundation in accordance with the guidelines of the National Institutes of Health and the Public Health Service Policy of the Humane Use and Care of Laboratory Animals.
Experimental groups
Syngeneic, male Lewis rats (Harlan, Indianapolis, IN, USA) were used in all experiments; use of syngeneic, inbred rats was especially important after jejunoileal intestinal allotransplantation to avoid confounding effects of immune phenomena or need for pharmacological immunosuppression. Anaesthesia was achieved by initial inhalation of isoflurane 2% (Abbott Laboratories, North Chicago, IL, USA) and maintained by subsequent intraperitoneal sodium pentobarbital (30–50 mg kg−1; AmproPharmacy, Arcadia, CA, USA). To accomplish complete extrinsic denervation of the jejunoileum, orthotopic small bowel isotransplantation (SBT) was performed in eight rats using standard microvascular techniques as described previously.7 In brief, the entire jejunoileum was removed together with a segment of aorta from the donor rat after flushing the intestinal lumen and the graft vasculature with chilled 154 mmol L−1 NaCl. The donor aorta of the graft was anastomosed to the recipient aorta (end-to- side) and the donor portal vein to the recipient inferior vena cava (end-to-side). After resecting the recipient’s jejunoileum, intestinal continuity was re-established by end-to-end jejunojejunostomy and ileoileostomy. Rats were allowed free access to water immediately postoperatively and rat chow 24 h later. Rats were maintained with normal rat chow and water and studied about 1 year after SBT.
To eliminate effects of ageing on our results, age-matched, non-operated rats served as naïve controls (NC; n = 6). These rats were 15 months of age, because SBT was performed in 3-month-old rats in our study. The weight of the rats at the time of the study did not differ between groups and was 550 g (500–600 g) [median (range)] in NC and 500 g (350–600 g) in SBT.
Recording of contractile activity
A segment of jejunum 10 cm distal to either the ligament of Treitz or the prior jejunojejunostomy (after SBT) was harvested and immersed in chilled, modified Krebs–Ringer’s bicarbonate solution (concentrations in mmol L−1: NaCl 116.4, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 23.8, calcium disodium edetate 0.26, and glucose 11.1) pre-oxygenated with 95% oxygen/5% carbon dioxide (Praxair, Burr Ridge, IL, USA). The jejunal segment was then opened along its mesenteric border, and eight full-thickness muscle strips (8 mm long and 2 mm wide) cut in the direction of the longitudinal muscle layer were suspended vertically in 10-mL tissue chambers filled with modified Krebs–Ringer’s bicarbonate solution, which was bubbled continuously with 95% oxygen/5% carbon dioxide and maintained at 37.5 °C. One end of the muscle strip was attached to a fixed hook, whereas the other end was connected to a non-compliant force transducer (Kulite Semiconductors Products, Inc., Leonia, NJ, USA) to measure isometric force.
Contractile activity was monitored on a chart recorder (Grass 7D polygraph; Grass Instrument Co, Quincy, MA, USA) while in parallel being saved digitally on a personal computer using dedicated software (MP-100A-CE and AcqKnowledge; Biopac Systems, Inc., Goleta, CA, USA) to allow later detailed computer analysis.
Experimental design
After a 90-min equilibration period with intervening washouts of the bath solution every 15 min, each strip was stretched incrementally at 10-min intervals to its optimal length (L0) beyond which further stretching did not increase the amplitude or frequency of spontaneous contractile activity.7 Thereafter, atropine (10−7 mol L−1), phentolamine (10−5 mol L−1), and propranolol (5 × 10−6 mol L−1) were added into the bath solution to establish NANC conditions. All subsequent experiments were performed at L0 under NANC conditions. Strips without spontaneous contractile activity were excluded from the study (<2% of strips). Each experimental condition was studied in at least two muscle strips per rat.
Two muscle strips per rat were exposed to three different, escalating concentrations of VIP (10−8, 10−7, 10−6 mol L−1) with washout of the bath solution between doses. After studying the effect of the VIP antagonist ([D-p-Cl-Phe6,Leu17]-VIP; 10−6 mol L−1) on baseline contractile activity, the effects of the VIP antagonist alone and in combination with the nitric oxide (NO) synthase inhibitor L-NG-nitro arginine (L-NNA; 10−4 mol L−1) were studied on the response to exogenous VIP (10−6 mol L−1).
Three different concentrations of substance P (10−8, 10−7, 10−6 mol L−1) were evaluated in two other strips per rat. After evaluating the effect on baseline contractile activity of the substance P antagonist ([D-Pro2, D-Trp7,9]-substance P; 10−5 mol L−1), we evaluated the effect of the substance P antagonist on the response to the middle dose (10−7 mol L−1) of exogenous substance P. Afterwards, the effect of VIP (10−6 mol L−1) was studied with and without the VIP antagonist (10−6 mol L−1) after each muscle strip was first precontracted with substance P (10−7 mol L−1) for 90 s.
Four strips from each rat were subjected to EFS at 3, 6 and 50 Hz with a constant voltage (10 V), pulse width (0.5 ms), and duration of stimulation (10 s). Between each EFS, 10 min were allowed for spontaneous activity to recover before the next EFS was applied. After each series of stimulations, the bath solution was exchanged. Two of the four strips were exposed to EFS with and without the VIP antagonist and L-NNA alone and in combination. The other two strips were studied with and without the substance P antagonist.
At the conclusion of each experiment, the muscle strips were blotted on filter paper and weighed to normalize the motility data per milligram tissue weight.
Immunohistofluorescence microscopy
Immunohistofluorescence microscopy for tyrosine hydroxylase (TH), which is found mainly in sympathetic neurons, was performed in both groups to study sympathetic re-innervation after SBT; the NC rats served as controls. Whole mounts of the duodenum (serving as non-denervated control tissue) and jejunum (each 1 × 1 cm) were prepared by removing the mucosa, pinning the tissue flat on a piece of Sylgard (Dow Corning Corporate, Midland, MI, USA), and fixing the tissue overnight in 4% paraformaldehyde. After rinsing the tissue with 0.1 mol L−1 phosphate-buffered saline (PBS), the tissue was incubated for 1 h in 10% normal donkey serum (NDS) containing PBS and 0.3% Triton X-100 (blocking solution). The tissue was then incubated for 48 h at 4 °C in the primary antibody solution containing 5% NDS and the affinity- purified polyclonal sheep anti-TH antibody (dilution 1:400); the tissue was washed with 0.1 mol L−1 PBS and incubated for 36 h at 4 °C in the secondary antibody solution containing 2.5% NDS and Cy3-conjugated rabbit anti-sheep antibody (1:200). After washing the tissue again in 0.1 mol L−1 PBS, confocal microscopy was performed using a laser scanning microscope (Carl Zeiss LSM 5 Pascal, Version 3.2, Heidelberg, Germany) with digital pictures taken at appropriate levels.
Data analysis
Baseline contractile activity was measured for 5 min under NANC conditions for each strip at L0 and represents the area under the contractile curve (g × 5 min mg−1 tissue). Typically, the effects of the drugs on contractile activity in a 5-min interval were compared to baseline contractile activity during an equally long interval immediately before the drug was administered. The effects of the VIP and substance P antagonists were evaluated in the same fashion. When the strips were precontracted with substance P, the effect of VIP during a 5-min interval was compared to the 90 s of precontraction directly before VIP was administered. Therefore, the drug responses are given as the percentage change from baseline contractile activity (defined as 0%), which represents the contractile activity 5 min or 90 s before drug administration. Positive values represent an increase, whereas negative values signify a decrease in contractile activity.
During EFS, we determined the response to EFS only during the 10-s period of stimulation and did not evaluate the response after termination of EFS (the so-called ‘off contraction’). In control rat jejunum, EFS at low frequencies is associated with a dominant, net inhibitory effect.6 EFS at 3 and 6 Hz inhibited contractile activity (Figs 1 and 2). A complete lack of contractile activity persisted for the first 4–6 s of EFS after which some contractile activity recurred. Because of this pattern, we evaluated the effect of EFS separately not only for the entire 10 s of EFS but also for the first 4 s and the last 6 s of EFS. Contractile activity, measured as area under the contractile curve, was expressed as the percentage change of baseline contractile activity for an equally long interval measured during the 20 s immediately before EFS. Additionally, the duration of complete inhibition of contractile activity during EFS was analysed visually for each stimulation and is given in seconds of inhibition. The response to EFS at 50 Hz was evaluated accordingly.
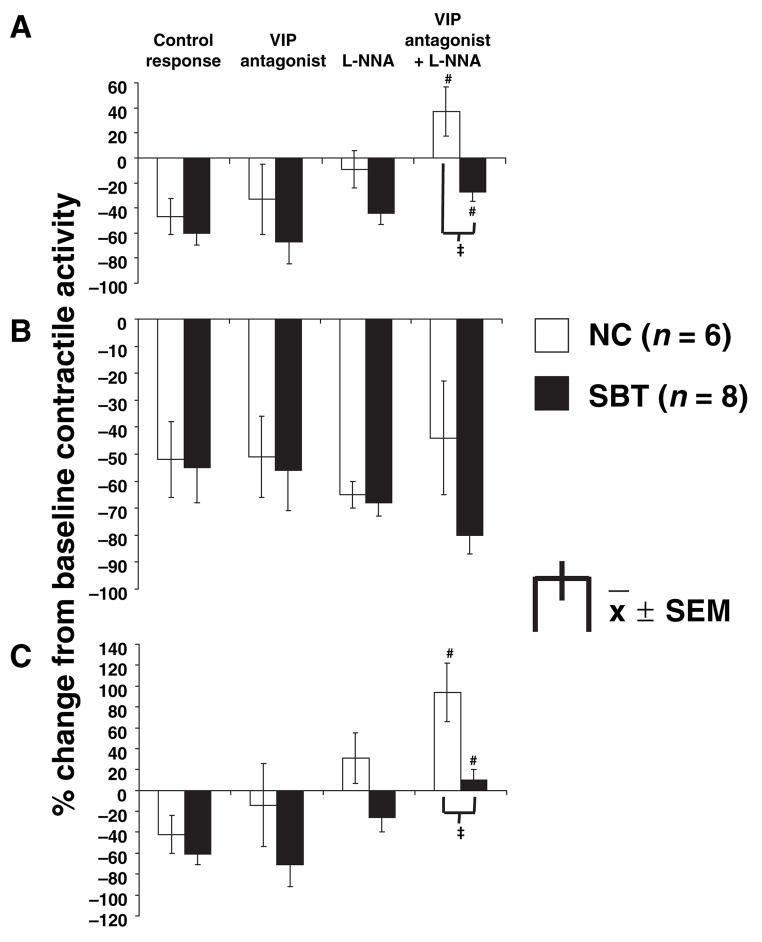
Figure 1.
Effect of vasoactive intestinal polypeptide (VIP) antagonist (10−6 mol L−1) and L-NG-nitro arginine (L-NNA) (10−4 mol L−1) on the inhibitory electrical field stimulation (EFS) response at 3 Hz for the entire 10-s interval (A), the first 4 s (B) and the last 6 s (C) of EFS; #P < 0.05 (ANOVA) compared to control response, L-NNA alone, and VIP antagonist alone. ‡P < 0.01 compared to naïve control (NC) under same condition (VIP antagonist + L-NNA). The combination of the VIP antagonist and L-NNA reduced the inhibitory EFS response during the entire 10 s and the last 6 s of EFS in both groups, greater in NC than in small bowel transplantation (SBT), causing net excitation during the last 6 s in NC. L-NNA alone attenuated the EFS-induced inhibition during the entire 10 s and the last 6 s of EFS. EFS-induced inhibition was unaffected by the VIP antagonist and L-NNA during the first 4 s of EFS.
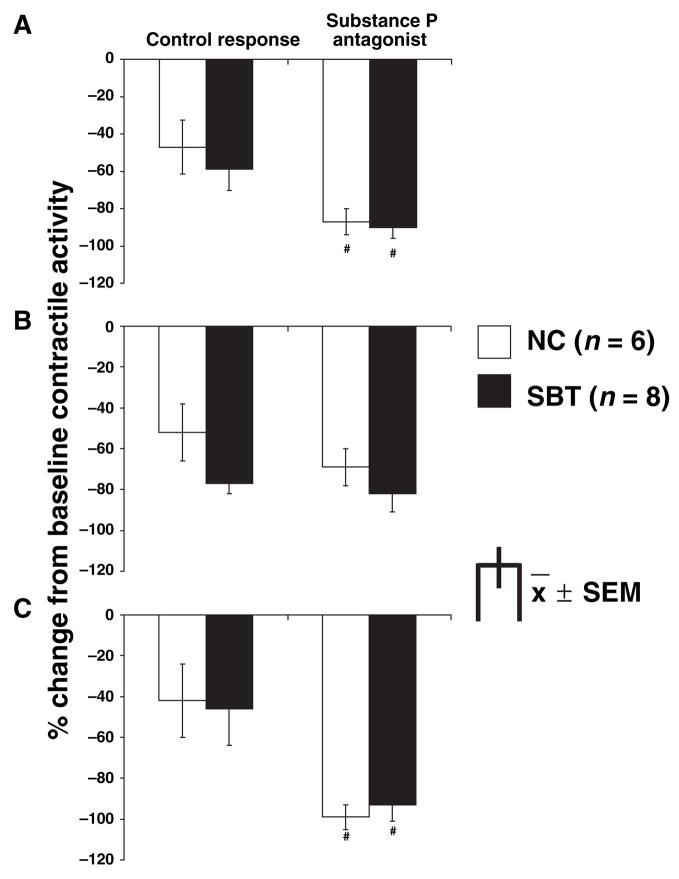
Figure 2.
Effect of substance P antagonist (10−5 mol L−1) on the inhibitory electrical field stimulation (EFS) response in naïve control (NC) (3 Hz) and small bowel transplantation (SBT) (6 Hz) for the entire 10-s interval (A), the first 4 s (B) and the last 6 s (C) of EFS; #P < 0.05 (ANOVA) compared to control response. The substance P antagonist increased EFS-induced inhibition during the entire 10 s and the last 6 s of EFS to a similar extent in both groups. EFS-induced inhibition was unaffected by the substance P antagonist during the first 4 s of EFS.
Data are summarized as mean ± SEM. Analysis of variance (ANOVA) was used to examine the effect of the different drugs or EFS on each response variable (contractile activity or duration of inhibition during EFS). Repeated measures ANOVA was used for repeated measurements within the same rat and thus were treated as repeated factors (e.g. for dose–response to VIP, substance P, or EFS response under different conditions). The different groups of rats were independent factors. When the overall main effect was statistically significant when analysed by ANOVA, pairwise comparisons were performed post hoc using paired t-tests (for repeated factors) or two-sample t-tests (for independent group factors). Additional t-tests were used for single comparisons (e.g. vs baseline contractile activity). A Bonferroni correction was applied when evaluating the statistical significance of multiple t-tests. ANOVA on Ranks was used when data were not normally distributed.
Drugs
Atropine sulphate, phentolamine hydrochloride, DL-propranolol hydrochloride, L-NNA, substance P, [D-Pro2,D-Trp7,9]-substance P, VIP and [D-p-Cl-Phe6, Leu17]-VIP were purchased from Sigma-Aldrich (St Louis, MO, USA), Triton X-100 from Fisher Scientific (Fair Lawn, NJ, USA), and NDS, affinity-purified polyclonal sheep anti-TH, and the Cy3 conjugated rabbit anti-sheep antibody from Chemicon International Inc.(Temecula, CA, USA).
RESULTS
Spontaneous contractile activity
After equilibration, stretching, and establishing NANC conditions, the area under the contractile curve was measured during a 5-min interval and normalized by the tissue weight. The spontaneous contractile activity did not differ between groups and was 6.0 ± 0.9 g ×5 min mg−1 tissue inNCand 4.9 ± 0.4 g ×5 min mg−1 tissue in SBT (P = ns).
Response to exogenous vasoactive intestinal polypeptide
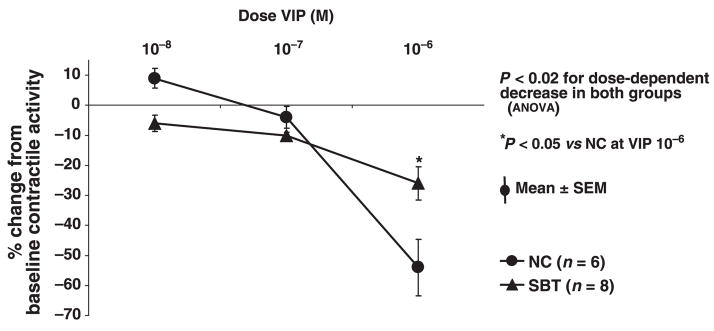
VIP inhibited spontaneous contractile activity dose-dependently in both groups (Fig. 3). The inhibition of contractile activity (% change from baseline) by VIP (10−6 mol L−1) was greater in NC compared to SBT (P < 0.05). The VIP antagonist had minimal effect on contractile activity in NC (7 ± 2%; P < 0.02, paired t-test) and in SBT (8 ± 4%; P = 0.09), but prevented in part the inhibitory effect of VIP (10−6 mol L−1). L-NNA reversed the inhibition caused by VIP (10−6 mol L−1) completely in SBT and in part in NC. The combination of the VIP antagonist and L-NNA prevented VIP-induced inhibition partially in both groups but had no additive effect (Table 1).
Figure 3.
Response of spontaneous contractile activity to escalating concentrations of vasoactive intestinal polypeptide (VIP) (10−8, 10−7, 10−6 mol L−1). VIP induced a dose-dependent inhibition of contractile activity in both groups, greater in naïve control (NC) than in small bowel transplantation (SBT).
Table 1.
Effect of VIP antagonist (10−6 mol L−1) and nitric oxide synthase inhibitor (L-NNA; 10−4 mol L−1) on response to exogenous VIP (10−6 mol L−1)
| Contractile activity (% change from baseline)** | ||||
|---|---|---|---|---|
| Control response | VIP antagonist | L-NNA | VIP antagonist + L-NNA | |
| NC | −54 ± 9* | −29 ± 4*,† | −18 ± 4*,† | −20 ± 4*,† |
| SBT | −26 ± 5* | −14 ± 4*,† | −1 ± 5† | −9 ± 2*,† |
VIP, vasoactive intestinal polypeptide; L-NNA, L-NG-nitro arginine; NC, naïve control; SBT, small bowel transplantation.
Differs from baseline contractile activity.
Differs from control response; P < 0.05 (ANOVA).
x̄ ± SEM; n ≥ 6 rats.
When the muscle strips were precontracted with a non-maximal dose of substance P (10−7 mol L−1), the relative per cent inhibitory effect of VIP (10−6 mol L−1) was increased markedly (P < 0.05; Table 2). This inhibitory effect of VIP on substance P-stimulated contractile activity was blocked partially by the VIP antagonist in NC and completely in SBT, however, a slight inhibition of contractile activity (−13 ± 7%) remained in SBT (P = ns).
Table 2.
Effect of VIP antagonist (10−6 mol L−1) on response to exogenous VIP (10−6 mol L−1) after precontraction with substance P (10−7 mol L−1)
| Contractile activity (% change from baseline)
| ||
|---|---|---|
| Control response | VIP antagonist | |
| NC | −82 ± 1*,† | −31 ± 4*,‡ |
| SBT | −81 ± 1*,† | −13 ± 7‡ |
VIP, vasoactive intestinal polypeptide; NC, naïve control; SBT, small bowel transplantation.
Differs from baseline contractile activity; P < 0.05.
Differs from control response without precontraction; P < 0.05 (ANOVA).
Differs from control response with precontraction; P < 0.05 (ANOVA).
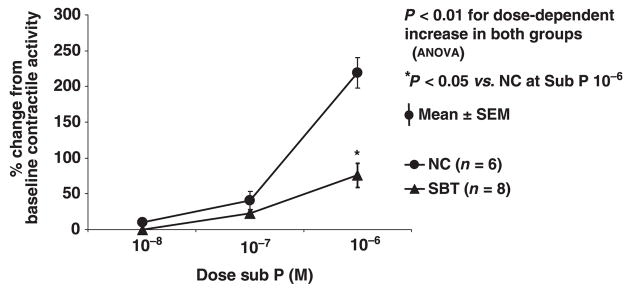
Response to exogenous substance P
Substance P increased contractile activity dose-dependently in both groups (Fig. 4). The response to substance P (10−6 mol L−1) was greater in NC than in SBT (P < 0.05). The substance P antagonist (10−5 mol L−1) increased spontaneous contractile activity in NC (43 ± 7%; P < 0.02, paired t-test) but not in SBT (17 ± 7%; P = ns). In NC, the procontractile effect of 10−7 mol L−1 substance P was prevented in part by the substance P antagonist (41 ± 12% vs 25 ± 7%; P < 0.01). In contrast, in SBT, the substance P antagonist had no effect on 10−7 mol L−1 substance P (23 ± 3% vs 21 ± 6%; P = ns).
Figure 4.
Response of spontaneous contractile activity to escalating concentrations of substance P (10−8, 10−7, 10−6 mol L−1). Substance P stimulated contractile activity in a dose-dependent fashion in both groups, greater in naïve control (NC) than in small bowel transplantation (SBT).
Response to electrical field stimulation
EFS at 3 Hz under NANC conditions inhibited overall contractile activity in both groups when evaluated for the entire 10 s, the first 4 s, and the last 6 s of EFS stimulation compared to the control response (P < 0.05; Fig. 1). The VIP antagonist alone had no obvious effect on EFS-induced inhibition in either group or at any of the individual time periods of EFS. L-NNA alone attenuated the inhibition by EFS at 3 Hz when quantitated over the entire 10 s and the last 6 s of EFS compared to the control response under NANC-conditions in both groups. The combination of L-NNA with the VIP antagonist prevented EFS-induced inhibition at 3 Hz when quantitated over the entire 10 s and the last 6 s of EFS in both groups, and inhibition was reversed to a net stimulation of contractile activity during the last 6 s in NC. When L-NNA and the VIP antagonist were combined, contractile activity was greater in NC compared to SBT during the entire 10 s as well as during the last 6 s. Although the inhibitory EFS response during the first 4 s of EFS at 3 Hz was unaffected by L-NNA and the VIP antagonist, L-NNA and the combination with the VIP antagonist shortened the duration of inhibition during EFS at 3 Hz but only in NC (Table 3). A comparable but less pronounced effect of L-NNA and the VIP antagonist was seen on the EFS response at 6 Hz (data not shown).
Table 3.
Effect of VIP antagonist (10−6 mol L−1) and L-NNA (10−4 mol L−1) on the duration of inhibition during the 10 s of EFS at the inhibitory EFS frequency of 3 Hz
| Duration of inhibition (s)
| ||||
|---|---|---|---|---|
| Control response | VIP antagonist | L-NNA | VIP antagonist + L-NNA | |
| NC | 6.6 ± 0.4 | 6.4 ± 0.5 | 4.6 ± 0.3* | 3.7 ± 0.8* |
| SBT | 6.7 ± 0.7 | 6.8 ± 0.7 | 6.0 ± 0.5 | 5.1 ± 0.3 |
VIP, vasoactive intestinal polypeptide; L-NNA, L-NG-nitro arginine; EFS, electrical field stimulation; NC, naïve control; SBT, small bowel transplantation.
Differs from control response; P < 0.05 (ANOVA).
EFS at 3 Hz in NC and at 6 Hz in SBT caused a net inhibition of contractile activity (Fig. 2; P < 0.05). The substance P antagonist increased this inhibitory effect of EFS quantitated over the entire 10 s as well as during the last 6 s of EFS stimulation. The response during the first 4 s of EFS as well as the duration of inhibition were unaffected by the substance P antagonist (data not shown). The effect of the substance P antagonist on the EFS response was studied at two different frequencies for NC and SBT, because the antagonist did not cause a significant change in the EFS response for both groups at the same frequency, although a clear trend was present (data not shown).
EFS at 50 Hz inhibited contractile activity in NC (−24 ± 25%; P < 0.05 vs baseline) but not in SBT (14 ± 19%; ns). The EFS response at 50 Hz was not affected by L-NNA, the VIP antagonist, or substance P antagonist (data not shown).
Immunohistofluorescence microscopy
Immunohistofluorescence staining for TH was performed in the duodenum and jejunum in both groups (Fig. 5). Staining for TH-positive neurons was present in the myenteric plexus of the duodenum and jejunum of NC and SBT with comparable immunopositivity, although we have shown previously that TH staining is absent in the jejunoileum early (≤2 months) after SBT.15 No subjective differences were evident in the jejunum between NC and SBT.
Figure 5.
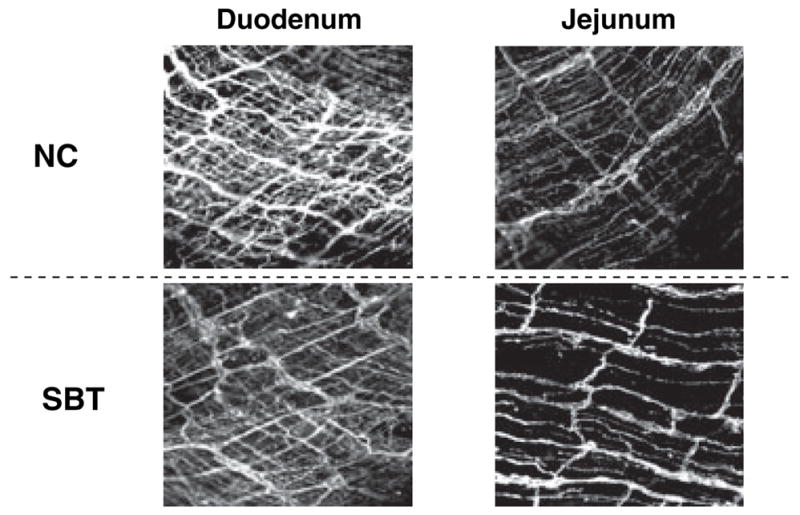
Immunohistofluorescence for tyrosine hydroxylase (TH) in the duodenum and jejunum of naïve control (NC) and small bowel transplantation (SBT). Immunohistofluorescence for TH was comparable in the jejunum of NC and SBT, showing quantitatively normal sympathetic innervation 1 year after SBT suggesting extrinsic reinnervation.
DISCUSSION
Our study was designed to determine long-term effects of chronic extrinsic denervation 1 year after SBT on intrinsic neural control of contractile activity mediated by inhibitory (VIP) and excitatory (substance P) NANC neurotransmitters in longitudinal smooth muscle of rat jejunum. We hypothesized that the extrinsic denervation mandated by SBT leads to long-term changes in enteric neural modulation of contractile activity mediated by these neurotransmitters. By using age-matched NC rats, we aimed to avoid changes secondary to ageing. We evaluated responses to exogenously applied neuropeptides and to EFS to explore indirectly any alterations in receptor expression, downstream signalling, or endogenous release of VIP and substance P (by EFS) which might contribute to enteric motor dysfunction after SBT.1,3–5 The inhibitory effect of exogenous VIP, found in both groups, was decreased 1 year after SBT and was blocked by either L-NNA or the VIP antagonist, but these antagonists had no additive effects. The relative inhibitory effect of VIP was increased when contractile activity was induced by substance P. Endogenous release of VIP during EFS was preserved but decreased 1 year after SBT. Endogenous release of substance P during EFS was also preserved 1 year after SBT, but the sensitivity to exogenously applied substance P was decreased.
The role of VIP in control of enteric contractile activity is complex and incompletely understood. VIP and NO are believed to be the dominant mediators of NANC inhibitory neural regulation of enteric contractile activity and are often released together and interact intensely; however, their role differs considerably between species19–21 and across anatomic regions of the gut.19,22–24 Our previous work in rat6 and dog20 showed that NO has a prominent inhibitory effect in longitudinal muscle of jejunum, but there was no effect in circular muscle of rat jejunum.13 We and others were unable to show any inhibitory effect of VIP on spontaneous contractile activity of longitudinal muscle of the jejunum in 2- and 3-month-old control rats as well as in 5-month-old rats 8 weeks after SBT,23,24 although an inhibitory potential of VIP was unveiled when muscle strips were precontracted with substance P.15 In contrast, in the current study, we demonstrated a prominent inhibitory effect of exogenous VIP on spontaneous contractile activity in 15-month-old control rats and in rats of the same age 1 year after SBT. These observations suggest that development of VIP sensitivity, in comparison to young rats, is secondary to ageing rather than to SBT. Indeed, age-related changes have been shown for adrenergic, cholinergic and nitrergic neurotransmission in the rat.25–28 Concerning VIP and substance P, although immunohistological studies in rat and human tissue were unable to demonstrate any effect of ageing on number of immunopositive neurons for these neuropeptides,29,30 agerelated changes in functional innervation with VIP and substance P cannot be excluded. The increase in the VIP-sensitivity prevented in part by SBT raises the question about the role of extrinsic innervation in the process of ageing in the intestine and enteric nervous system; our study cannot further elucidate this concept.
Precontraction of muscle strips with substance P increased the relative inhibitory effect of VIP. This observation suggests a potentially important role of VIP in inhibition of stimulated contractile activity; indeed, others have implicated VIP in the aboral (descending) relaxation during the peristaltic reflex.14 Whether a direct interaction between VIP and substance P is involved in this effect is possible but to the best of our knowledge has not been described yet.
The inhibitory effect of VIP was blocked in part by both the VIP antagonist and by L-NNA, but the combination of these drugs had no additive effect, and a discrete VIP-induced inhibition persisted. VIP induces its receptor-mediated inhibitory effect on the smooth muscle cell by activating adenylate cyclase and stimulating cyclic adenosine monophosphate (cAMP) production through VPAC1 and VPAC2 receptors, both of which are blocked by the competitive VIP antagonist we used.31,32 But VIP has also been reported to induce production and release of NO from presynaptic neurons, possibly via the natriuretic peptide clearance receptor, and even the induction of NO production in the smooth muscle cell is postulated.19 The residual effect of VIP in the presence of the VIP antagonist might be due to action via the natriuretic peptide clearance receptor, which is not blocked by the VIP antagonist,19 or by incomplete inhibition of the VIP effect by the antagonist at the dose we used (10−6 mol L−1). Lack of an additive effect of the combination of VIP antagonist and L-NNA suggests that both drugs might block incompletely different steps of the same ultimate pathway of VIP-induced inhibition; indeed the mechanism of action of VIP is possibly mediated via release of NO.
Under NANC conditions, EFS at low frequencies (<10 Hz) causes a net inhibition of contractile activity via endogenous release of inhibitory neurotransmitters. Previous work suggested these neurotransmitters to be primarily NO and VIP. Our past works on the effect of SBT have addressed release of NO.6,8,13 The current study was designed to explore endogenous release of VIP through use of a VIP antagonist. When the effect of endogenously released NO was eliminated by L-NNA, the inhibitory effect of endogenously released VIP was uncovered and could be blocked by the VIP antagonist in both groups, albeit to a lesser extent after SBT. The diminished effect of the VIP antagonist after SBT could result from a decreased sensitivity to VIP, as suggested by the experiments with exogenous VIP, and/or due to a decrease of endogenous release of VIP. Preservation of endogenous release of VIP is consistent with several morphological studies in rats, piglets, dogs, and human tissue which showed that immunoreactivity for VIP (and substance P) is preserved after SBT.17,33–38 These immunohistochemical data do not exclude alterations in functional release and/or response to endogenously released VIP that could explain the different response after SBT.
As with our previous study,15 both the VIP antagonist and L-NNA were unable to block the EFS-induced inhibition in the early part (first 4 s) of the EFS. Although inhibiting NO production shortened the duration of inhibition more in control tissue than after SBT, nevertheless, a short duration of net inhibition of contractile activity persisted. This inhibition was likely due to release of other NANC-inhibitory neurotransmitters, such as pituitary adenylate cyclase activating peptide (PACAP), adenosine triphosphate (ATP), carbon monoxide (CO), or hydrogen sulphide (H2S). The release of presynthesized NO appears unlikely, as NO is produced ‘on demand’.19 Our experiments, however, do not allow us to further differentiate these possibilities.
In a previous study, we demonstrated a decrease of substance P sensitivity in longitudinal muscle of rat jejunum 8 weeks after SBT.15 The current study shows that this effect of SBT persists even 1 year after SBT. In contrast to our work in rat, Tomita et al.22 described an increase in sensitivity to substance P 18 weeks after SBT in rat jejunal circular muscle, again highlighting the differences in response to extrinsic denervation within various muscle layers of small intestine. Endogenous release of substance P, as for VIP, was preserved after SBT in our study as shown by the increased inhibitory response during EFS at 3 and 6 Hz, demonstrating that even during the strong, EFS-induced net inhibition of contractile activity, other procontractile neurotransmitters like substance P are released and exert a procontractile effect despite the dominant, NO-mediated net inhibition.39 As with the persistent inhibitory response during the first 4 s of EFS in the presence of VIP and NO blockade, the substance P antagonist had no effect, potentially because substance P is not released during the first 4 s of EFS or more likely the net inhibitory response dominates any effect of endogenous release of substance P. Our study cannot differentiate these possibilities.
It was shown previously that SBT leads to thickening of the longitudinal and circular muscle layer of rat jejunum associated with hyperplasia and hypertrophy of smooth muscle cells 90 days after SBT.40 Although, this effect was more pronounced in animals experiencing chronic rejection after allotransplantation, it was also present when immune phenomena and rejection were avoided by syngeneic SBT; therefore, this tissue response appears to be a general response to the operative trauma, ischaemia/reperfusion injury, and extrinsic denervation. We cannot exclude that morphological alterations in the architecture of the bowel wall contributed to the functional changes in innervation shown in this study; however, because we used a syngeneic model of SBT, these effects should be minimized.
Finally, we wanted to explore the sympathetic reinnervation of the muscularis of the jejunum 1 year after SBT. Other studies in the rat have shown that sympathetic re-innervation occurs 3–4 weeks after SBT initially along the mesenteric vessels,18 after 15 weeks on the mesenteric side of the transplanted small bowel, and by 27 weeks after SBT on the anti-mesenteric side.16,17 Our study at 1 year after SBT further supports and extends this work by showing a rich reinnervation with an ostensibly normal subjective pattern of TH immunoreactivity. The changes in neurotransmission with VIP and substance P described in our study occurred despite sympathetic reinnervation, which demonstrates that extrinsic reinnervation after SBT does not necessarily reverse SBT-related alterations in enteric neural control.
In conclusion, we have demonstrated a decrease in sensitivity to exogenous VIP and substance P, as well as a decrease in effect of endogenous VIP release 1 year after SBT, despite quantitatively normal extrinsic innervation. These changes did not entail alterations in spontaneous contractile activity. Because VIP and substance P play important roles in enteric reflexes and enteric neural control of contractile activity, these alterations in NANC neurotransmission might contribute to enteric motor dysfunction after SBT.
Acknowledgments
We want to thank Deborah I. Frank for her expert assistance in the preparation of the manuscript.
This work was supported by grant DK 39337 from the National Institutes of Health, United States Public Health Services (M.G.S.) and grant KA 2329/1-1 from the Deutsche Forschungsgemeinschaft, Germany (M.S.K.).
Abbreviations
- ANOVA
analysis of variance
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
- CO
carbon monoxide
- EFS
electrical field stimulation
- H2S
hydrogen sulphide
- IACUC
Institutional Animal Care and Use Committee
- L-NNA
L-NG-nitro arginine
- L0
optimal length
- NANC
non-adrenergic, non-cholinergic
- NC
naïve control
- NO
nitric oxide
- NDS
normal donkey serum
- PACAP
pituitary adenylate cyclase activating peptide
- SBT
small bowel transplantation
- TH
tyrosine hydroxylase
- VIP
vasoactive intestinal polypeptide
- VPAC1 + 2
receptors binding mainly VIP and PACAP with different affinities
Footnotes
Parts of this work were presented at the American Motility Society Meeting in Freeport, Grand Bahama Island, March 1–4, 2007 and published in abstract form (Neurogastroenterology and Motility 2007;19:426).
References
- 1.Asfar S, Atkison P, Ghent C, et al. Small bowel transplantation. A life-saving option for selected patients with intestinal failure. Dig Dis Sci. 1996;41:875–83. doi: 10.1007/BF02091526. [DOI] [PubMed] [Google Scholar]
- 2.Rovera GM, DiMartini A, Graham TO, et al. Quality of life after intestinal transplantation and on total parenteral nutrition. Transplant Proc. 1998;30:2513–4. doi: 10.1016/s0041-1345(98)00704-0. [DOI] [PubMed] [Google Scholar]
- 3.Todo S, Reyes J, Furukawa H, et al. Outcome analysis of 71 clinical intestinal transplantations. Ann Surg. 1995;222:270–80. doi: 10.1097/00000658-199509000-00006. discussion 80–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rovera G, Furukawa H, Reyes J, Todo S, Hutson W. The use of clonidine for the treatment of high intestinal output following small bowel transplantation. Transplant Proc. 1997;29:1853–4. doi: 10.1016/s0041-1345(97)00096-1. [DOI] [PubMed] [Google Scholar]
- 5.Sarr MG, Duenes JA, Walters AM. Jejunal and ileal absorptive function after a model of canine jejunoileal autotransplantation. J Surg Res. 1991;51:233–9. doi: 10.1016/0022-4804(91)90100-z. [DOI] [PubMed] [Google Scholar]
- 6.Balsiger BM, Ohtani N, Anding WJ, Duenes JA, Sarr MG. Chronic extrinsic denervation after small bowel transplantation in rat jejunum: effects and adaptation in nitrergic and non-nitrergic neuromuscular inhibitory mechanisms. Surgery. 2001;129:478–89. doi: 10.1067/msy.2001.112070. [DOI] [PubMed] [Google Scholar]
- 7.Murr MM, Miller VM, Sarr MG. Contractile properties of enteric smooth muscle after small bowel transplantation in rats. Am J Surg. 1996;171:212–7. doi: 10.1016/S0002-9610(99)80102-0. discussion 7–8. [DOI] [PubMed] [Google Scholar]
- 8.Ohtani N, Balsiger BM, Anding WJ, Duenes JA, Sarr MG. Small bowel transplantation induces adrenergic hypersensitivity in ileal longitudinal smooth muscle in rats. J Gastrointest Surg. 2000;4:77–85. doi: 10.1016/s1091-255x(00)80036-0. [DOI] [PubMed] [Google Scholar]
- 9.Shibata C, Balsiger BM, Anding WJ, Sarr MG. Adrenergic denervation hypersensitivity in ileal circular smooth muscle after small bowel transplantation in rats. Dig Dis Sci. 1997;42:2213–21. doi: 10.1023/a:1018850214119. [DOI] [PubMed] [Google Scholar]
- 10.Murr MM, Sarr MG. Small bowel transplantation: effects on function of nonadrenergic, noncholinergic nerves. J Gastrointest Surg. 1997;1:439–45. doi: 10.1016/s1091-255x(97)80131-x. [DOI] [PubMed] [Google Scholar]
- 11.Shibata C, Murr MM, Balsiger B, Anding WJ, Sarr MG. Contractile activity of circular smooth muscle in rats one year after small bowel transplantation: differing adaptive response of the jejunum and ileum to denervation. J Gastrointest Surg. 1998;2:463–72. doi: 10.1016/s1091-255x(98)80038-3. [DOI] [PubMed] [Google Scholar]
- 12.Shibata C, Balsiger BM, Anding WJ, Duenes JA, Miller VM, Sarr MG. Functional changes in nonadrenergic, noncholinergic inhibitory neurons in ileal circular smooth muscle after small bowel transplantation in rats. Dig Dis Sci. 1998;43:2446–54. doi: 10.1023/a:1026630115009. [DOI] [PubMed] [Google Scholar]
- 13.Balsiger BM, Duenes JA, Ohtani N, et al. Nitric oxide pathways in circular muscle of the rat jejunum before and after small bowel transplantation. J Gastrointest Surg. 2000;4:86–92. doi: 10.1016/s1091-255x(00)80037-2. [DOI] [PubMed] [Google Scholar]
- 14.Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–15. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- 15.Kasparek MS, Fatima J, Iqbal CW, Duenes JA, Sarr MG. Role of VIP and substance P in NANC innervation in the longitudinal smooth muscle of the rat jejunum—influence of extrinsic denervation. J Surg Res. 2007;141:22–30. doi: 10.1016/j.jss.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 16.Kiyochi H, Ono A, Yamamoto N, Ohnishi K, Shimahara Y, Kobayashi N. Extrinsic sympathetic reinnervation after intestinal transplantation in rats. Transplantation. 1995;59:328–33. [PubMed] [Google Scholar]
- 17.Hirose R, Taguchi T, Hirata Y, Yamada T, Nada O, Suita S. Immunohistochemical demonstration of enteric nervous distribution after syngeneic small bowel transplantation in rats. Surgery. 1995;117:560–9. doi: 10.1016/s0039-6060(05)80256-9. [DOI] [PubMed] [Google Scholar]
- 18.Pernthaler H, Pfurtscheller G, Klima G, et al. Regeneration of sympathetic activities in small bowel transplants. Eur Surg Res. 1993;25:316–20. doi: 10.1159/000129295. [DOI] [PubMed] [Google Scholar]
- 19.Van Geldre LA, Lefebvre RA. Interaction of NO and VIP in gastrointestinal smooth muscle relaxation. Curr Pharm Des. 2004;10:2483–97. doi: 10.2174/1381612043383890. [DOI] [PubMed] [Google Scholar]
- 20.Zyromski NJ, Duenes JA, Sarr MG. Inhibition by nitric oxide and nonadrenergic, noncholinergic (NANC) nerves is preserved in a canine model of extrinsic denervation: implications for small bowel transplantation. Surgery. 2005;138:905–12. doi: 10.1016/j.surg.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 21.Stark ME, Bauer AJ, Sarr MG, Szurszewski JH. Nitric oxide mediates inhibitory nerve input in human and canine jejunum. Gastroenterology. 1993;104:398–409. doi: 10.1016/0016-5085(93)90407-4. [DOI] [PubMed] [Google Scholar]
- 22.Tomita R, Fujisaki S, Park E, Ikeda T, Koshinaga T. Substance P and vasoactive intestinal peptide in rat small-bowel isografts. Am J Surg. 2005;189:63–70. doi: 10.1016/j.amjsurg.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Okishio Y, Niioka S, Yamaji M, et al. Mediators of non-adrenergic, noncholinergic relaxation in Sprague Dawley rat intestine: comparison with the mediators of other strains. J Vet Med Sci. 2000;62:821–8. doi: 10.1292/jvms.62.821. [DOI] [PubMed] [Google Scholar]
- 24.Niioka S, Takeuchi T, Kishi M, et al. Nonadrenergic, noncholinergic relaxation in longitudinal muscle of rat jejunum. Jpn J Pharmacol. 1997;73:155–61. doi: 10.1254/jjp.73.155. [DOI] [PubMed] [Google Scholar]
- 25.Baker DM, Watson SP, Santer RM. Evidence for a decrease in sympathetic control of intestinal function in the aged rat. Neurobiol Aging. 1991;12:363–5. doi: 10.1016/0197-4580(91)90023-d. [DOI] [PubMed] [Google Scholar]
- 26.Yu O, Ouyang A. Distribution of beta-adrenoceptor subtypes in gastrointestinal tract of nondiabetic and diabetic BB rats. A longitudinal study. Dig Dis Sci. 1997;42:1146–53. doi: 10.1023/a:1018877318101. [DOI] [PubMed] [Google Scholar]
- 27.Tezuka A, Ishihata A, Aita T, Katano Y. Aging-related alterations in the contractile responses to acetylcholine, muscarinic cholinoceptors and cholinesterase activities in jejunum and colon of the male Fischer 344 rats. Exp Gerontol. 2004;39:91–100. doi: 10.1016/j.exger.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 28.Takeuchi T, Niioka S, Yamaji M, et al. Decrease in participation of nitric oxide in nonadrenergic, noncholinergic relaxation of rat intestine with age. Jpn J Pharmacol. 1998;78:293–302. doi: 10.1254/jjp.78.293. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RJ, Schemann M, Santer RM, Cowen T. The effects of age on the overall population and on sub-populations of myenteric neurons in the rat small intestine. J Anat. 1998;192:479–88. doi: 10.1046/j.1469-7580.1998.19240479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch TR, Carney JA, Go VL, Szurszewski JH. Inhibitory neuropeptides and intrinsic inhibitory innervation of descending human colon. Dig Dis Sci. 1991;36:712–8. doi: 10.1007/BF01311226. [DOI] [PubMed] [Google Scholar]
- 31.Fizanne L, Sigaudo-Roussel D, Saumet JL, Fromy B. Evidence for the involvement of VPAC1 and VPAC2 receptors in pressure-induced vasodilatation in rodents. J Physiol. 2004;554:519–28. doi: 10.1113/jphysiol.2003.053835. Epub 2003 Oct 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rekik M, Delvaux M, Frexinos J, Bueno L. Role of vasoactive intestinal polypeptide in the adaptation of intestinal smooth muscle cells to mechanical distension. J Pharmacol Exp Ther. 1998;287:832–8. [PubMed] [Google Scholar]
- 33.Larosa CA, Blank MA, Kimura K, Jaffe BM. Effect of transplantation on tissue levels of substance P, vasoactive intestinal polypeptide, and serotonin in rat small intestine. Ann N Y Acad Sci. 1990;594:336–46. doi: 10.1111/j.1749-6632.1990.tb40492.x. [DOI] [PubMed] [Google Scholar]
- 34.Taguchi T, Guo R, Masumoto K, et al. Chronological change of distribution in nitric oxide and peptidergic neurons after rat small intestinal transplantation. J Pediatr Surg. 1999;34:341–5. doi: 10.1016/s0022-3468(99)90205-1. [DOI] [PubMed] [Google Scholar]
- 35.Shen Z, Klover-Stahl B, Larsson LT, Malmfors G, Ekblad E, Sundler F. Peptide-containing neurons remain unaffected after intestinal autotransplantation: an experimental study in the piglet. Eur J Pediatr Surg. 1993;3:271–7. doi: 10.1055/s-2008-1063558. [DOI] [PubMed] [Google Scholar]
- 36.Malmfors G, Hakanson R, Okmian L, Sundler F. Peptidergic nerves persist after jejunal autotransplantation: an experimental study in the piglet. J Pediatr Surg. 1980;15:53–6. doi: 10.1016/s0022-3468(80)80403-9. [DOI] [PubMed] [Google Scholar]
- 37.Nelson DK, Sarr MG, Go VL. In vivo neural isolation of the canine jejunoileum: temporal adaptation of enteric neuropeptides. Gut. 1991;32:1336–41. doi: 10.1136/gut.32.11.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sugitani A, Reynolds JC, Todo S. Immunohistochemical study of enteric nervous system after small bowel transplantation in humans. Dig Dis Sci. 1994;39:2448–56. doi: 10.1007/BF02087666. [DOI] [PubMed] [Google Scholar]
- 39.Maggi CA, Patacchini R, Rovero P, Giachetti A. Tachykinin receptors and tachykinin receptor antagonists. J Auton Pharmacol. 1993;13:23–93. doi: 10.1111/j.1474-8673.1993.tb00396.x. [DOI] [PubMed] [Google Scholar]
- 40.Heeckt PF, Halfter WM, Schurer B, Schraut WH, Beger HG, Bauer AJ. Heterotopic intestinal transplantation aggravates the insult of chronic rejection. Transplantation. 1998;65:354–62. doi: 10.1097/00007890-199802150-00010. [DOI] [PubMed] [Google Scholar]