Abstract
In a well-studied model of social behaviour, male zebra finches sing directed song to court females and undirected song, used possibly for practice or advertisement. Although the two song types are similar, the level of neural activity and expression of the immediate early gene egr-1 are higher during undirected than during directed singing in the lateral part of the basal ganglia song nucleus AreaX (LAreaX) and its efferent pallial song nuclei lateral magnocellular nucleus of the anterior nidopallium (LMAN) and the robust nucleus of the arcopallium (RA). As social interactions are dependent on brain motivation systems, here we test the hypothesis that the midbrain ventral tegmental area–substantia nigra pars compacta (VTA–SNc) complex, which provides a strong dopaminergic input to LAreaX, is a source of this modulation. Using egr-1 expression, we show that GABAergic interneurons in VTA–SNc are more active during directed courtship singing than during undirected singing. We also found that unilateral removal of VTA–SNc input reduced singing-dependent gene expression in ipsilateral LAreaX during both social contexts but it did not eliminate social context differences in LAreaX. In contrast, such lesions reduced and eliminated the social context differences in efferent nuclei LMAN and RA, respectively. These results suggest that VTA–SNc is not solely responsible for the social context gene regulation in LAreaX, but that VTA–SNc input to LAreaX enhances the singing-regulated gene expression in this nucleus and, either through LAreaX or through direct projections to LMAN and RA, VTA–SNc is necessary for context-dependent gene regulation in these efferent nuclei.
Keywords: birdsong, dopamine, immediate early gene, sexual motivation, vocal production
Introduction
Songbirds possess the rare trait of vocal learning, which is controlled by a discrete neural network that consists of a vocal motor pathway [(HVC, a letter based name) → robust nucleus of the arcopallium (RA) → 12th nucleus, tracheosyringeal part (nXIIts) and other brainstem neurons; see Fig. 1A] involved in song production, and a vocal pallial–basal ganglia loop [lateral magnocellular nucleus of the anterior nidopallium (LMAN) → lateral AreaX of the striatum (LAreaX) → dorsal lateral nucleus of the medial thalamus (DLM) → LMAN] involved in song learning and modification (Fig. 1A; reviewed in Jarvis, 2004). Nuclei of these pathways show remarkable differences in activation depending upon the social context in which male zebra finches produce their songs; males sing a stereotyped directed song to females during courtship and a slightly more variable undirected song that is thought to be used for practice or advertisement (Sossinka & Bohner, 1980; Zann, 1996; Jarvis et al., 1998; Kao et al., 2005). During undirected singing (UD), the level of neural activity and expression of the immediate early gene (IEG) egr-1 (also known as ZENK; Mello et al., 1992) are high throughout both vocal pathways, whereas during female-directed singing (FD), activity and/ or egr-1 expression are low in the lateral part of the vocal pallial–basal ganglia loop (LAreaX and LMAN) and in RA of the motor pathway (Jarvis et al., 1998; Hessler & Doupe, 1999). Because the level of egr-1 expression in HVC, the primary song system input to both LAreaX and RA, was not dependent on social context, it has been proposed that the social context-dependent modulation of these vocal nuclei may be controlled by midbrain motivation-related brain areas (Jarvis et al., 1998; Hessler & Doupe, 1999). The suggested candidate areas included the ventral tegmental area (VTA)–substantia nigra pars compacta (SNc) complex, which sends dopaminergic input predominantly into the striatal vocal nucleus AreaX (Fig. 1A; Lewis et al., 1981) and the locus coeruleus (LoC), which sends norepinephrine input predominantly into pallial vocal nuclei HVC, RA and the magnocellular nucleus of the nidopallium (MAN); (Mello et al., 1998; Appeltants et al., 2002). These hypotheses are supported by several recent studies. In zebra finches, removal of all brain norepinephrine neurons causes the level of singing-induced egr-1 expression to be equally high in LAreaX in both social contexts (Castelino & Ball, 2005). Activity of most VTA neurons is modulated more during directed than during UD (Yanagihara & Hessler, 2006); similarly, dopamine levels in LAreaX are higher during directed than during UD (Sasaki et al., 2006). Studies in other songbird species have found singing-associated increases in IEG expression in VTA in other social contexts, such as during territorial singing in song sparrows (Maney & Ball, 2003) and during the breeding season singing in starlings (Riters et al., 2004; Heimovics & Riters, 2005). Here, we tested whether the VTA–SNc complex modulates vocal pathway nuclei function and their social context differences. We report that VTA–SNc has high egr-1 expression during FD but that VTA–SNc enhances the singing-driven gene activation of LAreaX in both social contexts, and that VTA–SNc is required, possibly via LAreaX, for the social context differences in efferent vocal nuclei.
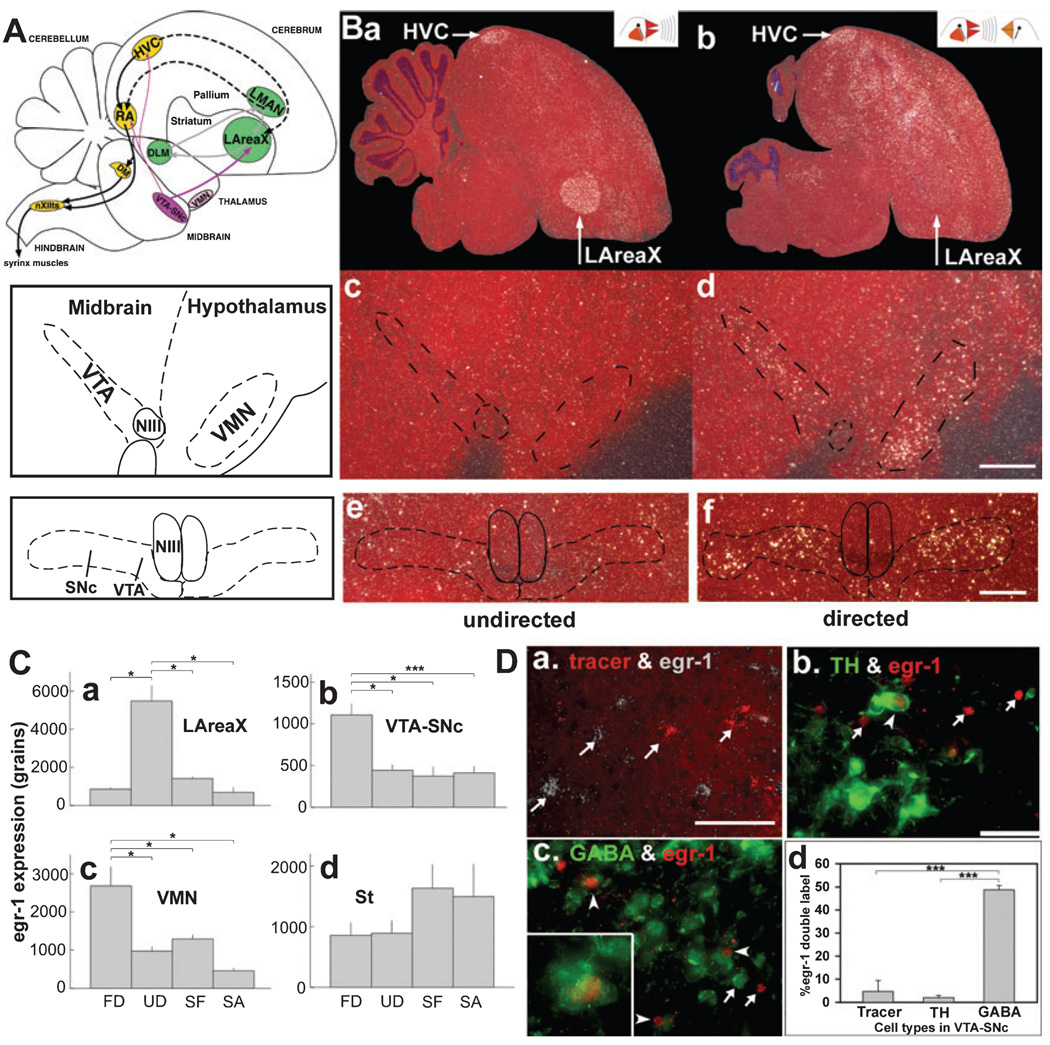
FIG. 1.
Social context-dependent gene expression. (A) Schematic diagram of songbird brain showing the song system and VTA–SNc, which sends a strong dopaminergic projection to LAreaX and weaker projections to HVC and RA. Black solid arrows and yellow nuclei, vocal motor pathway; grey arrows and green nuclei, vocal pallial–basal ganglia–thalamic loop; dashed arrows, connections between the two vocal pathways. (B) Egr-1 mRNA expression (white, silver grains) in sagittal brain sections (counterstained with cresyl violet) from animals that sang similar amounts (50 and 70 motifs) of (a, c and e) UD and (b, d and f) FD song. Camera lucida drawings on the left highlight the VMN, VTA and SNc regions; there is no distinct boundary between VTA and SNc. Sections in (a)–(d) are cut in the sagittal plane, and (e) and (f) in the coronal plane. NIII, third cranial nerve. (C) Quantification of egr-1 expression. FD, n = 14; UD, n = 10; SF, n = 4; SA, n = 5. * P < 0.05, *** P < 0.0001, one-way anova, Holm–Sidak post hoc test. (D) Cell types with FD-driven egr-1 expression in VTA–SNc. Arrows, single-labelled cells; arrowheads, double-labelled cells. (a) Retrograde tracer backfilled from LAreaX to VTA neurons (red) and egr-1 mRNA expression (white silver grains). (b) Expression of TH (green-labelled cytoplasm) and egr-1 protein (red-labelled nuclei). Only one double-labelled cell can be seen in this image. (c) GABA (green-labelled cytoplasm) and egr-1 protein (red-labelled nuclei); inset in lower left shows a 3× higher magnification of a double-labelled neuron. (d) Percentage of egr-1 positive neurons that were double-labelled with tracer from LAreaX (n = 11 sections), TH (n = 10 sections) or GABA (n = 7 sections) in VTA–SNc [out of 45–314 neurons across all sections in all birds (n = 3–5) for each single-labelled category]. *** P < 0.0001, one-way anova, Holm–Sidak post hoc test. Error bars for all panels represent SEM. Scale bars, 250 µm (B), 50 µm (D).
Materials and methods
Animals
We used adult male zebra finches (> 90 days old), bred at the RIKEN Institute and Duke University Medical Center. All experiments were performed according to the RIKEN BSI guidelines and were approved by the RIKEN Animal Experiments Committee and the Duke University Animal Care and Use Committee.
VTA–SNc gene expression experiments
Behaviour
For the gene expression experiment (Fig. 1B–C), strong adult male singers were selected from our aviaries. To select these males, a cage with multiple females was placed next to a cage with multiple males. Males that started to sing immediately to the females and continued to do so for at least 10 min were chosen. They were then housed individually in sound-attenuation chambers (75 × 27.5 × 28.8 cm) and singing behaviour was recorded in the next or subsequent mornings using Avisoft Recorder (Avisoft Bioacoustics, Berlin, Germany) or Sound Analysis Pro (http://ofer.sci.ccny.cuny.edu/html/sound_analysis.html; Tchernichovski et al., 2004). Birds were divided into four groups. These were, when including brain sections of similarly treated birds from a previous study (Jarvis et al., 1998): (i) FD (n = 14 total, three from this study); (ii) UD (n = 10 total, three from this study); (iii) silent with a female (SF; n = 4 from this study); and (iv) silent alone (SA; n = 5 total, three from this study). Each session lasted 45 min. For the FD group, a female was placed in the box with the male, separated by a cage wall barrier, on the night before the recording session. Males were presented with one female at first and then 10–15 min later a second female was introduced so that two females were present in the cage at the same time. We then replaced one of the two females within the 45-min time period, so that there were again two females in the cage to maintain high levels of FD. Only males that directed at least 98% of their songs to females were used; we determined this by observing them on a TV monitor. Males who did not sing were classified as SF birds. For UD, males remained alone during the recording session; males who did not sing were classified as SA birds. If a male did not sing within the first 2 h of the morning and we did not take it as a silent control, or if he did not sing the required amount of songs within the first 45 min of singing started in a 3-h period, then we repeated the behavioural experiment for two more days until the bird reached criterion; if not, we switched him for another bird. For all singing groups, brains were collected only if the birds performed 20 or more song bouts during the 45-min singing session; this is sufficient to induce high egr-1 mRNA levels in vocal nuclei (Jarvis & Nottebohm, 1997).
In situ hybridization and immunocytochemistry (ICC)
Birds were killed by decapitation and brains were removed, frozen in a tissue block mold with OCT Tissue-Tek compound (Sakura Fine Technical) and stored at −80 °C. Brain sections were cut at 12 µm thickness onto silanated glass slides. The previously collected brains (Jarvis et al., 1998) had been cut in the sagittal plane and the recent ones were cut in the frontal plane; all recent sections were cut on a similar model cryostat, Microm HM560 (Zeiss), to that used in the previous study. All sections were then processed for radioactive in situ hybridization with a zebra finch egr-1 riboprobe (Wada et al., 2006) or with a riboprobe containing the full-length egr-1 coding region (isolated by Dr Osceola Whitney, Duke University Medical Center) using a previously described procedure (Wada et al., 2004).
ICC was performed on alternate sections that were fixed with 4% paraformaldehyde for 10 min and rinsed three times with PBS. Sections were incubated in 5% normal donkey serum for 1 h at 4 °C, and then overnight at 4 °C with a mouse anti-tyrosine hydroxylase (TH) antibody (1 : 5000 or 1 : 2500; Chemicon, Temecula, CA, USA) to locate VTA and SNc (Lewis et al., 1981; Reiner et al., 2004) or a rabbit anti-aromatase antibody (1 : 2500; a kind gift from Dr Art Arnold) to locate the ventromedial nucleus of the hypothalamus (VMN; Supplementary material, Fig. S1; Saldanha et al., 2000). We included VMN in the gene expression analyses because its egr-1 expression was very prominent in the FD animals and it is directly adjacent to VTA–SNc. Sections were then rinsed three times with PBS and incubated in a mixture of secondary anti-mouse and anti-rabbit antibodies conjugated with Alexa 488 (green) or Alexa 568 (red; 1 : 200; Molecular Probes) for 2 h at room temperature, rinsed three times in PBS, and coverslipped with Vectashield DAPI solution (Vector Laboratories).
Gene expression quantification
Using the TH and aromatase staining on adjacent sections to locate VTA–SNc and VMN (Supplementary Fig. S1), we quantified the relative levels of egr-1 mRNA expression from the in situ hybridizations following a previously described procedure (Jarvis et al., 1998). We also quantified the expression in LAreaX and striatum immediately caudal (in sagittal sections) or lateral (in frontal sections) to LAreaX as control regions expected to show and not show egr-1 regulation by singing, respectively. In brief, two pictures per brain region from the same hemisphere were taken with a Leica DMXRA microscope using a 40× objective (290 × 210 µm area), and Spot III camera (Diagnostic Instruments) and software (Molecular Dynamics). Silver grains over 100–130 cells per field (depending upon region) were counted using the density slice and analyse functions of Scion Image software (NIH). Background counts on the glass slides were subtracted from the silver grain counts over the brain regions. In cases where we processed in situ hybridizations on different days, we included several brains that overlapped between the two different days, and used expression in selected brain regions (AreaX and VTA) to normalize between hybridizations on different days. The resultant values for each brain region of each bird were averaged across all birds to obtain one value per brain region per group. To assess differences between groups (FD, UD, SF and SA) in each brain region, a one-way anova followed by a post hoc Holm–Sidak test was conducted.
Colocalization of egr-1 in different neuron types of VTA–SNc
For the retrograde tracer and egr-1 double-labelling experiment (Fig. 1Da), five strongly singing males were anaesthetized with 1–3% isoflurane and placed in a stereotaxic apparatus. A retrograde tracer (0.2 µL fluorescent latex microspheres; Lumafluor) was pressure-injected either unilaterally or bilaterally into LAreaX using a Hamilton syringe (head angle 45°, injection location ~5.1 mm rostral and 1.8 mm lateral to the posterior divergence of the central sinus at the border of the forebrain and cerebellum, 3.15 mm below the brain surface). After 5–7 days to allow for bird recovery and tracer transport, the males were placed individually in the sound-attenuation chambers. Males were presented with females as in the experiment above. After they produced 30 or more bouts of FD in 45 min, the males were killed by decapitation. We chose 30 bouts as the lower limit because we found that this amount of singing was sufficient to induce high levels of both egr-1 mRNA and protein in VTA–SNc. Brains were frozen in OCT compound and cut at 12 µm. In situ hybridizations were performed with a riboprobe against egr-1 as described above, except alcohols and xylenes were not used in order to prevent removal of the tracer (Jarvis et al., 1998).
For ICC double-labelling of cells in VTA–SNc (Fig. 1, Db and c), four males were presented with females as described above. After 30 or more bouts of FD in 45 min, the males were anaesthetized with equithesin and perfused with 4% paraformaldehyde. Brains were removed, sunk overnight in 30% sucrose in PBS, and frozen in a tissue block mold with OCT. Frontal 35-µm-thick sections were cut on a cryostat, free-floated in PBS and stored at −20 °C in a mixture of 30% sucrose and 30% ethylene glycol in PBS. Selected sections were rinsed three times with PBS, incubated in 5% normal donkey serum for 1 h at 4 °C, and then 48 h at 4 °C in combinations of primary antibodies: rabbit anti-egr-1 (1 : 200; Santa Cruz Biotechnology) with mouse anti-TH (1 : 5000 or 1 : 2500; Chemicon) or with mouse anti-GABA (1 : 500; Sigma). Sections were then rinsed three times with PBS, and incubated in a mixture of anti-rabbit and anti-mouse secondary antibodies conjugated with Alexa 488 or Alexa 568 for 2 h at room temperature, rinsed three times with PBS, mounted onto silanated glass slides and coverslipped with Vectashield DAPI solution. The GABA antibody has been previously shown to be highly specific for GABA in zebra finch brain (Pinaud et al., 2004). Although in that study perfusion with a paraformaldehyde–glutaraldehyde mixture increased the intensity of labelling for GABA, we found similar staining with and without glutaraldehyde in the perfusate.
Quantification of colocalized neurons
For the retrograde tracer-injected birds, pictures of VTA–SNc were taken using a 63× objective (182 × 132 µm area) under brightfield (egr-1) and then red fluorescent (tracer) settings; the two images were then merged using Spot III software. Cells that contained a cluster of five or more silver grains were counted as egr-1-expressing. The number of cells expressing egr-1 alone, tracer alone, and both were manually counted in at least two separate merged images per section (n = 2 or 3 sections per bird). For ICC double-labelling of egr-1 (red) and TH or GABA (green), a similar procedure was used in which pictures of VTA–SNc in the red and green florescent channels were taken using a 40× objective (290 µm × 210 µm area) and a cooled CCD camera (cool SNAP ES Olympus microscope and video camera; Nippon Roper, Tokyo, Japan; detection of GABA required using a camera sensitive to low-level signals). The two images were then merged, and single-labelled and double-labelled cells were counted manually. To determine statistical differences between groups of egr-1 double-labelled cells, anova with post hoc Holm–Sidak test was conducted, using average data from individual sections as individual variables.
VTA–SNc lesion experiments
Surgery
Birds were anaesthetized with 1–3% isoflurane and placed in a stereotaxic apparatus. For VTA–SNc lesions (n = 24) we used 0.004% (low concentration, n = 5) or 0.4% (high concentration, n = 19) of 6-hydroxydopamine (6-OHDA; Sigma), dissolved in 0.2% ascorbic acid in sterile saline. The 6-OHDA solutions were injected unilaterally to VTA and SNc using a pressure injector (Nanoject II; Drummond Scientific). 6-OHDA is a neurotoxin that selectively destroys catecholaminergic neurons (Nagatsu & Ichinose, 1999). To locate VTA and SNc, we used a double-barrelled glass micropipette (two barrels, one filament, 1.2 × 150 mm; A–M Systems Inc.), with one barrel containing a silver wire electrode in 1 m NaCl and the other barrel containing 6-OHDA solution separated from the Nanoject pressure plunger by mineral oil. We used activity detected by the electrode to locate the dorsal–ventral position of VTA and SNc; in anaesthetized rats, the region of VTA–SNc has higher spontaneous activity than surrounding areas (Dr Regina Correlli, University of North Carolina, personal communication). We found a similar activity pattern in zebra finches, so we used the pattern as a guide to inject into VTA–SNc. To target the entire VTA–SNc, we made injections into anterior and posterior locations. For the anterior location (VTA), four injections of 50.6 nL each were made in two dorsal–ventral sites (D–V 5.8–6.45 mm range; R-C 2.1 mm, M-L 0.4 mm; two injections per site). Because the posterior location (SNc) is larger, we made four to nine injections at two or three dorsal–ventral sites (D–V 5.8–6.3 mm range; R-C 1.7 mm, M-L 0.8 mm; two or three injections per site). After surgery, birds were placed in the sound-attenuation chamber to recover and to record their songs.
Behaviour, in situ hybridization and ICC
Pre-surgery UD and FD behaviour of the 24 male birds were observed and recorded for at least 45 min with Sound Analysis Pro and a video camera inside the sound-attenuation chambers. After 5–7 days from surgery, the males were assigned to one of three behavioural groups: FD for 45 min (n = 11), UD for 45 min (n = 9), and SA controls (n = 4). The males were then killed by decapitation and brains were processed for egr-1 in situ hybridization as described above. To evaluate the lesion efficiency, alternate sections were incubated with mouse anti-TH (Chemicon) or rabbit anti-dopamine beta-hydroxylase (DBH) antibody (1 : 1000, Chemicon; Mello et al., 1998), followed by a secondary antibody conjugated with Alexa 488. TH is an enzyme required for dopamine synthesis and is expressed at high levels in the VTA–SNc neurons that project to the striatum (Nagatsu & Ichinose, 1999), including to AreaX (Lewis et al., 1981). It is also present in other catecholaminergic neurons that synthesize norepinephrine. DBH is an enzyme that converts dopamine to norepinephrine (Nagatsu & Ichinose, 1999) and is expressed at high levels in the LoC, which provides a strong norepinephrine input to the pallium, including to RA, and weak input to AreaX (Mello et al., 1998; Appeltants et al., 2002). Somata that are TH-positive and DBH-negative, as is the case in VTA–SNc, are referred to as dopaminergic, following standard practices (Bayer & Pickel, 1991; Liprando et al., 2004).
Quantification
To determine lesion sizes, pictures of VTA–SNc and LoC were taken from the intact and lesioned hemispheres, using a 40× objective with the Olympus microscope and video camera. Using the TH staining, the areas of VTA–SNc and LoC in intact and lesion sides were measured with Neurolucida (Microbrightfield). The area of the remaining VTA–SNc on the lesion side was divided by the area of the VTA–SNc on the intact side to obtain a ratio that reflected lesion size. The same measurement was made for LoC. To quantify gene expression levels, pictures of silver grains for egr-1 in vocal nuclei were taken with a 63× objective and analysed as described above for the gene expression experiments. Simple regression analyses were used to determine the proportional relationship of egr-1 increases in vocal nuclei between the intact and VTA–SNc-lesioned or sham hemispheres. Multiple regressions were used to determine whether there were significant differences in this relationship between the VTA–SNc-lesioned and sham-operated birds. To determine the effects of VTA–SNc lesions on social context differences in vocal nuclei independent of singing amount, we calculated ratios of egr-1 expression in LAreaX, RA and LMAN, each relative to HVC, using a previously described approach (Jarvis et al., 1998). We used HVC as the normalizing brain region because it does not show changes in egr-1 expression as a result of social context (Jarvis et al., 1998) or VTA–SNc lesions (see Results). The ratio measure not only eliminates the amount of singing as a variable but it also serves as a robust internal bird and hemispheric control. To determine statistical differences in ratios between groups we used unpaired t-tests.
Song quantification for 6-OHDA-treated animals
To quantify potential lesion effects on singing behaviour we compared singing rate, syllable similarity and sequence stereotopy before and after the unilateral VTA–SNc lesions. To quantify singing rate we calculated the mean number of song motifs produced per minute in the 45-min singing sessions before and after lesions. To measure syllable similarity, we calculated the mean similarity score (% similarity and mean accuracy) with Sound Analysis Pro from 10 aligned comparisons of representative song motifs before and after lesions; the Sound Analysis Pro similarity function is an aggregate measure of five features: frequency modulation, entropy, continuity, duration and pitch (Tchernichovski et al., 2000). For the syllable similarity score, we discovered that we could not directly compare the similarity values of pre- and post-lesion songs due to technical limitations of slight changes in recording conditions (variations in microphone quality and attenuation box acoustics) before and after surgery. Therefore, to normalize against this technical variable we computed the ratios of the mean percentage syllable similarity of pre- vs. post-lesion divided by pre- vs. pre-lesion for lesioned and sham-operated birds. To measure sequence stereotopy, we used the equations (sequence linearity and consistency) in Scharff & Nottebohm (1991) as modified in Foster & Bottjer (2001). First we scored the sequence of a minimum of 30 song motifs per bird before and after surgery. We did not have presurgery song for three of the sham-treated birds and therefore these birds were not included in the analysis. Song sequence linearity was calculated as the number of syllable types per motif minus 1, divided by the number of different transitions between syllables. A group of song motifs with no variability in pattern would produce a linearity score of 1.0. Sequence consistency for each pair of syllables of each motif was calculated by calculating syllables across all song motifs as the fraction of the most common subsequent syllable transition type among all transition types for that syllable. Then the average sequence consistency across all syllables was calculated for each bird. The terminal syllable of song motifs was not included in the consistency calculation, and introductory syllables were excluded from both calculations. A single stereotypy score was calculated for each bird by averaging the linearity and consistency scores. A highly stereotyped sequence has a score close to 1.0. In order to quantify the similarity of this measure between the two recording sessions for each bird (e.g. pre- vs. post-lesion), we calculated the ratio of the stereotypy score before and after surgery. To determine statistical differences in all three measures above we used the t-test.
Results
To determine a possible role of VTA–SNc in the modulation of vocal system nuclei function by social context, we performed two types of experiments: (i) descriptive experiments relating singing behaviour with VTA–SNc egr-1 expression; and (ii) manipulation experiments studying the effect that VTA–SNc lesions have on vocal nuclei egr-1 expression.
VTA–SNc gene expression experiments
Social context during singing altered egr-1 expression in VTA–SNc
We first measured egr-1 mRNA expression levels in VTA–SNc after singing in different social contexts, using the expression changes in vocal nuclei as our control measures. As expected (Jarvis et al., 1998), egr-1 mRNA expression in LAreaX was ~5× higher after UD than after FD (Fig. 1, Ba and b, Ca). In contrast, we found that in the VTA (Fig. 1, Bc and d) as well as in the adjacent SNc (Fig. 1, Be and f), egr-1 mRNA expression was ~2× higher after FD than after UD (Fig. 1, Cb). There was no consistent difference between VTA and SNc (P = 0.93; average ± SEM VTA : SNc ratio, 0.95 ± 0.16, n = 3 FD animals, frontal sections), and thus we have presented our results with the two regions combined as VTA–SNc. We also noted robust differences in a rostrally adjacent nucleus, the VMN, in which, as in VTA–SNc, egr-1 expression was higher after FD (Fig. 1, Bc and d, 1Cc). Although we did not study the role of VMN with lesions in this study, the descriptive expression results in this paragraph apply to VMN as well. The egr-1 increases in VTA–SNc (as well as VMN) were specific to FD, as the level of egr-1 expression in males that could see females but did not sing (SF) was similar to that of males who were silent alone and did not sing (SA; Fig. 1, Cb and c). The expression changes in VTA–SNc and LAreaX were not a result of general changes in brain gene regulation as, for example, the egr-1 expression in the striatum adjacent to LAreaX was not significantly different between the behavioural conditions (Fig. 1, Cd). The relationship of VTA–SNc egr-1 expression and song production was not present as it was for LAreaX. In LAreaX, the level of egr-1 expression was linearly proportional to the number of song motifs produced during UD (r2 = 0.795, P < 0.0001, n = 10 UD and n = 5 SA; simple regression), as expected (Jarvis & Nottebohm, 1997), whereas in VTA–SNc there was no detectable correlation during FD (r2 = 0.173, P = 0.0862; n = 13 FD and n = 5 SA). The levels of egr-1 expression between VTA–SNc and LAreaX were also not correlated (r2 = 0.025 and 0.25, P = 0.14 and 0.9 for UD and FD, respectively; simple regressions). Together, these results suggest that the high levels of egr-1 expression in VTA–SNc were stimulated by a combination of singing and courtship.
Singing-associated egr-1 was expressed in GABAergic neurons of VTA–SNc
We next assessed which neuronal type in VTA–SNc expressed high levels of egr-1 during FD. Zebra finch VTA–SNc is thought to contain mainly two neuron types: type 1 neurons which are dopaminergic, express TH and project to the striatum, including to AreaX, and type 2 neurons, which are inhibitory, presumably GABAergic, and are thought to synapse locally (Lewis et al., 1981; Gale & Perkel, 2006). In order to identify type 1 neurons, the dopaminergic projection neurons, we retrogradely labelled them by injecting a tracer into LAreaX and elicited FD as described in the methods. We found that < 5% of egr-1 expressing neurons in VTA–SNc were LAreaX-projecting neurons (Fig. 1, Da and d). As this low number of double-labelled egr-1 neurons in VTA–SNc could be due to the possibility that the localized tracer injections labelled a limited number of dopaminergic projection neurons, we further performed double-label ICC with antibodies against egr-1 and TH. We found that only ~2.0% of the egr-1-positive neurons were TH-positive (Fig. 1, Db and d). To test whether the remaining egr-1 cells were of type 2 neurons in VTA–SNc, we performed egr-1 double-labelling with a marker of inhibitory interneurons, GABA. We found that nearly half of the egr-1-positive neurons were GABA-positive and that this was significantly more than the number of egr-1 neurons colocalized with the tracer or TH (Fig. 1, Dc and d). These results suggest that, during FD, egr-1 expression is induced mainly in local inhibitory GABAergic interneurons of VTA–SNc.
VTA–SNc lesion experiments
VTA–SNc was required for normal singing-driven egr-1 expression in LAreaX, LMAN and RA
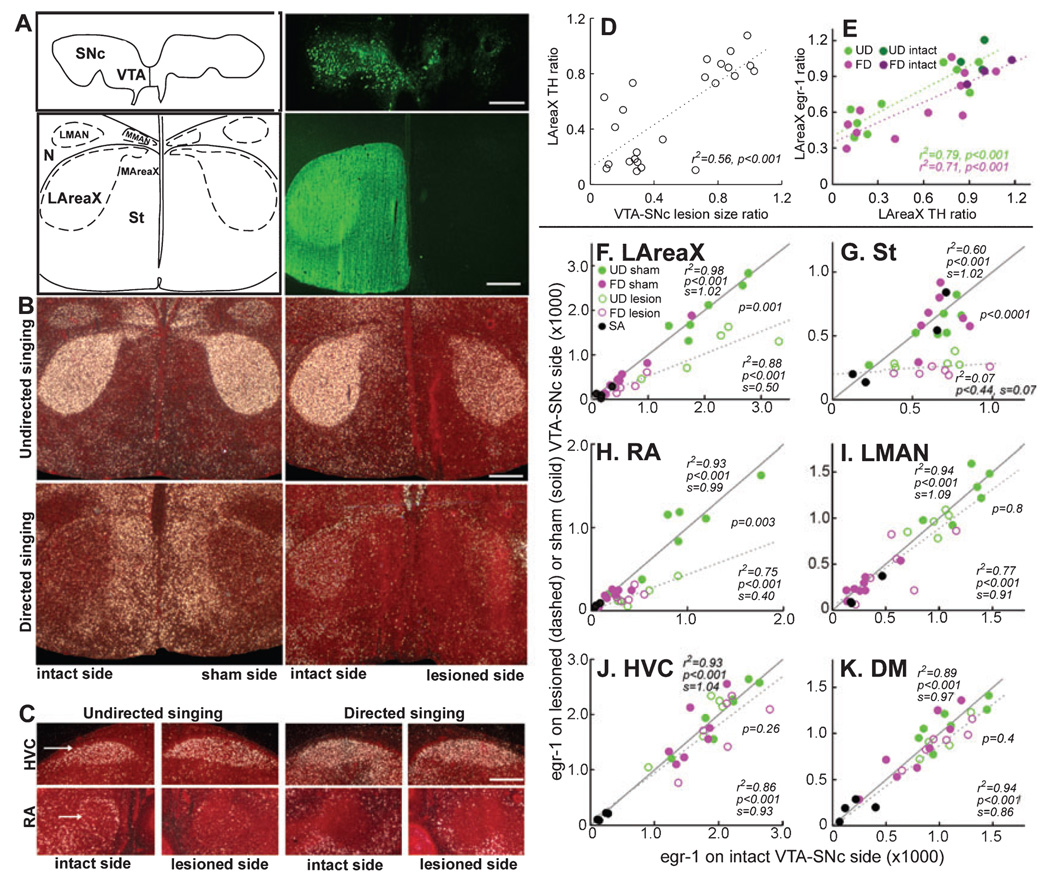
To directly test whether VTA–SNc influences the singing-regulated egr-1 expression in LAreaX, we performed unilateral lesions using 6-OHDA and compared singing-regulated egr-1 expression in the ipsilateral and contralateral vocal nuclei (Fig. 2A–C). We used TH staining in VTA–SNc to assess lesion size ratios. Similar to birds that did not undergo surgery (n = 3 FD, n = 3 UD), birds that received the low-concentration dose of 6-OHDA (n = 3 FD, n = 2 UD) and those with lesions dorsal to VTA–SNc (n = 2 FD, n = 2 UD) had VTA–SNc lesion size ratios at or near 1.0 (0.8–1.2; remaining unlesioned part : intact side). The latter two groups were thus designated sham-operated controls (n = 5 FD, n = 4 UD of the 24 birds that underwent surgery). The remaining birds that received the high-concentration dose of 6-OHDA in VTA–SNc (n = 6 FD, n = 5 UD, n = 4 SA) had lesion size ratios that ranged from 0 to 0.7. Proportional to VTA–SNc lesion size ratios was a reduction in TH ratios in the ipsilateral : contralateral LAreaX and surrounding striatum (Fig. 2A and D). Proportional to the reduction in the TH ratios in ipsilateral : contralateral LAreaX was a concomitant reduction of the egr-1 expression ratios in ipsilateral : contralateral LAreaX after both UD and FD (Fig. 2B and E). The level of the singing-driven egr-1 expression in ipsilateral LAreaX was ~50% lower than in the intact contralateral hemisphere (Fig. 2F; compare the solid line of intact animals, slope = 1.02, with the dashed line of the VTA–SNc-lesioned animals, slope = 0.5). The level of egr-1 expression was also reduced in the surrounding striatum (Fig. 2B and G) though, unlike in vocal nuclei, the level of striatal expression was not related to the amount of singing (r2 = 0.083, P = 0.217, UD; r2 = 0.145, P = 0.09, FD). The TH and egr-1 expression ratios in ipsilateral : contralateral LAreaX of sham-operated birds were not significantly different from birds that did not undergo surgery (P > 0.11, t-test, n = 9 shams, n = 4 intacts), indicating that the above results are specific to the VTA–SNc lesion group.
FIG. 2.
VTA–SNc requirements for singing-regulated gene expression in the song system. (A) Top panels, camera lucida drawing (left) and TH somata immunostaining (right, green) showing unilateral lesion of VTA–SNc. Bottom panels, camera lucida drawing and TH fibre immunostaining (green) showing ipsilateral removal of TH input to AreaX and surrounding striatum. Sections are frontal. (B) Egr-1 expression (white silver grains) in LAreaX after UD and FD of sham-operated (left panels) and unilateral VTA–SNc-lesioned (right panels) males, showing decreased expression in LAreaX and striatum on the lesioned side. The sham-operated bird that sang directed song has high expression in the medial striatum, which may be due to locomotor activity (G. Feenders, H. Mouritsen and E. D. Jarvis, personal communication). (C) Egr-1 expression in HVC and RA of VTA–SNc-lesioned animals, showing decreased expression in ipsilateral RA but not HVC. (D) Correlation of VTA–SNc lesion size ratio and TH fibre intensity ratios in ipsilateral : contralateral LAreaX (n = 24 sham and lesion surgery-treated animals; simple regression). (E) Correlation of ratios of TH fibre intensity and egr-1 expression in ipsilateral : contralateral LAreaX (n = 11 FD and n = 9 UD surgery treated animals; and n = 3 FD and n = 3 UD intact animals; simple regression). (F–K) Correlations of egr-1 expression in different brain regions of intact (n = 3 FD, n = 3 UD) and sham-operated (n = 5 FD, n = 4 UD) animals (solid lines and circles) vs. VTA–SNc-lesioned (n = 6 FD, n = 5 UD) animals (dashed lines and open circles). SA animals that received VTA–SNc lesions were included in the correlations as baseline nonsinging control egr-1 levels. In these graphs, expression levels increased in vocal nuclei of both hemispheres with increasing amounts of song production (no. of songs not shown), except for the striatum (St). The r2, P, and slope (s) values (obtained from simple regressions) above the solid lines are for the intact + sham-operated animals; those below the dashed line are for the VTA–SNc-lesioned animals. The P-values between the solid and dashed lines (obtained from multiple regression) show significant differences between the curves of intact + sham-operated and lesioned groups in LAreaX, RA and striatum adjacent to LAreaX, but not in LMAN, HVC or DM. Equal expression between hemispheres results in a slope of 1. Scale bars, 0.25 mm (A, top panel), 0.5 mm (all others).
Similar to LAreaX, the VTA–SNc lesions also resulted in a 40% lower singing-driven egr-1 expression in ipsilateral song nucleus RA relative to the intact contralateral hemisphere (Fig. 2C and H). In contrast, there was no consistent effect of VTA–SNc lesions on egr-1 expression in LMAN (Fig. 2I) or HVC (Fig. 2C and J), nuclei that project to both LAreaX and RA (Fig. 1A). We noted that expression in medial AreaX, which sits directly below medial MAN, may have been affected (Fig. 2A and B), but we did not assess this quantitatively due to difficulty in locating these small nuclei. TH expression in RA was slightly, but significantly, reduced by lesions of VTA–SNc (average ratio 0.89 ± 0.1, P < 0.001, paired t-test between hemispheres, n = 16 lesioned animals). However, unlike in LAreaX, the TH reduction in RA was not linearly proportional to the amount of egr-1 reduction (r2 = 0.31, P = 0.4, UD; r2 = −0.25, P = 0.44, FD). The reduced egr-1 in RA did not appear to reflect a gross reduction in overall activity levels in RA during singing, as its efferent target vocal nucleus of the midbrain, the dorsal medial nucleus of the midbrain (DM; Fig. 1A), still showed singing-driven egr-1 expression that was similar on the two sides of the brain (Fig. 2K).
To determine whether the observed changes in egr-1 and/ or TH expression in LAreaX and RA were specific to the removal of dopaminergic neurons and not to the removal by 6-OHDA of adjacent catecholaminergic cell groups, such as the more caudally located LoC, we reacted adjacent sections with an antibody against DBH. We found that of the 16 VTA–SNc-lesioned birds, 10 had intact LoC (Fig. 3A), three had very small lesions (DBH intensity ratios of 0.7–0.8, lesion : intact side), and three (two UD singers and one SA) had large LoC lesions (ratios ~0.1; Fig. 3C). Unlike the VTA–SNc lesions (Fig. 2E, F and H), there was no relationship between the degree of LoC damage and the level of egr-1 or TH expression in ipsilateral LAreaX or RA (P > 0.05, r2 = 0.189–0.04).
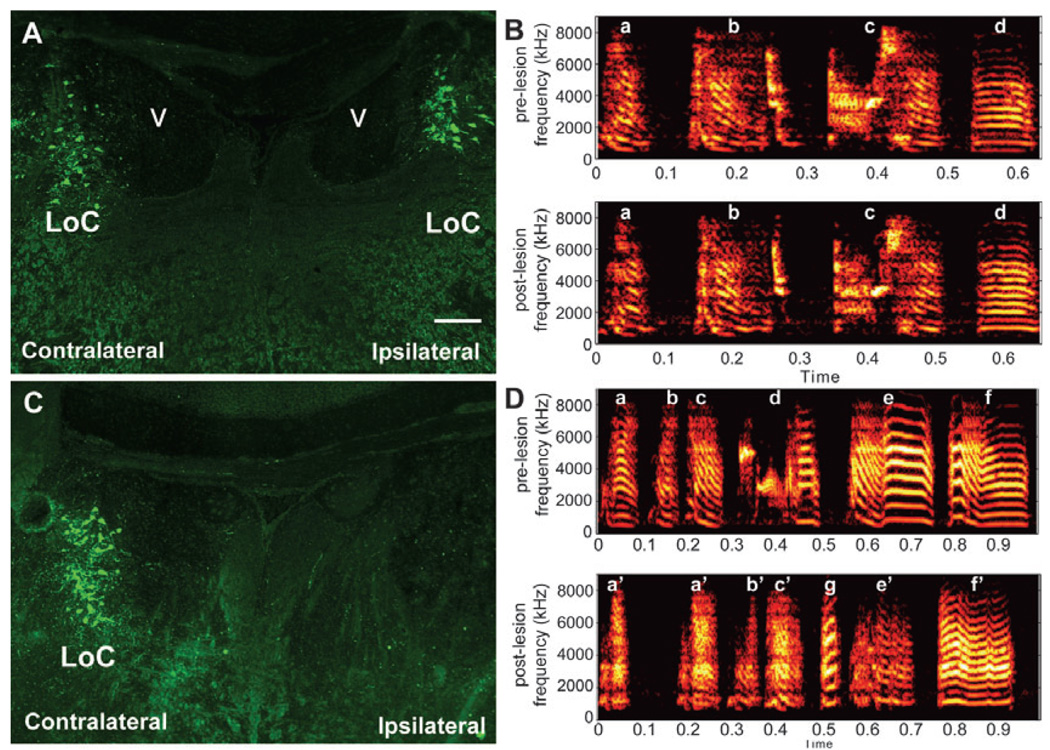
FIG. 3.
Differential effects of VTA–SNc lesions alone and combined VTA–SNc and LoC lesions on singing behaviour. Song motifs of two birds before (pre) and after (post) lesions are available as Supplementary Audio S1–Supplementary Audio S4 (.wav files). (A) Unilateral VTA–SNc-lesioned animal with LoC intact bilaterally (DBH-stained neurons, green). Frontal section. (B) Song motif structure and syntax of the bird in (A) did not show major changes; pre- and post-lesion song motif sequence and syllable structure (a–d) were similar. (C) Unilateral VTA–SNc-lesioned animal with a unilateral LoC lesion (DBH-stained neurons, green). Frontal section. (D) Song structure and syntax of the bird in (C) showed major changes; song syllable structure was degraded (syllables a′, c′, e′ and f′) and motif sequence had deletions (syllable b) and additions (syllable g; Supplementary audio S4). Scale bar, 0.25 mm.
VTA–SNc was required for social context-dependent differences in LMAN and RA
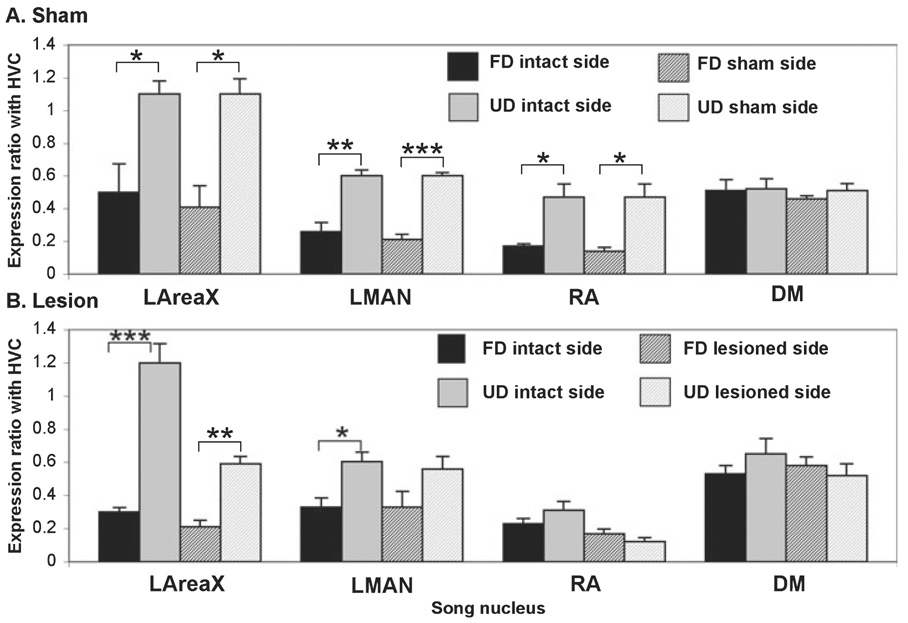
The above analyses assess relative differences between hemispheres but do not assess either each hemisphere independently or social context differences. Based upon the overlap in values of the VTA–SNc-lesioned hemispheres in LAreaX (Fig. 2F, open circles), RA (Fig. 2H) and LMAN (Fig. 2I) it is possible that social context-dependent egr-1 regulation was modified in these nuclei, but these results are also influenced by singing amount. To determine the effects of social context independent of singing amount, we created ratios of egr-1 expression in LAreaX, RA and LMAN relative to HVC (see Materials and Methods). As expected (Jarvis et al., 1998), in the VTA–SNc sham-operated birds, the LAreaX : HVC, LMAN : HVC and RA : HVC egr-1 expression ratios in both hemispheres (sham and intact) were lower after FD than after UD (Fig. 4A), and there was no social context difference in the DM : HVC ratios (Fig. 4A). In the VTA–SNc-lesioned birds, although the LAreaX : HVC expression ratio was reduced relative to sham-lesioned birds (compare lesioned side of Fig. 4B with sham side of Fig. 4A; P = 0.002, t-test), a significant social context-dependent difference remained in LAreaX between FD and UD in the VTA–SNc-lesioned hemisphere (Fig. 4B, patterned bars). In contrast, there was a reduction in the social context differences in LMAN of the VTA–SNc-lesioned hemisphere (Fig. 4B). The reduction was due to a heterogeneous result of higher bilateral expression in LMAN in some of the FD lesioned birds and lower bilateral expression in some of the UD birds, such that the UD and FD expression values were more similar and were no longer significantly different (Fig 2I and Fig 4B). In RA there was an elimination of the social context differences in both the VTA–SNc-lesioned and intact hemispheres (Fig. 4B). The elimination appeared to be due to reduced egr-1 expression during UD (Fig. 4B). In DM, there were still high expression levels in both social contexts (Fig. 4B). Thus the effect of the VTA–SNc lesions on social context-dependent gene regulation appears to be specific to LMAN and RA.
FIG. 4.
Social context-dependent regulation of egr-1 in sham-operated and VTA–SNc-lesioned animals. (A) Expression ratios in vocal nuclei relative to HVC in sham-operated birds (FD, n = 5; UD, n = 4). (B) Expression ratios in vocal nuclei relative to HVC in VTA–SNc-lesioned birds (FD, n = 6; UD, n = 5). Ratios > 1.0 indicate expression levels higher than HVC; ratios < 1.0 indicate expression levels lower than HVC. * P < 0.05, ** P < 0.001, *** P < 0.0001, t-test between FD and UD groups.
Lesion effects on singing behaviour
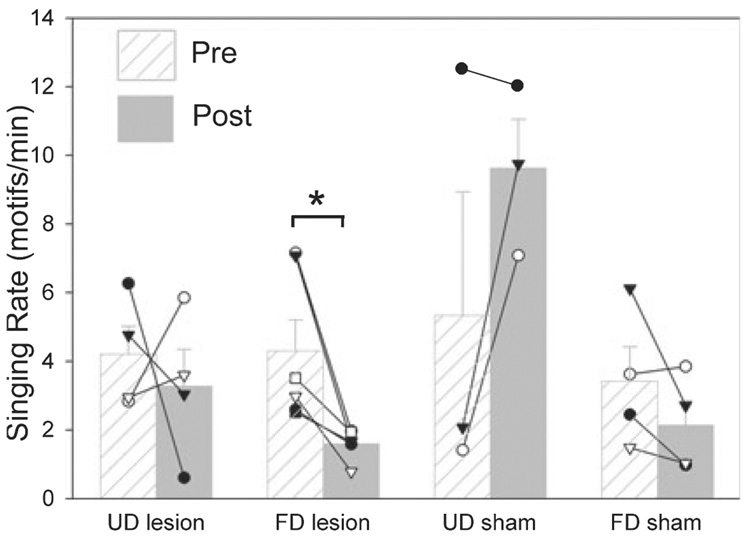
We next investigated whether the unilateral VTA–SNc lesions affected singing behaviour. Following unilateral lesions, the singing rate was significantly decreased when the males produced FD but not when they produced UD (Fig. 5). Relative to sham controls, songs of birds with unilateral lesions only in VTA–SNc did not have noticeable alterations in syllable structure (average ± SEM similarity of pre- vs. post-lesion ratio for sham birds, 0.93 ± 0.01, n = 6; average for VTA–SNc lesion birds, 0.93 ± 0.01, n = 9; P = 0.75, t-test). The relative stereotypy of sequential syllable production was also not affected by unilateral VTA–SNc lesions (average ± SEM ratio of post-vs. pre- for sham birds, 1.00 ± 0.01, n = 6; average for lesioned birds, 0.98 ± 0.01, n = 9; P = 0.23, t-test). In contrast, the three birds that had both unilateral VTA–SNc and LoC lesions showed major changes in song. Two of these birds had marked spectrographic and audible changes in song motif syllable structure (average similarity of pre- vs. post-lesion ratio, 0.69 and 0.28; Fig. 3D and Supplementary audio file, Audio S1–Supplementary audio file, Audio S4). The syllables were nosier and more variable in structure. For the bird with the largest syllable structure change, the overall pattern of syllable stereotypy and sequencing was also altered; the average stereotypy score was reduced from 1.0 before the lesion to 0.80 after, and included deleting old syllables and sometimes inserting a new one in a motif (Fig. 3D, Supplementary Audio S3 and Supplementary Audio S4). The third bird with a combined unilateral lesion to VTA–SNc and LoC did not sing at all in the 7 days after the lesion, during or outside the 45-min recording sessions (as detected with Sound Analysis Pro automated song recording).
FIG. 5.
Quantification of singing rate before and after VTA–SNc lesions. Singing was recorded 1 day before and 5–7 days after VTA–SNc lesions. Birds with the large LoC lesions were not included in this analysis. * P < 0.05, paired t-test.
Discussion
Our gene expression studies show that neurons in VTA–SNc are differentially active during singing in different social contexts. Further, our lesion studies suggest that VTA–SNc is involved in modulating the singing-related activation of the song system. Below we discuss potential mechanisms of the social context-dependent differences in VTA–SNc and its modulation of the song system.
Previous studies in other songbird species (song sparrows and starlings) have shown that expression of IEGs (egr-1 or c-fos) increases in VTA when the birds sing in specific contexts, such as during territorial encounters and during the breeding season (Maney & Ball, 2003; Riters et al., 2004; Heimovics & Riters, 2005). However, the context dependence of directed vs. undirected song was not examined. Here we show in zebra finches that during FD there was high activation of egr-1 in VTA and SNc and that during UD there was very little egr-1 activation. We further found that the high egr-1 activation did not occur within the dopaminergic neurons of VTA–SNc that project to the striatum, but instead in the GABAergic interneurons. The egr-1-expressing cells that were not labelled by either TH- or GABA-specific antibodies could represent neurons that contained subthreshold levels of either antigen, most probably GABA as its signal intensity was lower. This result is consistent with another study of ours conducted in parallel that found singing-regulated neural firing in VTA neurons, where ~80% of these neurons show greater modulation of neural firing during FD than during UD (Yanagihara & Hessler, 2006); the spike widths of these singing-activated neurons were similar to those of GABAergic interneurons recorded in VTA of zebra finch brain slices (Gale & Perkel, 2006). Interestingly, our gene expression results are also parallel to the effect of drugs of abuse on c-fos and delta FosB expression in mammalian VTA–SNc; systemic administration of d-amphetamine or cocaine (dopamine reuptake inhibitors) causes induction of c-fos or delta FosB in the GABAergic interneurons of SNc with no induction in the dopaminergic projection neurons (Hebb & Robertson, 2000; Perrotti et al., 2005). Both drug administration in mammals and FD in zebra finches are accompanied by higher levels of dopamine in the striatum than those seen during drug-free conditions (Phillips et al., 2003) or UD (Sasaki et al., 2006). Taken together, these findings suggest that directed courtship singing and drugs of abuse that affect dopamine transporters may lead to activation of an inhibitory circuit in VTA–SNc. For a possible mechanism of how this occurs during singing, we offer the following hypothesis.
During UD, neurons in VTA–SNc slightly change their firing rate from the tonic firing seen during rest (Yanagihara & Hessler, 2006). This tonic firing may result in the steady low dopamine levels seen in LAreaX (Sasaki et al., 2006) and, along with the presumed singing-related excitatory motor inputs from HVC (Ding et al., 2003), it could result in high-activity-dependent induction of egr-1 in LAreaX (Jarvis et al., 1998; Hessler & Doupe, 1999); tonic firing and low dopamine levels are thought to activate the D1 dopamine receptor which is known to induce egr-1 expression (Gerfen, 2000). However, during FD there appears to be relatively high phasic firing of the presumed GABAergic interneurons in VTA–SNc (this study and Yanagihara & Hessler, 2006), which would be expected to influence the firing pattern of the dopaminergic neurons to become phasic. This activity might not cause egr-1 induction in the dopaminergic projection neurons because such neurons mainly express the D2 and not the D1 subtype of dopamine receptors (Gale & Perkel, 2006) and the D2 receptor inhibits egr-1 expression (Gerfen, 2000). The phasic firing rate could also result in the high levels of dopamine seen in LAreaX during FD (Sasaki et al., 2006). High levels of dopamine are thought to preferentially bind to the D2 receptor (Gerfen, 2000), which may explain the lower firing rates and egr-1 expression in LAreaX during FD (Jarvis et al., 1998; Hessler & Doupe, 1999).
Such a model is consistent with the correlation studies. However, it cannot fully account for the findings of our lesion study. Clearly, the results of the lesion experiments show that, after input from VTA–SNc is removed, some social context differences still remain in LAreaX. The main effect is that LAreaX shows decreased egr-1 expression during singing in both social contexts. Two possible explanations for the discrepancy between the correlation and lesion findings are: (i) there is redundancy such that both VTA–SNc and another brain area modulate the social context-dependent differences of the song system, and when VTA–SNc is lesioned the other source still functions; or (ii) VTA–SNc does not modulate the social context differences of the song system, but instead it enhances the activation of the song system in both contexts and another source of input to VTA–SNc and LAreaX independently regulates the social context differences in VTA–SNc and LAreaX. Another source could be norepinephrine input, as removal of norepinephrine input in the brain prevents the social context differences in egr-1 expression in LAreaX (but not in LMAN or RA; Castelino & Ball, 2005); egr-1 expression in LAreaX during FD was as high as that found during UD. Because the largest source of norepinephrine input to the forebrain is from the LoC (Mello et al., 1998), one possible explanation is that the LoC suppresses neural activity and IEG up-regulation in LAreaX during FD, whereas the VTA–SNc enhances the levels during both FD and UD. Before this scenario can be accepted, several caveats need to be resolved. The LoC was not directly tested in the studies of Castelino & Ball (2005), and the two of our UD animals that had combined LoC and VTA–SNc lesions still showed decreased expression in LAreaX, similar to that which occurs with VTA–SNc lesions alone. Further, although norepinephrine and norepinephrine receptors have been found in AreaX (Harding et al., 1998), norepinephrine fibre labelling in LAreaX and the surrounding striatum is very sparse (Mello et al., 1998). However, a recent study reported that AreaX does receive a projection from the LoC (Castelino et al., 2007), indicating potential direct modulation of LAreaX by both the LoC and the VTA–SNc. Testing these ideas will require further studies involving direct manipulations of LoC and combined VTA–SNc and LoC lesions.
The effects of VTA–SNc lesions on efferent pallial nuclei LMAN and RA are partially consistent with recent findings of ours for LAreaX lesions (Kubikova et al., 2007). In that study, unilateral lesions of LAreaX reduced egr-1 expression in both ipsilateral and contralateral LMAN and RA during UD, and eliminated the social context differences in LMAN and RA. It was proposed that the contralateral effects were due to bilateral transmission through brainstem vocal nuclei. In the current VTA–SNc lesion study, it is possible that the reduced activation of ipsilateral LAreaX caused bilateral effects to downstream vocal nuclei LMAN and RA, with the largest effect on the nucleus furthest downstream, RA. Alternatively for RA, it is possible that loss of the direct sparse projection from VTA–SNc (Appeltants et al., 2002) caused a concomitant indirect effect through LAreaX, resulting in a bigger effect than that seen in LMAN. Interestingly, no effect of our lesions was seen on HVC, even though VTA sends a relatively sparse projection to HVC (Appeltants et al., 2002).
Our behavioural findings suggest that the VTA–SNc may not directly affect song motor output but, rather, may influence the motivation to sing to females. After unilateral VTA–SNc lesions, the rate of FD but not UD was reduced, with no clear alteration in song structure or consistent change in song sequencing. Further support for the lack of direct influence of VTA–SNc on singing motor behaviour is the lack of a correlation between the level of egr-1 expression in VTA–SNc and the number of songs produced. The prior studies that have reported increased egr-1 or c-fos expression in VTA of other songbird species showed that in some cases (Maney & Ball, 2003; Riters et al., 2004) but not others (Heimovics & Riters, 2005) the levels were proportional to the amount of singing. In conjunction with this finding, another recent study showed that c-fos can be increased in zebra finch VTA–SNc during a variety of socially motivated behaviours other than singing (Bharati & Goodson, 2006). Finally, another recent report on starlings showed that dopamine agonists stimulate and antagonists suppress male singing in the presence of females (Schroeder & Riters, 2006). We interpret these findings to indicate that the motivation to sing and singing amount are sometimes related but can also be separate, and that activation of VTA may be more closely related to the motivation level rather than to the motor behaviour. This idea is consistent with findings in mammals, including humans, which show that VTA activation is associated with motivated behaviours (Young & Wang, 2004; Aron et al., 2005; Esch & Stefano, 2005). Confirming this idea will require bilateral VTA and SNc lesions in songbirds.
The main effect we noted on song motor behaviour was in the several birds that had combined unilateral VTA–SNc and LoC lesions. This finding is intriguing as removal of all norepinephrine neurons in the brain, including the LoC, does not cause such changes to song (Castelino & Ball, 2005). Thus, our preliminary results indicate that further detailed investigation will be required to characterize the possible intriguing effects that combined unilateral VTA–SNc and LoC lesions could have on motor and social aspects of singing behaviour.
Finally, other candidate brain regions that are known to be involved in motivation, reward and arousal need to be tested for their possible roles in social context-dependent activation of the song system and singing behaviour. Such candidates include the VMN as revealed by IEG regulation in this study, which is required for courtship cooing of male ring doves (Chen et al., 2006), the preoptic nucleus which is required for the motivation to sing regardless of context (Riters & Ball, 1999), and the mesencephalic central grey which shows increased c-fos expression during territorial intrusion singing and sends dopaminergic projections to HVC and RA (Appeltants et al., 2002; Maney & Ball, 2003). The regulation of the social context gene expression and activity differences is likely to be controlled by an array of brain areas that modulate the song system specifically and the forebrain generally.
Supplementary Material
The following supplementary material may be found on www.blackwell-synergy.com
Audio S1. Pre VTA lesion.
Audio S2. Post VTA lesion.
Audio S3. Pre LoC VTA lesion.
Audio S4. Post LoC VTA lesion.
Fig. S1. Identification of VTA–SNc and VMN.
Acknowledgements
We thank Dr Kazuhiro Wada and Dr Osceola Whitney for use of their egr-1 clones, Dr Wada for advice on experimental procedures, Dr Arthur Arnold for the aromatase antibody, Maurice Anderson for assistance with cutting brain sections, Dr Whitney and Dr Shin Yanagihara for useful discussions, and Dr Whitney for critical reading of the manuscript. Support contributed by RIKEN Brain Science Institute to N.A.H., and NIH R01MH62083 and NIH Director’s Pioneer Award to E.D.J.
Abbreviations
- 6-OHDA
6-hydroxydopamine
- AreaX
area X of the striatum
- DBH
dopamine beta-hydroxylase
- DLM
dorsal lateral nucleus of the medial thalamus
- DM
dorsal medial nucleus of the midbrain
- FD
female-directed singing
- GABA
gamma-aminobutyric acid
- ICC
immunocytochemistry
- IEG
immediate–early gene
- LMAN
lateral MAN
- LAreaX
lateral AreaX
- LoC
locus coeruleus
- MAN
magnocellular nucleus of the nidopallium
- nXIIts
12th nucleus, tracheosyringeal part
- RA
robust nucleus of the arcopallium
- SA
silent alone
- SF
silent with a female
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- UD
undirected singing
- VMN
ventromedial nucleus of the hypothalamus
- VTA
ventral tegmental area
References
- Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J. Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- Bharati IS, Goodson JL. Fos responses of dopamine neurons to sociosexual stimuli in male zebra finches. Neuroscience. 2006;143:661–670. doi: 10.1016/j.neuroscience.2006.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata) Eur. J. Neurosci. 2005;21:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Diekamp B, Ball GF. Noradrenergic projections to the song control nucleus Area X of the medial striatum in male zebra finches (Taeniopygia guttata) J. Comp. Neurol. 2007;502:544–562. doi: 10.1002/cne.21337. [DOI] [PubMed] [Google Scholar]
- Chen G, Bonder EM, Cheng MF. Lesion-induced neurogenesis in the hypothalamus is involved in behavioral recovery in adult ring doves. J. Neurobiol. 2006;66:537–551. doi: 10.1002/neu.20247. [DOI] [PubMed] [Google Scholar]
- Ding L, Perkel DJ, Farries MA. Presynaptic depression of glutamatergic synaptic transmission by D1-like dopamine receptor activation in the avian basal ganglia. J. Neurosci. 2003;23:6086–6095. doi: 10.1523/JNEUROSCI.23-14-06086.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esch T, Stefano GB. The neurobiology of love. Neuro. Endocrinol. Lett. 2005;26:175–192. [PubMed] [Google Scholar]
- Foster EF, Bottjer SW. Lesions of a telencephalic nucleus in male zebra finches: Influences on vocal behavior in juveniles and adults. J. Neurobiol. 2001;46:142–165. doi: 10.1002/1097-4695(20010205)46:2<142::aid-neu60>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. Physiological properties of zebra finch ventral tegmental area and substantia nigra pars compacta neurons. J. Neurophysiol. 2006;96:2295–2306. doi: 10.1152/jn.01040.2005. [DOI] [PubMed] [Google Scholar]
- Gerfen CR. Molecular effects of dopamine on striatal-projection pathways. Trends Neurosci. 2000;23:S64–S70. doi: 10.1016/s1471-1931(00)00019-7. [DOI] [PubMed] [Google Scholar]
- Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J. Neurobiol. 1998;34:329–346. [PubMed] [Google Scholar]
- Hebb MO, Robertson HA. Identification of a subpopulation of substantia nigra pars compacta gamma-aminobutyric acid neurons that is regulated by basal ganglia activity. J. Comp. Neurol. 2000;416:30–44. doi: 10.1002/(sici)1096-9861(20000103)416:1<30::aid-cne4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J. Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat. Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Jarvis ED. Learned birdsong and the neurobiology of human language. Ann. NY Acad. Sci. 2004;1016:749–777. doi: 10.1196/annals.1298.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc. Natl Acad. Sci. USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kubikova L, Turner EA, Jarvis ED. The pallial–basal ganglia pathway modulates the behaviorally-driven gene expression of the motor pathway. Eur. J. Neurosci. 2007;25:2145–2160. doi: 10.1111/j.1460-9568.2007.05368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis JW, Ryan SM, Arnold AP, Butcher LL. Evidence for a catecholaminergic projection to area X in the zebra finch. J. Comp. Neurol. 1981;196:347–354. doi: 10.1002/cne.901960212. [DOI] [PubMed] [Google Scholar]
- Liprando LA, Miner LH, Blakely RD, Lewis DA, Sesack SR. Ultrastructural interactions between terminals expressing the norepinephrine transporter and dopamine neurons in the rat and monkey ventral tegmental area. Synapse. 2004;52:233–244. doi: 10.1002/syn.20023. [DOI] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J. Neurobiol. 2003;56:163–170. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: immunocytochemical study of dopamine-beta-hydroxylase. J. Comp. Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc. Natl Acad. Sci. USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagatsu T, Ichinose H. Molecular biology of catecholamine-related enzymes in relation to Parkinson’s disease. Cell. Mol. Neurobiol. 1999;19:57–66. doi: 10.1023/A:1006912523846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Bolanos CA, Choi KH, Russo SJ, Edwards S, Ulery PG, Wallace DL, Self DW, Nestler EJ, Barrot M. DeltaFosB accumulates in a GABAergic cell population in the posterior tail of the ventral tegmental area after psychostimulant treatment. Eur. J. Neurosci. 2005;21:2817–2824. doi: 10.1111/j.1460-9568.2005.04110.x. [DOI] [PubMed] [Google Scholar]
- Phillips PE, Stuber GD, Heien ML, Wightman RM, Carelli RM. Subsecond dopamine release promotes cocaine seeking. Nature. 2003;422:614–618. doi: 10.1038/nature01476. [DOI] [PubMed] [Google Scholar]
- Pinaud R, Velho TA, Jeong JK, Tremere LA, Leao RM, von Gersdorff H, Mello CV. GABAergic neurons participate in the brain’s response to birdsong auditory stimulation. Eur. J. Neurosci. 2004;20:1318–1330. doi: 10.1111/j.1460-9568.2004.03585.x. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce L, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter GF, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J. Comp. Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Hormones Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behav. Brain Res. 2004;155:307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J. Comp. Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J. Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J. Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder MB, Riters LV. Pharmacological manipulations of dopamine and opioids have differential effects on sexually motivated song in male European starlings. Physiol. Behav. 2006;88:575–584. doi: 10.1016/j.physbeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Sossinka R, Bohner J. Song types in the zebra finch (Poephila guttata castanotis) Z. Tierpsychol. 1980;53:123–132. [Google Scholar]
- Tchernichovski O, Lints TJ, Deregnaucourt S, Cimenser A, Mitra PP. Studying the song development process: rationale and methods. Ann. NY Acad. Sci. 2004;1016:348–363. doi: 10.1196/annals.1298.031. [DOI] [PubMed] [Google Scholar]
- Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP. A procedure for an automated measurement of song similarity. Anim. Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- Wada K, Howard JT, McConnell P, Whitney O, Lints T, Rivas MV, Horita H, Patterson MA, White SA, Scharff C, Haesler S, Zhao S, Sakaguchi H, Hagiwara M, Shiraki T, Hirozane-Kishikawa T, Skene P, Hayashizaki Y, Carninci P, Jarvis ED. A molecular neuroethological approach for identifying and characterizing a cascade of behaviorally regulated genes. Proc. Natl Acad. Sci. USA. 2006;103:15212–15217. doi: 10.1073/pnas.0607098103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Sakaguchi H, Jarvis ED, Hagiwara M. Differential expression of glutamate receptors in avian neural pathways for learned vocalization. J. Comp. Neurol. 2004;476:44–64. doi: 10.1002/cne.20201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur. J. Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nat. Neurosci. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]
- Zann RA. The Zebra Finch: A Synthesis of Field and Laboratory Studies. New York: Oxford University Press; 1996. Chapter 10: Vocalizations; pp. 196–247. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The following supplementary material may be found on www.blackwell-synergy.com
Audio S1. Pre VTA lesion.
Audio S2. Post VTA lesion.
Audio S3. Pre LoC VTA lesion.
Audio S4. Post LoC VTA lesion.
Fig. S1. Identification of VTA–SNc and VMN.