Abstract
4-1BB and 4-1BBL can control adaptive immunity, but we demonstrated that their interaction also suppressed myelopoiesis. 4-1BBL was found to be expressed on hematopoietic stem cells, and differentiating common myeloid (CMPs) and granulocyte-macrophage progenitors (GMPs), and 4-1BB was inducible on activated myeloid progenitors. Steady state numbers of GMPs, myeloid-lineage cells, and mature dendritic cells, were elevated in 4-1BB- and 4-1BBL-deficient mice, indicative of a negative functional role, and this was confirmed in bone marrow chimeras and in vitro where the absence of 4-1BB/4-1BBL interactions led to enhanced differentiation into dendritic cell lineages. The regulatory activity was mediated through 4-1BBL, with binding by 4-1BB inhibiting differentiation of myeloid progenitors. Thus, 4-1BB and 4-1BBL have a novel function in limiting myelopoiesis and dendritic cell development.
INTRODUCTION
4-1BB (also known as CD137 and TNFRSF9), a member of the tumor-necrosis factor receptor (TNFR) superfamily, was first identified as an inducible costimulatory molecule on activated T cells1. The ligand of 4-1BB (which is variously called 4-1BBL, CD137L or TNFSF9), also a TNF superfamily member, was later found expressed on activated antigen-presenting cells (APCs) such as B cells, macrophages and dendritic cells (DCs)2,3. The interaction of 4-1BB with 4-1BBL strongly regulates immunity and has been proposed to preferentially control T cell responses based on studies in various murine models of cancer, infectious disease and autoimmune disease4,5. Much of the early literature on 4-1BB–4-1BBL interactions was derived from studies of agonist 4-1BB antibodies and Tnfsf9−/−mice5, demonstrating a positive regulatory role in T cell priming6,7, and suggesting that signals from 4-1BB costimulate T cells. In contrast, more recent data suggest that the biology of 4-1BB is much more complex than simply positively regulating T cell immunity. Splenocytes from Tnfrsf9−/− mice displayed hyperproliferation to mitogens8 and adoptive transfer experiments with antigen-specific T cells that could not express 4-1BB demonstrated enhanced rather than suppressed initial CD4+ and CD8+ T cell responses in vivo9,10. Furthermore, other publications with agonist anti-4-1BB have demonstrated suppression of inflammation and autoimmunity in multiple settings11, data that might be explained by promoting regulatory mechanisms or through a bona fide suppressive activity.
Although originally thought to be exclusive to T cells, expression of 4-1BB in vivo might be quite broad. It has been found on natural killer (NK) cells12 and additionally inducible on certain myeloid lineage cells such as DCs13–15, granulocytes16 and mast cells17. Furthermore, DCs and mast cells express both 4-1BB and 4-1BBL upon activation, possibly at the same time, and crosslinking of 4-1BB on these cells can regulate cellular function14,17. Tnfrsf9−/− and Tnfsf9−/− mice show no apparent defects in the development of T and B lymphocytes and lymphoid organs8,18, whereas transgenic expression of 4-1BBL under the control of the major histocompatibility complex (MHC) class II promoter resulted in selective depletion of B cells and expansion of Mac-1+ cells19. Intriguingly, Tnfrsf9−/− cells from spleen, blood and bone marrow showed enhanced myeloid colony-forming potential in vitro8. Collectively, these results have suggested the possibility that 4-1BB–4-1BBL interactions might play a role in cellular development and differentiation, particularly within the myeloid lineage. Here, we report that 4-1BBL was found to be expressed on hematopoietic stem cells (HSCs), myeloid progenitor cells including common myeloid and granulocyte-macrophage progenitors (CMPs and GMPs), as well as differentiating myeloid-lineage cells, and 4-1BB was inducible on the latter cells by cytokines that regulate myelopoiesis. We show that the interaction of these molecules functionally suppressed early steps of myelopoiesis, and limited the differentiation of monocytes and DCs, including conventional and plasmacytoid DC lineages. Our results define a novel previously unidentified regulatory role of 4-1BB–4-1BBL interaction in the development of myeloid-lineage cells and DCs.
RESULTS
4-1BBL is expressed on HSCs and progenitors
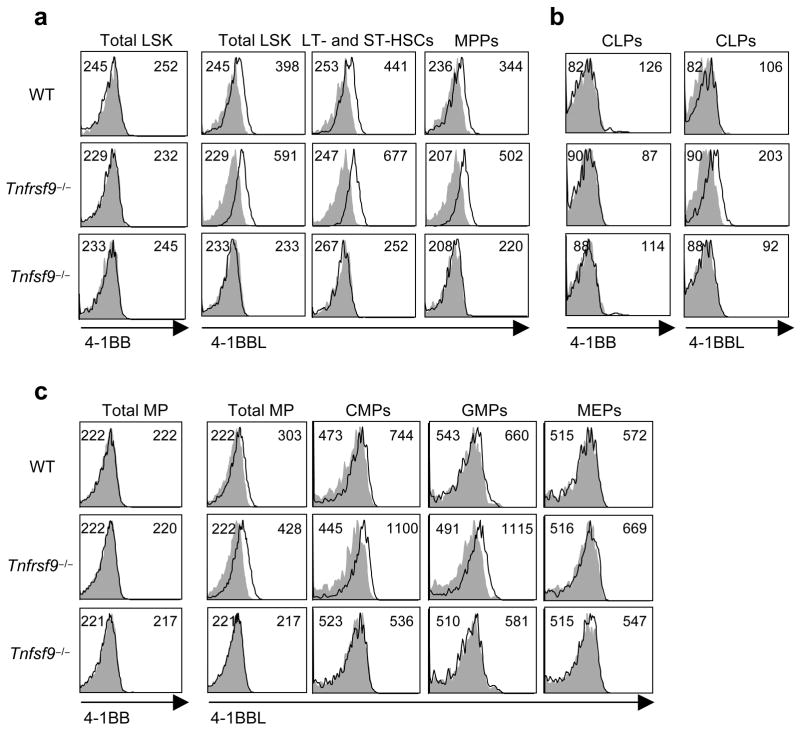
We assessed the expression of 4-1BB and 4-1BBL in the bone marrow. Recent characterization of bone marrow cells has enabled identification of the stepwise development of HSCs and progenitors20–22. Bone marrow cells were grouped into several populations (Fig. 1). These included LSK cells (Lin−Sca-1+c-Kithi interleukin 7 receptorα−; IL-7Rα−), encompassing long and short-term hematopoietic stem cells (LT- and ST-HSCs) and multipotent progenitors (MPPs); myeloid progenitors (Lin−Sca-1−c-KithiIL-7Rα−) that include common myeloid progenitors (CMPs), granulocyte-macrophage progenitors (GMPs) and megakaryocyte-erythrocyte progenitors (MEPs); and common lymphoid progenitors (CLPs, Lin−Sca-1loc-KitloIL-7Rα+). Membrane expression of 4-1BBL is regulated upon binding to 4-1BB, a common phenomenon in the TNF family, likely through cleavage from the cell membrane, and therefore cells from both wild-type and Tnfrsf9−/− mice were analyzed (Fig. 1). Importantly, we detected 4-1BBL on LT- and ST-HSCs and MPPs (Fig. 1a), as well as CLPs (Fig. 1b) and CMPs and GMPs, but not MEPs (Fig. 1c). Upon differentiation, 4-1BBL expression was lost in mature cells of those specific lineages (B and T cells, granulocytes, and monocytes) in the bone marrow or periphery (Supplementary Fig. 1 online and data not shown). Thus, 4-1BBL is available on HSCs and progenitor cells within the bone marrow, suggesting a possible role for in early steps of hematopoiesis.
Figure 1.
4-1BBL is expressed on hematopoietic stem cells and progenitors. Wild-type (WT), Tnfrsf9−/− and Tnfsf9−/− bone marrow cells were analyzed with multi-color staining. Lineage positive cells were gated out by staining with CD11b, CD3, B220, TER-119, Gr-1, and CD11c. Lineage negative cells were further characterized as described in methods. The empty histograms represent the staining of 4-1BB or 4-1BBL and the shaded histograms represent isotype controls. Mean fluorescent intensities (MFI) of isotype control and 4-1BB or 4-1BBL are indicated in the top left and right of each histogram, respectively. Data are representative of three to five independent experiments.
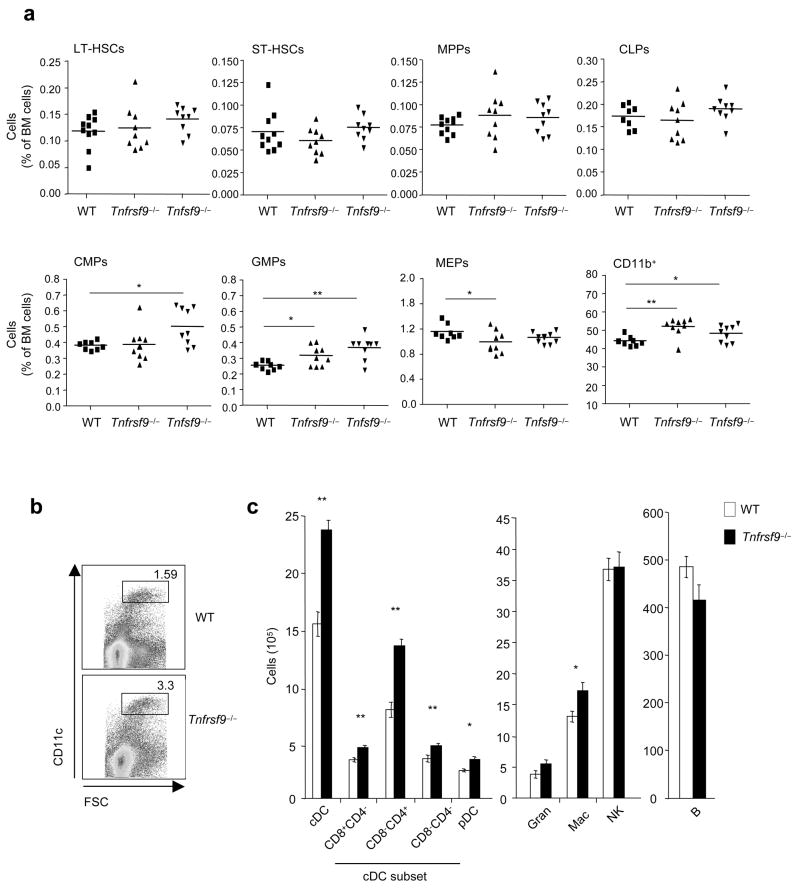
4-1BBL was found on HSCs and MPPs regardless of whether cells were derived from wild-type or Tnfrsf9−/− mice, but expression on CLPs, CMPs, and GMPs was largely confined to the situation where 4-1BB was absent (Fig. 1). This result implies that 4-1BBL on CLPs, CMPs, and GMPs is being actively engaged in situ by 4-1BB within the bone marrow, but 4-1BBL on HSCs and MPPs might not be active under steady-state conditions. In line with 4-1BB–4-1BBL playing a role in hematopoiesis, analysis of steady-state numbers of stem cells and progenitor cells within the bone marrow of adult 4-1BB- and 4-1BBL-deficient mice revealed enhanced percentages of GMPs and CD11b+ myeloid lineage cells (Fig. 2a). This finding promoted the unexpected notion that these molecules have a suppressive or rate limiting function in early steps of myelopoiesis. Further supporting this conclusion, enhanced percentages of CD11chi dendritic cells were found in the spleen in the absence of 4-1BB–4-1BBL interactions (Fig. 2b), predominantly reflected by increased numbers of CD4+CD8− conventional DCs, although statistically significant differences in numbers were found for all DC subsets including pDCs (Fig. 2c). These data suggest that 4-1BBL on CMPs or GMPs, or differentiating progeny of these cells, via engagement of 4-1BB, represents a rate-limiting step in myelopoiesis leading to differentiation of DCs that accumulate in lymphoid organs. Although CLPs were found to express 4-1BBL, no difference in their steady-state numbers was evident (Fig. 2a), and no difference in peripheral B lineage (follicular, marginal zone, B-1a) cells (Fig. 2c) or T lineage (conventional T, regulatory T, NKT) cells (not shown) was found in Tnfrsf9−/−animals, implying that 4-1BBL does not play a major role on CLPs under normal conditions of lymphopoiesis.
Figure 2.
Enhanced frequencies of GMPs and myeloid lineage cells in the bone marrow, and dendritic cells in the spleen, in the absence of 4-1BB–4-1BBL interaction. (a) Wild-type (WT), Tnfrsf9−/− and Tnfsf9−/− bone marrow cells were analyzed from 7–9 week-old female mice with multi-color staining as indicated in methods. Frequency of stem cells (LT- and ST-HSCs), progenitors (MPPs, CLPs, CMPs, GMPs and MEPs), and myeloid-lineage cells (CD11b+) as a percentage of total bone marrow cells were analyzed. Mean value shown as well as data from individual mice. *, P < 0.05, and **, P < 0.01, versus wild-type control. (b,c) Splenocytes from wild-type and Tnfrsf9−/− mice (10–11 week-old female) were analyzed for various lineages of cells with multi-color staining as indicated in methods. (b) A representative plot of CD11c vs. FSC, showing percent CD11chi cells. (c) Total numbers of each cell subset per spleen. Each bar represents mean value ± S.E.M. from eight mice. *, P < 0.05, and **, P < 0.01, versus control. Data are representative of two to three independent experiments.
4-1BB on myeloid progenitors limits myelopoiesis
The source(s) of 4-1BB then became of interest. We could not detect 4-1BB on any stem cell or progenitor population in the bone marrow in situ, except for a small percentage (2–3%) of CLPs (Fig. 1b). Bone marrow NK cells and some CD8+ T cells were found to express 4-1BB (Supplementary Fig. 1 and data not shown), likely reflecting peripherally activated cells trafficking in and out of this organ. These cells then might represent one potential source of 4-1BB to ligate 4-1BBL.
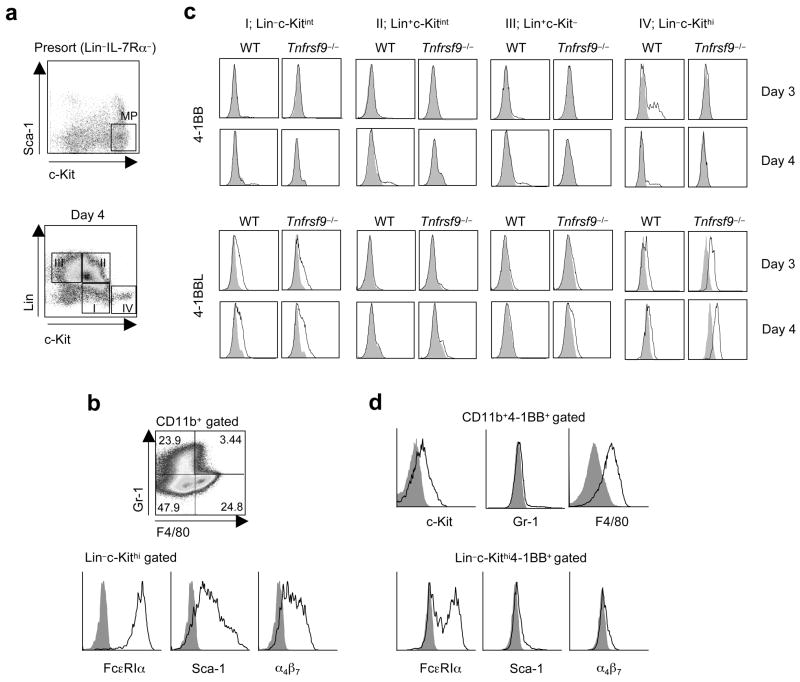
Another potential source might be a differentiating progenitor cell that participates in cell intrinsic or extrinsic regulation and a lack of detection of 4-1BB in the bone marrow in situ reflects very transient expression induced by cytokines that promote myelopoiesis. To test this notion, we cultured purified total myeloid progenitors (Lin−Sca-1−c-KithiIL-7Rα−; Fig. 3a) with a cytokine cocktail (SCF, IL-11, IL-3, TPO and GM-CSF) known to support in vitro myelopoiesis23,24. The majority of myeloid progenitors downregulated c-Kit expression to intermediate amounts after 1 day and showed a stepwise differentiation into myeloid-lineage cells (Lin+c-Kit−; group III) via a transitional cell population (Lin−c-Kitint; group I and Lin+c-Kitint (group II); Fig. 3a and Supplementary Fig. 2a online). We also found a subpopulation of cells that retained myeloid progenitor-like markers, in being Lin−c-Kithi (group IV), that were visualized from days 2–6, while maintaining their surface phenotype (Fig. 3a and Supplementary Fig. 2a). Further characterization of Lin+ cells showed that 95% were CD11b+ (not shown) and some gained F4/80 or Gr-1 expression at day 4 (Fig. 3b) indicative of differentiating into the monocyte and granulocyte lineages. The majority of Lin−c-Kithi cells expressed immunoglobulin receptor FcεRIα, Sca-1, and integrin α4β7, suggesting that these cells were differentiating precursors of mature mast cells25 (Fig. 3b).
Figure 3.
4-1BBL and 4-1BB are expressed on differentiating myeloid-lineage cells. Total myeloid progenitors (MP, Fig. 1) were sorted from wild-type (WT) and Tnfrsf9−/− bone marrow and cultured with SCF, IL-11, IL-3, GM-CSF, and TPO. (a) Lin−IL-7Rα− cells were stained with Sca-1 versus c-Kit, and cells subsequently sorted as MP as indicated (top, insert). Cells were further analyzed for lineage markers (CD11b, Gr-1 and TER-119) versus c-Kit 4 days after cytokine stimulation (bottom). Cells were divided into four groups as Lin−c-Kitint (I), Lin+c-Kitint (II), Lin+c-Kit− (III), and Lin−c-Kithi (IV). (b) Characterization of Lin+ (CD11b+) (top) and Lin−c-kithi cells (bottom) at d4. (c) Expression of 4-1BB or 4-1BBL on various subsets on d3 and d4. (d) Characterization of 4-1BB+ cells within Lin+(CD11b+) and Lin−c-Kithi populations at d4 and d3 respectively. Data are representative of two to three independent experiments.
4-1BB was induced on sub-populations of Lin+ cells increasing from days 3–6 (Fig. 3c and Supplementary Fig. 2b,c). Additionally, 4-1BB was prominent on the surface of Lin−c-Kithi cells at day 3, but expression was more transient. Lin+ cells that expressed 4-1BB were CD11bhic-Kit+/− and the majority expressed F4/80 with only 3–5% being Gr-1+ (Fig. 3d), and therefore represented myeloid progenitor cells differentiating into the monocyte lineage. Lin−c-Kithi cells that expressed 4-1BB were FcεRIα+/− and had low surface expression of Sca-1 and α4β7 (Fig. 3d), confirming that 4-1BB was also inducible on differentiating mast cell precursors. 4-1BBL was expressed on the Lin−c-Kitint population throughout culture, but down-regulated almost completely on Lin+c-Kitint cells (Fig. 3c and Supplementary Fig. 2b,c). When cells further differentiated into Lin+c-Kit− cells, 4-1BBL was again visualized but at lower abundance. 4-1BBL was also present on the surface of Lin−c-Kithi cells throughout culture. In most cases, 4-1BBL expression was higher on 4-1BB-deficient cells, suggesting active ligation with 4-1BB was occurring in these cultures. Interestingly, when myeloid progenitors were cultured with different cytokine combinations that can stimulate proliferation but less differentiation, we found that both Lin−c-Kithi and myeloid-lineage cells lacked 4-1BB, and expression depended on GM-CSF (Supplementary Fig. 3 online). In contrast, 4-1BBL was observed on the Lin−c-Kitint cells, regardless of cytokines added in culture (data not shown).
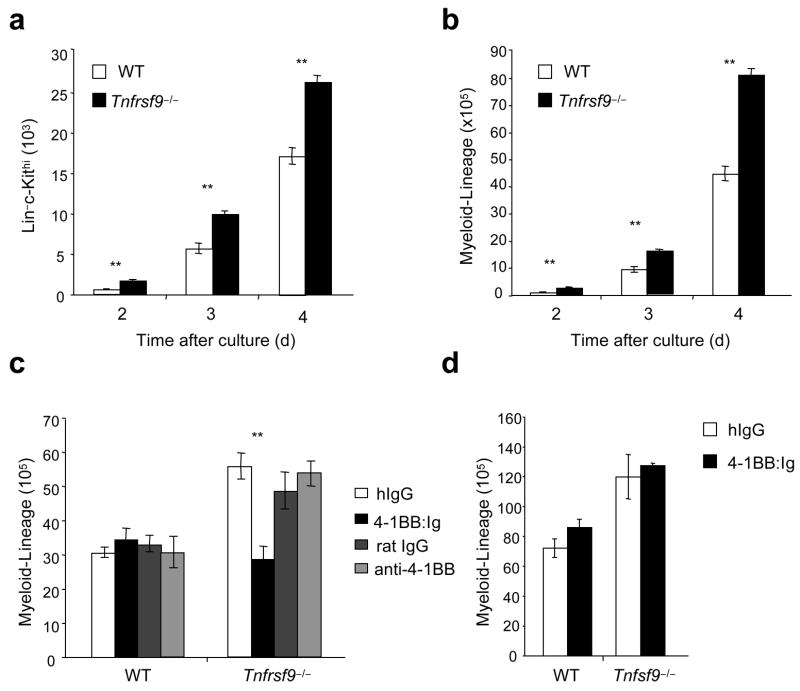
Thus, 4-1BB expressed on differentiating myeloid-lineage cells and mast cells precursors, along with 4-1BB on bone marrow resident CD8+ T cells and NK cells, could partner with 4-1BBL on CMPs, GMPs and/or cells derived from these progenitors differentiating into the monocyte or DC lineage. Supporting the notion that the source of 4-1BB in vivo is likely to be differentiating myeloid and mast cell precursors and confirming a regulatory rather than a stimulatory role, we found enhanced numbers of Lin−c-Kithi mast cell precursors (Fig. 4a) and Lin+c-Kit− myeloid-lineage cells (Fig. 4b–d) were generated in vitro over time when purified Tnfrsf9−/− or Tnfsf9−/− myeloid progenitors were differentiated in culture. To further explore this regulatory event, an agonistic antibody to 4-1BB26, and 4-1BB:Ig delivered in plate-bound form to crosslink 4-1BBL, were added into myeloid progenitor cultures. Agonistic stimulation of 4-1BB had no substantial effect on myelopoiesis. In contrast, 4-1BB:Ig strongly reduced the accumulation of Tnfrsf9−/− myeloid-lineage cells back to numbers seen with wild-type cells (Fig. 4c), but could not reverse outgrowth of Tnfsf9−/− myeloid progenitors (Fig. 4d). Thus, 4-1BB binding to 4-1BBL on differentiating myeloid progenitors (CMPs and GMPs) and/or myeloid-lineage cells has a suppressive effect on the accumulation of myeloid-lineage cells, brought about by inhibitory signaling through 4-1BBL. This action is analogous to previous results that showed that crosslinking 4-1BBL suppressed the differentiation and/or outgrowth of osteoclasts from bone marrow macrophages in response to RANKL27,28.
Figure 4.
4-1BB–4-1BBL interactions negatively regulate myelopoiesis in vitro. Myeloid progenitors from wild-type (WT), Tnfrsf9−/− and Tnfsf9−/− mice were cultured with cytokines as in Fig. 3. (a,b) Numbers of Lin−c-Kithi (a) and myeloid-lineage (Lin+c-Kit−) cells (b) over time. (c,d) Myeloid progenitors were stimulated with plate-bound 4-1BB:Ig and control human Ig, or soluble anti-4-1BB (3H3) and control rat Ig. Numbers of myeloid-lineage cells at day 4 (c) and day 5 (d) are shown. Each bar represents mean ± S.E.M. of triplicates. **, P < 0.01, versus control. Data are representative of two to three independent experiments.
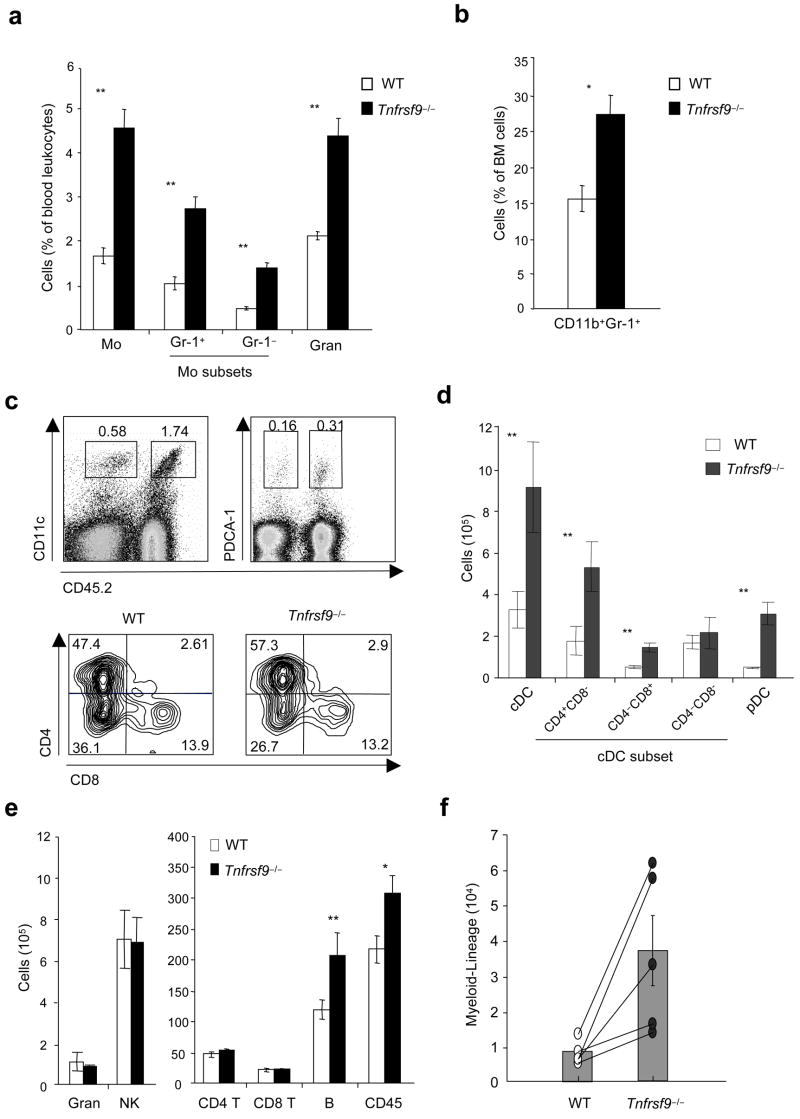
4-1BB negatively regulates myelopoiesis and DC development
To further show a suppressive role for 4-1BB–4-1BBL interactions in myelopoiesis in vivo, we performed competitive repopulation experiments with 1:1 mixtures of wild-type (CD45.1+) and Tnfrsf9−/− (CD45.2+) B- and T cell-depleted bone marrow cells transferred into lethally irradiated (CD45.1+) recipients. Eight weeks after reconstitution, the major population of blood monocytes appeared as CD45.2+ originating from Tnfrsf9−/− bone marrow (Fig. 5a). This population included both Gr-1+ and Gr-1− subsets that have been suggested to possess alternate potential to differentiate into DCs and macrophages29. In parallel, development of blood granulocytes was favored in the absence of 4-1BB. Analysis of bone marrow, in which initial myelopoiesis takes place leading to output into the blood, further demonstrated a greater proportion of CD11b+Gr-1+ cells of Tnfrsf9−/− origin (Fig. 5b).
Figure 5.
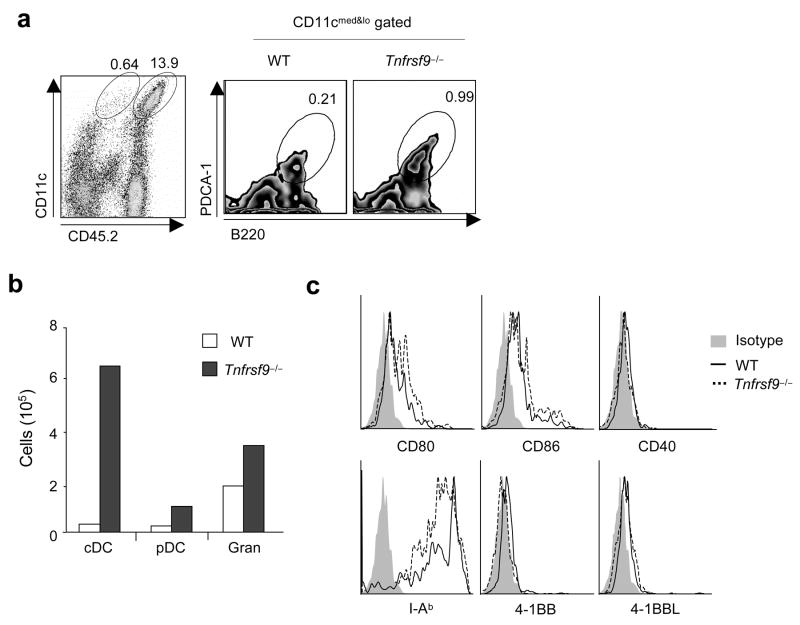
4-1BB limits myelopoiesis and development of dendritic cells and B cells. (a–e) Mixed bone marrow chimeras were developed by injecting lethally irradiated wild-type (WT) mice with a 1:1 mixture of wild-type (CD45.1+) and Tnfrsf9−/− (CD45.2+) bone marrow, and were analyzed for the reconstitution of cells in blood and bone marrow (after 8 weeks), and spleen (after 10 weeks). (a) Percentages of monocytes and granulocytes within total white cells in blood. Monocyte subsets were further divided into Gr-1+ or Gr-1−. (b) Proportion of myeloid-lineage cells (CD11b+Gr-1+) within total leukocytes in bone marrow. (c) Percentages of cDCs (left) and pDCs (right) within total splenocytes (top). cDC subsets in spleen (bottom), gating on CD11chi and subdivided based on CD8 and CD4 expression. Percentages within CD11chi cells indicated. (d,e) Actual total numbers of each indicated cell subset in spleen. White and black bars represent cells originating from wild-type bone marrow (CD45.1+) and Tnfrsf9−/− bone marrow (CD45.2+), respectively. P value was calculated with paired Student’s t-test. Each bar represents mean value ± S.E.M. from three to four mice. *, P < 0.05, and **, P < 0.01, versus control. All data are representative of two to three experiments. (f) Common myeloid progenitors (CMPs) were sorted from wild-type (CD45.1+) and Tnfrsf9−/− (CD45.2+) mice and a 1:1 mixture was injected into lethally irradiated Rag1−/− (CD45.2+) mice. Development of myeloid-lineage cells was analyzed at day 8 after transfer. Empty and closed circles, representing numbers of wild-type and Tnfrsf9−/− myeloid-lineage cells, respectively, from the same recipient mice are linked as lines. P value was calculated with paired Student’s t-test (p = 0.038). Histograms show mean values ± S.E.M. Data are representative of two independent experiments.
Supporting the idea that 4-1BB limits homeostasis of myeloid-lineage cells, analysis of splenocytes showed that a substantially greater number of DCs in both conventional (cDCs, CD11chi and high MHC class II expression, I-A&I-Ehi) and plasmacytoid (pDCs, CD11cloPDCA-1+I-A&I-Eint) lineages originated from Tnfrsf9−/− bone marrow (Fig. 5c,d). Parallel data were observed in peripheral lymph nodes (data not shown). The lack of 4-1BB favored the development of CD4+CD8− cells with an increased percentage within the cDC population (Fig. 5c), although both CD4+CD8− and CD4−CD8+ populations increased in number (Fig. 5d). No difference in maturation was observed on the DCs, based on expression of CD80, CD86, CD40, and MHC class II molecules (data not shown). This finding replicates and confirms the steady-state phenotype in 4-1BB-deficient mice (Fig. 2c), although the suppressive effect was more pronounced, likely reflective of the increased rate of hematopoiesis in the bone marrow chimeras. In lymphoid organs, similar numbers of granulocytes (CD11b+Gr-1hi) and NK cells (CD3−NK1.1+DX5+), and CD4+ and CD8+ T cells were found derived from wild-type and Tnfrsf9−/− bone marrow. Almost the same pattern of cell development was seen in Rag1−/− recipients of unseparated bone marrow cells (Supplementary Fig. 4a online) and in congenic mice at different time points (Supplementary Fig. 4b and data not shown). Thus, 4-1BB limits the development of DCs that accumulate in lymphoid organs under steady-state conditions and under conditions of strong myelopoiesis. Interestingly, we also observed enhanced numbers of B cells of Tnfrsf9−/− origin in the mixed chimeras in wild-type and Rag1−/− recipients (Fig. 5e and Supplementary Fig. 4a,c), a result not replicated in the steady-state in 4-1BB-deficient animals (Fig. 2c). This result shows that expression of 4-1BBL on CLPs (Fig. 1), or their progeny, can become active and suppressive for B cell development in conditions of strong lymphopoiesis.
Expression of 4-1BB on wild-type bone marrow cells did not compensate for the lack of 4-1BB on the knockout cells in the mixed chimeras. This finding suggested that 4-1BB–4-1BBL interactions are cell intrinsic to differentiating myeloid progenitors and that 4-1BB-expressing T cells and NK cells resident in the bone marrow are unlikely to be the primary mediators of regulation. To extend this notion, we found that Tnfrsf9−/− CMPs, separated from CLPs and HSCs, still exhibited enhanced differentiation into the myeloid lineage in comparison to wild-type CMPs, when transferred into a T cell-deficient Rag1−/− environment (Fig. 5f).
4-1BBL suppresses myelopoiesis and DC development
To verify that the suppressive role of 4-1BB in myelopoiesis and DC development was due to the interaction with 4-1BBL, and to further support the idea that any interaction might be cell intrinsic in responding and differentiating progenitor cells, we generated mixed chimeras with bone marrow from Tnfsf9−/− mice. Replicating the results with Tnfrsf9−/− bone marrow, approximately 70% of DCs in the spleen, in both conventional and plasmacytoid lineages, were found to derive from Tnfsf9−/− bone marrow (Fig. 6a and Supplementary Fig. 4d). Similarly, approximately 5 times as many cDCs in the lymph nodes were of Tnfsf9−/−origin (Fig. 6b). Moreover, a greater proportion of CD11b+Gr-1+ cells of Tnfsf9−/− origin were found within the bone marrow (Fig. 6c). T cell numbers were similar, however, again we found enhanced development of B cells from Tnfsf9−/− bone marrow (Fig. 6a). Collectively, these results show that under conditions of strong hematopoiesis, 4-1BB interacting with 4-1BBL negatively regulates myelopoiesis and B lymphopoiesis in the bone marrow and development of DC and B cells that populate peripheral lymphoid organs.
Figure 6.
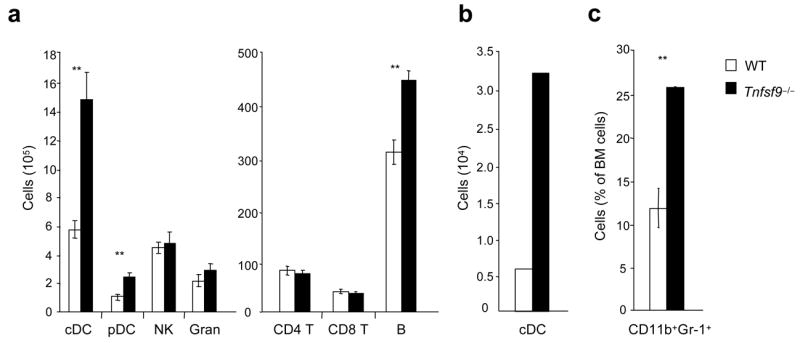
Enhanced myelopoiesis and development of dendritic cells and B cells in the absence of 4-1BBL. Mixed bone marrow chimeras (wild-type, CD45.1+ versus Tnfsf9−/−, CD45.2+) were analyzed for reconstitution of cell populations after 8 weeks. (a,b) Total numbers of each cell subset in spleen (a) and in peripheral lymph nodes (normalized per 107 cells) (b). Lymph nodes were pooled from four mice per group. (c) Percentages of myeloid-lineage cells within total bone marrow leukocytes. Each bar represents mean value ± S.E.M. from four mice (a,c). **, P < 0.01, versus control. Data are representative of two independent experiments.
4-1BB regulates development of DCs in the lung
DCs also reside in many non-lymphoid tissues, such as the lung, liver, gut, kidney and skin30,31, and such DCs have been proposed to arise peripherally from monocytes under the directive influence of inflammatory cytokines such as tumor necrosis factor (TNF) and GM-CSF32,33. In particular, the lung harbors DCs in both conventional and plasmacytoid lineages that regulate pulmonary inflammation34,35. Strikingly, approximately 95% of cDCs (CD11chiI-A&I-Ehi) and 80% of pDCs (CD11cloB220+PDCA-1+) in the lungs of mixed wild-type and Tnfrsf9−/− bone marrow chimeras developed from Tnfrsf9−/− bone marrow (Fig. 7a,b), as well as many granulocytes (Fig. 7b). The lung cDCs exhibited an immature phenotype (CD80loCD86loCD40loI-A&I-Ehi) regardless of origin, although interestingly lung cDC expressed low amounts of both 4-1BB and 4-1BBL that were not found on splenic cDCs (Fig. 7c, and data not shown). These results suggest that 4-1BB and 4-1BBL might also limit peripheral DC development, for example, from monocytes within the lung as well as regulating myeloid progenitor development within the bone marrow.
Figure 7.
4-1BB regulates the accumulation of dendritic cells in the lung. Mixed bone marrow chimeras, set up as in Fig. 5, were analyzed for the reconstitution of cells in the lung at 10 weeks. (a) Flow analysis of cDCs and pDCs (wild-type, CD45.1+ versus Tnfrsf9−/−, CD45.2+). For pDCs, CD11cint populations were gated and then further characterized with PDCA-1 and B220. Percentages within total lymphocyte populations are shown. (b) Actual numbers (normalized per 107 cells) of each cell subset. (c) Expression of surface markers on cDCs. The shaded histograms represent the staining of isotype controls and solid and dashed lines represent the staining of specific markers on cDC originated from wild-type or Tnfrsf9−/−bone marrow cells, respectively. Lung cells were pooled from four mice. Data are representative of two experiments.
4-1BB–4-1BBL limits the accumulation of BM-DCs
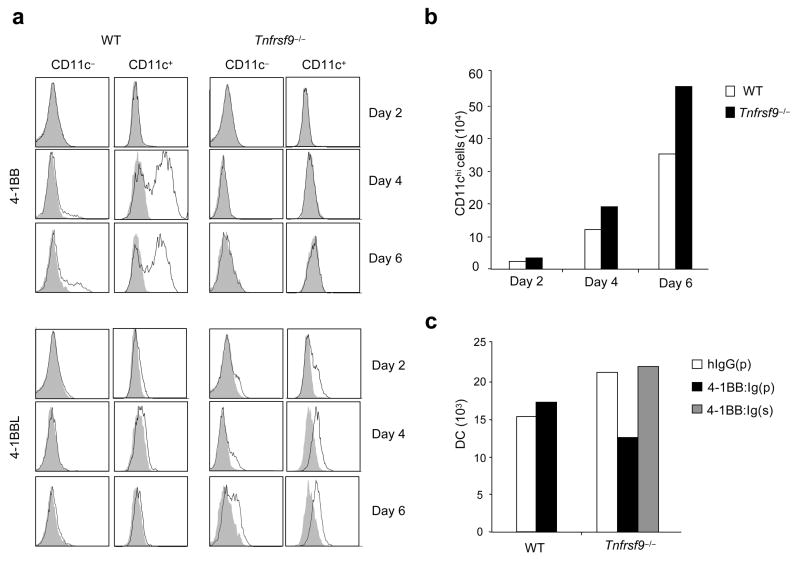
Wild-type or Tnfrsf9−/− bone marrow cells were then stimulated with GM-CSF in vitro32. This culture system has been proposed to resemble peripheral differentiation of monocytes into DCs, with bone marrow progenitors transitioning from a CD11b−CD11c−phenotype into a CD11b+CD11c− monocyte-like population, before expressing CD11c32,36. 4-1BBL was induced on a proportion of CD11c− and CD11c+ cells at day 2 and expression continued through to day 6 (Fig. 8a). Again, 4-1BBL was only detected easily when 4-1BB was not available for binding, providing evidence of active engagement. No 4-1BB could be visualized on day 2, but it was strongly upregulated by day 4 and retained through to day 6 on a small proportion of CD11c− cells and a large proportion of CD11c+ cells. In support of an active regulatory role, greater numbers of CD11chi bone marrow-derived DCs were generated over time from Tnfrsf9−/− bone marrow (Fig. 8b). The absence of 4-1BB again had no effect on maturation (not shown). Thus, both 4-1BB and 4-1BBL are expressed during the differentiation of bone marrow-derived DCs and their interaction limits DC differentiation or outgrowth.
Figure 8.
4-1BB–4-1BBL interactions limit the accumulation of bone marrow-derived dendritic cells (BM-DCs). (a,b) Wild-type (WT) and Tnfrsf9−/− BM cells were cultured with GM-CSF to generate BM-DCs. (a) Flow analysis of 4-1BB and 4-1BBL on either CD11c− or CD11c+ cells over time. Shaded histograms represent isotype controls. (b) Accumulation of BM-DCs (CD11bhiCD11c+) over time. (c) Sorted myeloid progenitors from wild-type and Tnfrsf9−/− mice were cultured with GM-CSF. Where indicated, either plate-bound (p) or soluble (s) 4-1BB:Ig or control Ig was added into cultures. The number of BM-DCs from pooled triplicate cultures were determined at day 7. All data are representative of two to four experiments.
Lastly, myeloid progenitors were sorted from bone marrow and also stimulated with GM-CSF37. Myeloid progenitors (CD11b−CD11c−) differentiated into a monocyte-like phenotype (CD11b+CD11c−) and then into DCs (CD11b+CD11c+ I-A&I-Ehi) and expressed both 4-1BB and 4-1BBL similar to cultures with unseparated bone marrow cells (Supplementary Fig. 5 online and data not shown). Again, accumulation of DCs was enhanced at day 7 in Tnfrsf9−/− myeloid progenitor cultures as compared to wild-type controls (Fig. 8c). Notably, crosslinking 4-1BBL with plate-bound 4-1BB:Ig reduced the accumulation of DCs from Tnfrsf9−/− myeloid progenitors to numbers found in wild-type myeloid progenitor cultures (Fig. 8c). Soluble 4-1BB:Ig, which cannot cross-link 4-1BBL, showed no substantial effect. These data confirm a suppressive role for 4-1BBL in controlling DC differentiation.
To further define the mechanism by which 4-1BBL plays an inhibitory role, we tested whether the ligation of 4-1BBL through 4-1BB:Ig suppresses intracellular signaling in bone marrow cells differentiating into DC. Plate-bound 4-1BB:Ig inhibited the phosphorylation of ERK and IκBα as compared to hIgG control (Supplementary Fig. 6a online). Casein kinase I is the only reported kinase activated by 4-1BBL27, but we found an inhibitor of this molecule could not reverse suppression mediated by 4-1BB:Ig in myeloid-lineage and DC differentiation (Supplementary Fig. 6b and c). Previous work additionally reported that 4-1BBL control of osteoclast development from bone marrow macrophages involved interferon-β and IL-10 production28,38. However, we also failed to alter inhibition through cross-linking 4-1BBL on myeloid progenitors by blocking these cytokines or TGF-β (Supplementary Fig. 6d and e).
DISCUSSION
In this report we show that 4-1BBL was expressed on common myeloid and granulocyte-macrophage progenitor cells that are fundamental to myelopoiesis, and that 4-1BB could be induced on multiple subsets of differentiating myeloid-lineage cells. The interaction of 4-1BB with 4-1BBL negatively regulated the development of myeloid-lineage cells from CMPs and GMPs and limited the differentiation of both conventional and plasmacytoid lineages of DCs. We also demonstrate that 4-1BBL was expressed on common lymphoid progenitors and suppressed development of B cells under conditions of strong lymphopoiesis. Collectively, the results reveal an unappreciated role for these TNF superfamily molecules in controlling highly organized pathways of cellular development and provide new insight into the control of distinct lineages of immune cells.
Although 4-1BBL was ubiquitously expressed on HSCs, the fact that steady-state numbers of these cells and MPPs were not substantially altered in the 4-1BB- or 4-1BBL-deficient animals, suggests that 4-1BBL is not normally active on HSCs. In contrast, steady-state numbers of GMPs and CD11b+ myeloid-lineage cells were elevated in situ in the absence of 4-1BB and 4-1BBL. Additionally, in vitro and in vivo studies with total myeloid progenitors, or purified CMPs, still revealed the suppressive influence of 4-1BBL on myelopoiesis. Thus, we conclude that 4-1BB–4-1BBL interactions regulate the early steps of myelopoiesis, targeting CMPs or GMPs, and/or cells undergoing transition from these cells into myeloid-lineage cells. However, 4-1BBL expression on HSCs might still be highly relevant under certain inflammatory conditions, a subject which awaits future study.
Importantly, when we mimicked 4-1BB binding to 4-1BBL, by providing 4-1BB:Ig in a form that crosslinks 4-1BBL, this interaction suppressed the enhanced accumulation of Tnfrsf9−/− myeloid-lineage cells and DCs derived from unseparated bone marrow cells or from purified myeloid progenitors. In contrast, ligation of 4-1BB with an agonist antibody had no effect. Thus, ‘reverse signaling’ through 4-1BBL is responsible for regulation, providing complementary data to other reports describing a similar phenomenon through 4-1BBL, where impeded cellular proliferation was found in activated T cells and bone marrow macrophages differentiating into osteoclasts8,27,28,39. We have confirmed suppressive signaling by showing inhibition of activation of the growth-promoting NF-κB and ERK intracellular pathways in bone marrow cells differentiating into DCs. However, the nature of the intracellular signals transmitted through 4-1BBL that lead to reduced myelopoiesis is not clear and will need further analysis.
Unlike 4-1BBL that is detected in the bone marrow in situ, 4-1BB was highly regulated on progenitor cells, possibly because in this scenario 4-1BB functions like a typical ligand rather than a signaling receptor, fitting with the concept that its expression might be a rate-limiting factor for myelopoiesis. 4-1BB was found transiently on Lin−c-Kithi differentiating mast cell precursors and also on differentiating myeloid-lineage cells (Lin+c-Kitint) and expression depended on GM-CSF. Similarly, GM-CSF alone strongly promoted 4-1BB in myeloid progenitors differentiating into DC. Thus, it is likely that this sophisticated and transient regulation might explain why 4-1BB was not detected on bone marrow progenitor cells immediately ex vivo.
Our results with differentiating myeloid-lineage cells and DCs derived from unseparated BM cells or purified myeloid progenitors suggest that both 4-1BB and 4-1BBL can be expressed within these lineages, either separately or together, leading to the notion of cell intrinsic regulation and/or cross-talk between the same or alternate stages of cells. The spatio-temporal interactions between 4-1BB and 4-1BBL in the bone marrow might then be quite complex. While 4-1BBL appears to be readily available, 4-1BB could be delivered by multiple sub-populations of cells, either on neighboring cells perhaps in specialized niches, or on the same cell in a cell intrinsic fashion. We found the augmented outgrowth of Tnfrsf9−/−myeloid-lineage cells paralleled that of Tnfsf9−/− myeloid-lineage cells in mixed bone marrow chimeras where wild-type cells (that could express either molecule) were also responding, which supports the notion of cell intrinsic regulation. Furthermore, as total myeloid progenitors or purified CMPs from Tnfrsf9−/− mice still exhibited enhanced myeloid-lineage and DC differentiation, in vitro and in vivo, these results also favor differentiating myeloid-lineage and mast cell precursors as the primary source of 4-1BB in the bone marrow. However, it is still possible that 4-1BB on resident or recirculating mature T and NK cells might modulate myelopoiesis, especially in some inflammatory conditions.
We focused on DCs since these cells are thought largely to develop within the myeloid-lineage30,32,40. DC development is complex and not fully understood. Earlier work identified a clonogenic progenitor, termed a macrophage-DC progenitor (MDP; Lin−CX3CR1+c-Kit+)41, that is a subset of the GMP population (based on its phenotype of CD34+FcγRII/IIIhi) and gives rise to monocytes, macrophages and cDCs. Further data showed that MDPs can generate cDCs that reside in the spleen without transitioning through a monocytic intermediate42, whereas other reports have shown that cDCs in peripheral organs may derive from a monocyte precursor32. In contrast, both MDPs and monocytes could not give rise to pDCs, suggesting pDCs diverge from cDCs perhaps at the level of CMPs or GMPs32,40. Furthermore, it was proposed that a Flt3+ cell within either the CMP or CLP populations could develop into both cDCs and pDCs40,43. In the absence of 4-1BB or 4-1BBL, greater numbers of cDCs and pDCs in lymphoid and non-lymphoid organs were found, and greater numbers of monocytes were generated in both bone marrow and blood. Also, enhanced B cell development was found in the bone marrow chimeras. Collectively, this further supports the conclusion that primary regulation of myelopoiesis (and B lymphopoiesis) through 4-1BBL will occur in the bone marrow at the stages of CMPs, GMPs, or precursors of B lineage cells including CLPs.
Peripheral tissue DCs might derive from separate DC precursors that seed these organs before being committed to final differentiation into mature DCs32,40. Monocytes can be DC precursors and are suggested to give rise to cDCs in non-lymphoid organs42,44,45. It is not clear how this process is regulated, but the cytokine milieu, and specifically GM-CSF, might be crucial for monocyte differentiation32,33. In this regard, GM-CSF-induced bone marrow cultures are thought to mimic the stages of in vivo peripheral tissue DC differentiation36,46. We found that 4-1BB and 4-1BBL are expressed during GM-CSF driven differentiation and their interaction limited the accumulation of bone marrow derived DCs. Moreover, the majority of cDCs in the lung of bone marrow chimeras originated from the Tnfrsf9−/− population. This result, in part, might be related to the enhanced number of blood monocytes. However, the relative skewing of lung cDCs in favor of Tnfrsf9−/− derived populations was far greater than that of blood monocytes. Therefore, we suggest that 4-1BB and 4-1BBL could also participate in limiting more distal steps of cDC differentiation that potentially occur within the peripheral organ itself.
In conclusion, we demonstrate an unrecognized role for 4-1BB and 4-1BBL in myelopoiesis and the development of DCs. This work complements previous studies on other TNF superfamily members that have shown that CD27 is expressed on HSCs and progenitor cells and binding of CD70 negatively regulates myeloid colony-forming potential in vitro and B lymphopoiesis in vivo47,48, and that the lymphotoxin α1β249. Thus the TNF superfamily controls not only the responsiveness of mature lymphoid populations but also the balance between separate cell lineages.
METHODS
Mice
Tnfrsf9−/− mice and Tnfsf9−/− mice on a C57BL/6 background were bred at LIAI and Emory University, respectively. C57BL/6J (CD45.2+) and C57BL/6Pep3b/BoyJ (CD45.1+) and Rag1−/−mice were purchased from the Jackson Laboratories. All experiments were in compliance with the regulations of the LIAI animal care committee in accordance with guidelines of the Association for the Assessment and Accreditation of Laboratory Animal Care. Studies with Tnfsf9−/− bone marrow cells were performed at Emory University.
Cell suspensions
Spleens and lymph nodes and lung tissue were digested for 30 min at 37 °C with collagenase D (Roche) and DNase I (Sigma-Aldrich). Bone marrow was extracted from the tibia and femur in HEPES-buffered (pH 7.4) RPMI 1640 medium (Invitrogen) containing 1% FBS (Omega Scientific), and erythrocytes lysed with red blood cell lysing buffer (Sigma-Aldrich). Lung cells underwent density gradient centrifugation (density: 1.0875 ± 0.0010 g/cm3) with Lympholyte®-M (Cedarlane) to separate live cells from dead cells and red blood cells.
Reagents
The following antibodies and reagents were used; anti-CD16-CD32 (2.4G; 553140; BD Bioscience), anti-IL-10R (1B1.3a; 112708), anti-IFN-β (MIB-5E9.1; 508104; from BioLegend), and anti-TGF-β (1D11.16.8; isolatedmfrom hybridoma purchased from ATCC); biotin-conjugated anti-4-1BB (17B5; 106103) and anti-4-1BBL (TKS-1; 107103; all from BioLegend); fluorescein isothiocyanate (FITC)-conjugated anti-CD45.1 (A20; 553775), anti-CD45.2 (104; 553772), anti-B220 (RA3-6B2; 553088), anti-CD11b (M1/70; 553310), anti-CD11c (HL3; 553801; all from BD Bioscience), anti-CD3 (145-2C11; 100305), anti-TER119 (TER-119; 116205), anti-Gr-1 (RB6-8C5; 108907), anti-TCRβ (H57-597; 109206; all from BioLegend), anti-CD127 (A7R34; 11-1271), and anti-CD34 (RAM34; 11-0341; all from eBioscience); phycoerythrin (PE)-conjugated anti-CD4 (RM4-5; 553049), anti-CD80 (16-10A1; 553769), anti-CD19 (1D3; 553786), anti-α4β7 (DATK32; 553811; all from BD Bioscience), anti-Sca-1 (E13-161.7; 122507), anti-CD49b (DX5; 108907; all from BioLegend), anti-CD86 (GL1; 12-0862), anti-I-A/E (M5/114.15.2; 11-5321), anti-CD40 (1C10; 12-0401), anti-FcεRIα (MAR-1; 12-5898), anti-CD16/32 (FcγRII/III, 93; 12-0161), anti-Flt3 (A2F10; 12-1351; all from eBioscience), and anti-mouse pDC antigen (mPDCA-1, JF05-1C2.4.1; 130-091-962, Miltenyi Biotec); allophycocyanin (APC)-conjugated anti-CD11c (HL3; 550261), anti-Thy1.2 (53-2.1; 553007; all from BD Bioscience), anti-B220 (RA3-6B2; 103212), anti-c-Kit (2B8; 105812), anti-NK1.1 (PK136; 108709), anti-Sca-1 (E13-161.7; 122511; all from BioLegend), anti-F4/80 (BM8; 17-4801), anti-CD127 (A7R34; 17-1271; all from eBioscience), and anti-mPDCA-1 (JF05-1C2.4.1; 130-091-963, Miltenyi Biotec); peridinin chlorophyll protein (PerCP)-conjugated anti-CD45.2 (104; 552950), anti-CD8α (53-6.7; 553036; all from BD Bioscience); PerCP-Cy5.5-conjugated anti-CD11b (M1/70; 550993; BD Bioscience); pacific blue-conjugated anti-Sca-1 (E13-161.7; 122520) and anti-B220 (RA3-6B2; 103227; all from BioLegend); PE-Cy5-conjugated anti-c-Kit (2B8; 105809; BioLegend); PE-Cy7-conjugated anti-c-Kit (2B8; 105814), anti-CD11b (M1/70; 101216), and anti-CD3 (145-2C11; 100320; all from BioLegend). 7-amino-actinomycin D (7-AAD, 420401) was from BioLegend and anti-c-Kit (130-091-224), and anti-biotin (130-090-485) microbeads were from Miltenyi Biotec. All recombinant cytokines were from Peprotech. Human IgG:Fc (0160-14) and rat IgG (KLH/G1-2-2; 0116-14) were from R&D systems. 4-1BB:Ig and 3H3 mAb were purified as described26. PE-conjugated streptavidin and carboxyfluorescein succinimidyl ester (CFSE) were from Molecular Probes and the inhibitor of casein kinase I (CKI-7) was from Sigma-Aldrich.
Immunofluorescence labeling and sorting
Bone marrow cells were enriched over MACS LS columns (Miltenyi Biotec) through either positive isolation of c-Kit+ cells by labeling with anti-c-Kit magnetic beads, or negative depletion of lineage-negative cells by labeling with biotin-conjugated Abs specific for lineage markers followed by anti-biotin magnetic beads. Enriched BM cells were collected for further cell sorting. Cells were gated for flow cytometry or cell sorting as follows: LSK cells, Lin−(B220−, CD11b−, CD3−, TER-119−, Gr-1−, CD11c−) Sca-1+c-KithiIL-7Rα−; LT-HSC, Lin−Sca-1+c-KithiIL-7Rα−Flt-3−Thy1.2+; ST-HSCs, Lin−Sca-1+c-KithiIL-7Rα−Flt-3+Thy1.2+; MPPs, Lin−Sca-1+c-KithiIL-7Rα−Flt-3+Thy1.2−; myeloid progenitors, Lin−Sca-1−c-KithiIL-7Rα−; CMPs, Lin−Sca-1−c-KithiIL-7Rα−CD34+FcγRII/IIIlo; GMPs, Lin−Sca-1−c-KithiIL-7Rα−CD34+FcγRII/IIIhi; MEPs, Lin−Sca-1−c-KithiIL-7Rα−CD34−FcγRII/IIIlo; CLPs, Lin−Sca-1loc-KitloIL-7Rα+; monocytes, CD11bhiF4/80+Gr-1int; granulocytes, CD11bhiGr-1hiF4/80−; B cells, CD19+B220+; NK cells, CD3−NK1.1+DX5+; T cells, CD3+TCRβ+; CD4+ T cells, CD3+CD4+CD11c−; CD8+ T cells, CD3+CD8+CD11c−; cDCs, CD11c+I-A&I-Ehi; spleen and lymph node pDCs, CD11cloPDCA-1+I-A&I-Eint; lung pDCs, CD11cintPDCA-1+B220+. FACS-VantageSE DiVa (Becton Dickinson), LSR II (Becton Dickinson), and FACS-Calibur (Becton Dickinson) flow cytometers and cell sorters were used with FlowJo software (TreeStar).
Bone marrow chimeras
Recipient mice (CD45.1+ or CD45.2+ Rag1−/− were irradiated with two rounds (separated by a 3 h interval) of irradiation with 5.5 Gy (11 Gy total). Bone marrow cells from wild-type (CD45.1+), Tnfrsf9−/− (CD45.2+), and Tnfsf9−/− (CD45.2+) mice were labeled with biotin-conjugated anti-B220 and anti-CD3 followed by anti-biotin microbeads. Cells were passed over MACS LS columns and the flow-through (B and T cell depleted) fraction was collected for adoptive transfer. For CMP transfer, wild-type (CD45.1+) and Tnfrsf9−/− (CD45.2+) CMP were sorted as described earlier. Mixed bone marrow cells (2.5 × 106 of each strain, total 5 × 106) or CMPs (2.5 × 104 of each strain, total 5 × 104) were transferred i.v. one day after irradiation. Mice were administered with antibiotics (1.0 mg/ml neomycin and 0.1 mg/ml of polymyxin-B sulfate; Sigma-Aldrich) in the drinking water for 2–3 weeks post-reconstitution. Reconstitution of each lineage was analyzed at various time points (at 7–16 weeks for unseparated bone marrow transfers or at day 8 for CMP transfer). In mixed chimeras (CD45.1+ plus CD45.2+), the number of donor bone marrow-derived cells, particularly T cells, having the same congenic marker as host-derived cells (CD45.1+ in most cases) was normalized by subtracting the average number of host bone marrow-derived cells surviving after irradiation (around 3–5 % of total CD45+ cells in chimera, with > 90% being T cells). The average number of host bone marrow-derived cells at individual time points was calculated from control mice (n = 3 to 6) that received only one bone marrow population which had a congenic marker different from host. In parallel, the number of donor-derived CD45.2+ myeloid-lineage cells in mixed CMP chimera in Rag1−/− (CD45.2+) mice was normalized by subtracting the average number of host bone marrow-derived cells from three control Rag1−/− mice that were irradiated then injected with PBS.
Myeloid progenitor culture
Various numbers of cells, typically 2 × 104, were cultured with IMDM (GIBCO) containing 20 % FBS in the presence of cytokines; stem cell factor (SCF, 20 ng/ml), IL-3 (10 ng/ml), IL-11 (10 ng/ml), TPO (10 ng/ml), GM-CSF (10 ng/ml). Where indicated, myeloid progenitors were cultured in the presence of plate-bound human IgG:Fc (20 μg/ml) and 4-1BB:Ig (20 μg/ml), or soluble rat IgG (10 μg/ml) and anti-4-1BB (3H3, 10 μg/ml). Alternatively, myeloid progenitors were cultured in the presence of plate-bound human IgG:Fc or 4-1BB:Ig together with antibodies (20 μg/ml), such as rat IgG, anti-IL-10R, anti-IFN-β, and anti-TGF-β, or the inhibitor of casein kinase I (CKI-7, 20 μM). Cultures were initiated with 0.5 ml medium containing all reagents, including cytokines, antibodies, and inhibitor, in 24-well plates, and 0.5 ml fresh medium with reagents was added at day 3 and day 6.
Bone marrow-derived DCs
T and B cell depleted BM cells (3 × 105) were incubated with IMDM containing with 10 % FBS and GM-CSF (20 ng/ml) in 24-well plates. Cultures were initiated with 0.8 ml medium, and 0.8 ml fresh medium with GM-CSF (10 ng/ml) was added at day 3 and day 6. Various numbers of sorted myeloid progenitors, typically 1.0 × 105, were cultured with same protocol, and where indicated, plate-bound human IgG:Fc (20 μg/ml), 4-1BB:Ig (20 μg/ml), or soluble 4-1BB:Ig (20 μg/ml) were added into culture.
Western blots
Live cells were lysed in ice-cold RIPA Lysis Buffer (20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 2 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS, 50 mM NaF, 1 mM Na3VO4) containing protease inhibitor cocktail for mammalian tissues (P8340, Sigma-Aldrich) for 30 min. Insoluble material was removed and lysates used for Western blotting. Protein content was determined by bicinchoninic acid protein assay (Thermo scientific). Equal amounts (5–15μg) were loaded onto 4–12% NuPage Bis-Tris precasting gels (SDS-PAGE), transferred onto PVDF membrane (Invitrogen), and immunoblotted. All blots were developed with the ECL immunodetection system (Immobilon western chemilumiluminescent HRP, Millipore). Anti-p-Erk (#9101) and p-IκBα (#9241) were from Cell Signaling Technology and anti-Erk (K-23, sc-153) and IκBα (C-21, sc-371) from Santa Cruz Biotech.
Statistical analysis
Statistical significance was determined by two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We thank X. Tang, Y. Adams, Y. Kinjo, W. Xiao, C. Hutter and B. Zhang for technical assistance. Supported by NIH grants AI42944 (to M.C.) and CA85860 and AI059290 (to R.S.M). Additional support from NIH grant AI050265 (to H.C.), NIH grant EY013325 and an “Innovative Research Award” from the Arthritis Foundation (to B.S.K), and fellowships from the Diabetes and Immune Disease National Research Institute (to S.-W.L.) and the Crohn’s and Colitis Foundation of America (to Y.P.). This is manuscript 938 from the La Jolla Institute for Allergy and Immunology.
Footnotes
AUTHOR CONTRIBUTIONS
S.-W.L., Y.P., T.S. R.S.M. and M.C. designed the research and analyzed the data; S.-W.L., Y.P. and T.S. performed the experiments at LIAI, and S.-W.L. performed experiments as a visiting scientist with R.S.M. at Emory University; R.S.M, B.S.K., and H.C. contributed reagents and to the preparation of the manuscript,; S.-W.L. and M.C. wrote the manuscript.
References
- 1.Kwon BS, Weissman SM. cDNA sequences of two inducible T-cell genes. Proc Natl Acad Sci USA. 1989;86:1963–1967. doi: 10.1073/pnas.86.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goodwin RG, et al. Molecular cloning of a ligand for the inducible T cell gene 4-1BB: a member of an emerging family of cytokines with homology to tumor necrosis factor. Eur J Immunol. 1993;23:2631–2641. doi: 10.1002/eji.1830231037. [DOI] [PubMed] [Google Scholar]
- 3.Schwarz H. Biological activities of reverse signal transduction through CD137 ligand. J Leukoc Biol. 2005;77:281–286. doi: 10.1189/jlb.0904558. [DOI] [PubMed] [Google Scholar]
- 4.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3:609–620. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 5.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 6.Tan JT, Whitmire JK, Ahmed R, Pearson TC, Larsen CP. 4-1BB ligand, a member of the TNF family, is important for the generation of antiviral CD8 T cell responses. J Immunol. 1999;163:4859–4868. [PubMed] [Google Scholar]
- 7.Bertram EM, Lau P, Watts TH. Temporal segregation of 4-1BB versus CD28-mediated costimulation: 4-1BB ligand influences T cell numbers late in the primary response and regulates the size of the T cell memory response following influenza infection. J Immunol. 2002;168:3777–3785. doi: 10.4049/jimmunol.168.8.3777. [DOI] [PubMed] [Google Scholar]
- 8.Kwon BS, et al. Immune responses in 4-1BB (CD137)-deficient mice. J Immunol. 2002;168:5483–5490. doi: 10.4049/jimmunol.168.11.5483. [DOI] [PubMed] [Google Scholar]
- 9.Lee SW, Vella AT, Kwon BS, Croft M. Enhanced CD4 T cell responsiveness in the absence of 4-1BB. J Immunol. 2005;174:6803–6808. doi: 10.4049/jimmunol.174.11.6803. [DOI] [PubMed] [Google Scholar]
- 10.Lee SW, et al. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–4472. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 11.Vinay DS, Cha K, Kwon BS. Dual immunoregulatory pathways of 4-1BB signaling. J Mol Med. 2006;84:726–736. doi: 10.1007/s00109-006-0072-2. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J Immunol. 2002;169:4230–4236. doi: 10.4049/jimmunol.169.8.4230. [DOI] [PubMed] [Google Scholar]
- 13.Huang Q, et al. The plasticity of dendritic cell responses to pathogens and their components. Science. 2001;294:870–875. doi: 10.1126/science.294.5543.870. [DOI] [PubMed] [Google Scholar]
- 14.Wilcox RA, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168:4262–4267. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 15.Futagawa T, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14:275–286. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 16.Lee SC, et al. 4-1BB (CD137) is required for rapid clearance of Listeria monocytogenes infection. Infect Immun. 2005;73:5144–5151. doi: 10.1128/IAI.73.8.5144-5151.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimoto H, et al. Costimulation of mast cells by 4-1BB, a member of the tumor necrosis factor receptor superfamily, with the high-affinity IgE receptor. Blood. 2005;106:4241–4248. doi: 10.1182/blood-2005-04-1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeBenedette MA, et al. Analysis of 4-1BB ligand (4-1BBL)-deficient mice and of mice lacking both 4-1BBL and CD28 reveals a role for 4-1BBL in skin allograft rejection and in the cytotoxic T cell response to influenza virus. J Immunol. 1999;163:4833–4841. [PubMed] [Google Scholar]
- 19.Zhu G, et al. Progressive depletion of peripheral B lymphocytes in 4-1BB (CD137) ligand/I-Eα)-transgenic mice. J Immunol. 2001;167:2671–2676. doi: 10.4049/jimmunol.167.5.2671. [DOI] [PubMed] [Google Scholar]
- 20.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Laiosa CV, Stadtfeld M, Graf T. Determinants of lymphoid-myeloid lineage diversification. Annu Rev Immunol. 2006;24:705–738. doi: 10.1146/annurev.immunol.24.021605.090742. [DOI] [PubMed] [Google Scholar]
- 22.Kondo M, et al. Biology of hematopoietic stem cells and progenitors: implications for clinical application. Annu Rev Immunol. 2003;21:759–806. doi: 10.1146/annurev.immunol.21.120601.141007. [DOI] [PubMed] [Google Scholar]
- 23.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 24.Kozar K, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 25.Chen CC, Grimbaldeston MA, Tsai M, Weissman IL, Galli SJ. Identification of mast cell progenitors in adult mice. Proc Natl Acad Sci USA. 2005;102:11408–11413. doi: 10.1073/pnas.0504197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shuford WW, et al. 4-1BB costimulatory signals preferentially induce CD8+ T cell proliferation and lead to the amplification in vivo of cytotoxic T cell responses. J Exp Med. 1997;186:47–55. doi: 10.1084/jem.186.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saito K, et al. Infection-induced up-regulation of the costimulatory molecule 4-1BB in osteoblastic cells and its inhibitory effect on M-CSF/RANKL-induced in vitro osteoclastogenesis. J Biol Chem. 2004;279:13555–13563. doi: 10.1074/jbc.M303791200. [DOI] [PubMed] [Google Scholar]
- 28.Shin HH, Lee EA, Kim SJ, Kwon BS, Choi HS. A signal through 4-1BB ligand inhibits receptor for activation of nuclear factor-κ B ligand (RANKL)-induced osteoclastogenesis by increasing interferon (IFN)-beta production. FEBS Lett. 2006;580:1601–1606. doi: 10.1016/j.febslet.2006.01.091. [DOI] [PubMed] [Google Scholar]
- 29.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 30.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- 32.Shortman K, Naik SH. Steady-state and inflammatory dendritic-cell evelopment. Nat Rev Immunol. 2007;7:19–30. doi: 10.1038/nri1996. [DOI] [PubMed] [Google Scholar]
- 33.Naik SH, et al. Intrasplenic steady-state dendritic cell precursors that are distinct from monocytes. Nat Immunol. 2006;7:663–671. doi: 10.1038/ni1340. [DOI] [PubMed] [Google Scholar]
- 34.van Rijt LS, et al. In vivo depletion of lung CD11c+ dendritic cells during allergen challenge abrogates the characteristic features of asthma. J Exp Med. 2005;201:981–991. doi: 10.1084/jem.20042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Heer HJ, et al. Essential role of lung plasmacytoid dendritic cells in preventing asthmatic reactions to harmless inhaled antigen. J Exp Med. 2004;200:89–98. doi: 10.1084/jem.20040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nikolic T, de Bruijn MF, Lutz MB, Leenen PJ. Developmental stages of myeloid dendritic cells in mouse bone marrow. Int Immunol. 2003;15:515–524. doi: 10.1093/intimm/dxg050. [DOI] [PubMed] [Google Scholar]
- 37.Steptoe RJ, Ritchie JM, Harrison LC. Increased generation of dendritic cells from myeloid progenitors in autoimmune-prone nonobese diabetic mice. J Immunol. 2002;168:5032–5041. doi: 10.4049/jimmunol.168.10.5032. [DOI] [PubMed] [Google Scholar]
- 38.Shin HH, Lee JE, Choi HS. Absence of 4-1BB increases cell influx into the peritoneal cavity in response to LPS stimulation by decreasing macrophage IL-10 levels. FEBS Lett. 2007;581:4355–4360. doi: 10.1016/j.febslet.2007.08.015. [DOI] [PubMed] [Google Scholar]
- 39.Schwarz H, Blanco FJ, von Kempis J, Valbracht J, Lotz M. ILA, a member of the human nerve growth factor/tumor necrosis factor receptor family, regulates T-lymphocyte proliferation and survival. Blood. 1996;87:2839–2845. [PubMed] [Google Scholar]
- 40.Wu L, Liu YJ. Development of dendritic-cell lineages. Immunity. 2007;26:741–750. doi: 10.1016/j.immuni.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 41.Fogg DK, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 42.Varol C, et al. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–180. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Amico A, Wu L. The early progenitors of mouse dendritic cells and plasmacytoid predendritic cells are within the bone marrow hemopoietic precursors expressing Flt3. J Exp Med. 2003;198:293–303. doi: 10.1084/jem.20030107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Landsman L, Varol C, Jung S. Distinct differentiation potential of blood monocyte subsets in the lung. J Immunol. 2007;178:2000–2007. doi: 10.4049/jimmunol.178.4.2000. [DOI] [PubMed] [Google Scholar]
- 45.Leon B, Lopez-Bravo M, Ardavin C. Monocyte-derived dendritic cells formed at the infection site control the induction of protective T helper 1 responses against Leishmania. Immunity. 2007;26:519–531. doi: 10.1016/j.immuni.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 46.Inaba K, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiesmann A, et al. Expression of CD27 on murine hematopoietic stem and progenitor cells. Immunity. 2000;12:193–199. doi: 10.1016/s1074-7613(00)80172-7. [DOI] [PubMed] [Google Scholar]
- 48.Nolte MA, et al. Immune activation modulates hematopoiesis through interactions between CD27 and CD70. Nat Immunol. 2005;6:412–418. doi: 10.1038/ni1174. [DOI] [PubMed] [Google Scholar]
- 49.Kabashima K, et al. Intrinsic lymphotoxin-β receptor requirement for homeostasis of lymphoid tissue dendritic cells. Immunity. 2005;22:439–450. doi: 10.1016/j.immuni.2005.02.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.