Abstract
Objective
To determine the maximum stimulus levels at which a measured auditory steady-state response (ASSR) can be assumed to be a reliable measure of auditory thresholds.
Design
ASSR thresholds were measured at octave frequencies from 500 to 4000 Hz in 10 subjects with profound hearing loss. These subjects provided no behavioral responses to sound at the limits of pure-tone audiometers and at the limits of the stimulus levels produced by the ASSR device. Subjects were divided into two groups of five, with repeated measures obtained within the same session in one group and repeated measures obtained in a separate session on a different day in the other group.
Results
ASSR thresholds were observed in all 10 subjects at each of four frequencies and in both trials. On average, these ASSR thresholds were observed at 100 dB HL (SD = 5 dB). Because these responses were at least 18 to 22 dB below the limits of the equipment where all subjects had no behavioral responses, it is reasonable to conclude that the ASSRs were not generated by the auditory system.
Conclusions
An artifact or distortion may be present in the recording of ASSRs at high levels. These data bring into question the view that there is a wider dynamic range for ASSR measurements compared with auditory brain stem response measurements, at least with current implementation.
With the advent of universal newborn hearing screening (UNHS), clinicians are being asked to provide diagnostic audiological evaluations on infants within the first few weeks or months of life. This seems reasonable, since it is widely thought that the success of intervention will depend, at least in part, on the age at which intervention begins (Moeller, 2000; Yoshinaga-Itano et al., 1998). Thus, if UNHS identifies newborn infants with hearing loss, it will be important to initiate intervention as soon as possible after the identification. At these ages, however, it is not clinically feasible to rely on behavioral responses to sound to provide an estimate of the magnitude and configuration of hearing loss. Clinicians must rely on measurements that do not require a voluntary response on the part of the infant if intervention is to be initiated soon after identification.
The auditory brainstem response (ABR) is considered an objective electrophysiological measure and has been used to provide information about auditory sensitivity in patients who are either too young or who are functioning at a developmental level at which reliable behavioral response measurements are not possible. These responses are elicited by stimuli with rapid onsets. Such stimuli are characterized by a spread of energy, the extent of which depends on how these stimuli are generated. Several stimulus paradigms have been combined with ABR measurements to obtain frequency-specific responses. These methods include the derived-band technique (Don, Eggermont and Brackman, 1979), the notched-noise technique (Picton et al., 1979; Stapells, Gravel and Martin, 1995), and the use of tone bursts in quiet (Gorga et al., 1988; 1993). In these cases, stimuli are gated in such a way as to reduce the spread of energy or the stimuli are presented with a masking noise designed to restrict the cochlear region contributing to a response.
Determining ABR thresholds under routine conditions requires skill on the part of the observer. These responses are small and embedded in a background of ongoing brain activity (EEG noise) or muscle activity. Thus, signal averaging is required to reduce the noise and resolve the ABR. Furthermore, it is more difficult to observe a response at near-threshold levels, compared with suprathresh-old conditions. Finally, responses elicited by certain stimuli (tone bursts, and clicks or tone bursts combined with noise maskers) are more difficult to observe than click-evoked responses in quiet.
Because of these problems, there has been interest in developing alternative techniques for estimating sensitivity loss. One such technique is the auditory steady-state response (ASSR), which examines the responses to sinusoids that are either amplitude, frequency, or amplitude and frequency modulated. Data have begun to accumulate that suggest that ASSR threshold estimates are reasonably accurate at predicting behavioral thresholds (Cone-Wesson etal., 2002; Dimitrijevic et al., 2002; Rance et al., 1998; Rance & Rickards, 2002; Vander Werff et al., 2002).
There are several reported advantages to ASSR measurements. First, the response is elicited by a modulated pure tone. Thus, the stimulus is more narrowly defined in the frequency domain, compared with gated sinusoids or clicks. However, it is important to remember that once outer hair cell damage exists (which occurs in most cases of sensory hearing loss), the extent to which narrow cochlear regions can be excited is reduced. This is probably the case for gated tone bursts in quiet, tone bursts presented with a noise masker, and even for pure tones (see Gorga and Neely, 2002, for a discussion of the underlying physiology in support of this view).
ASSR measurements also lend themselves to automatic response detection. Such a feature is attractive in that it avoids problems associated with the experience and expertise of the observer. Automated algorithms have been developed for the detection of ABRs (most notably, the Fsp procedure developed by Don and Elberling, 1996 and the template-matching approach used in the screening device marketed by Natus). However, the Fsp procedure is not in wide-spread clinical use and the template approach has been applied only in UNHS.
The same calibration techniques used for pure-tone audiometry can be used for ASSR stimuli because the reference equivalent threshold sound pressure level (RETSPL) for ASSR stimuli should be the same as it is for pure tones. This means that national or international standards can be used to calibrate these stimuli. In contrast, no such standard exists for the stimuli used to elicit ABRs.
Finally, it has been suggested that ASSR measurements have a wider dynamic range than ABR measurements. That is, because of the stimulus’ long duration, higher levels can be achieved with ASSR stimuli (in units of hearing level, HL), compared with the case for the brief duration/rapid onset stimuli that are used with ABR measurements. Whereas for ABR measurements, the maximum output may be 90 to 100 dB HLn (referenced to the behavioral thresholds of a local jury of normal-hearing subjects), it is possible to produce ASSR stimuli of 115 dB HL or greater (referenced to either ANSI or ISO standards). Indeed, ASSRs have been reported for ears that did not produce an ABR, suggesting that the measurements can be applied with greater degrees of hearing loss (e.g., Rance et al., 1998).
The present paper focuses on the dynamic range for ASSR measurements by exploring the stimulus limits of system distortion or artifact for these measurements. In this study, ASSRs were measured in subjects for whom the ASSR stimuli at the maximum levels produced by the hardware were inaudible. If no ASSRs are observed in these subjects, then the dynamic range of the measurement is at least as great as the current instrument limitations in terms of the stimulus levels it can produce. If ASSRs are observed in these subjects, they presumably are not providing information related to peripheral auditory function. If this occurs, then the levels at which these responses are observed provide estimates of the upper limit of reliable ASSR measurements.
Methods
Subjects
Ten adult subjects with profound hearing loss participated in the present study. These subjects were recipients of cochlear implants. Subjects ranged in age from 30 to 77 yr. The cochlear implant was turned on during the process in which informed consent was obtained and instructions were given. It was turned off during all experimental measurements, including both behavioral and evoked potential (EP) threshold measurements. Only subjects who did not produce behavioral responses to both the pure-tone and ASSR stimuli (described below) participated in ASSR EP threshold measurements. This study was conducted under an approved institutional review board protocol.
Instrumentation
Pure-tone stimuli produced by clinical audiometers (calibrated to ANSI S3.2 – 1996 standards) were used to measure behavioral “thresholds” at the octave frequencies from 0.5 to 4 kHz. Since none of the subjects produced behavioral responses to sounds, these “threshold” values represent the output limits of each audiometer, which varied slightly for different audiometers. Thus, for the same frequency, the maximum output of one audiometer was 100 dB HL, whereas in other cases, the upper limit was 115 dB HL.
Behavioral “thresholds” also were measured for each subject, using the amplitude-modulated stimuli produced by the ASSR system (Bio-Logic, MASTER). These stimuli were delivered through an insert earphone whose output was calibrated in a 2 cm3 cavity. The output limits for these stimuli ranged from 118 dB HL at 0.5 kHz to 122 dB HL at 4 kHz. Table 1 provides the actual sound pressure levels at 0 dB HL for each frequency produced by the ASSR system.
TABLE 1.
Reference equivalent threshold sound pressure levels for 0 dB HL for auditory steady-state response stimuli
| Frequency | SPL for 0 dB HL |
|---|---|
| 500 | 9 |
| 1000 | 7 |
| 2000 | 14 |
| 4000 | 3 |
In the software version used for the present experiment, the ASSR system would not produce sound unless the electrode impedances were within a criterion limit (set internally in the software and not under our control). As a result, an impedance network was built, having resistances of 2 kOhms across any two nodes in the network. Electrodes were attached to these nodes so that the system would see an acceptable impedance and thus would produce sounds that could be calibrated. In the process of this calibration procedure, it was noted that ASSRs were detected across these resistive loads. Although these “responses” obviously are not of a biological origin, these measurements do not represent a good test of the upper limits of reliable ASSR measurements because the noise levels under these conditions are much lower than the levels measured across electrodes attached to human heads. For this reason, measurements were made in humans with no behavioral responses to sound because they represent a more realistic biological model of a system in which no ASSRs should be observed, yet they would be expected to produce noise levels similar to those seen under clinical conditions (at least when sedation is not used).
Procedures
Pure-tone audiometric thresholds and behavioral thresholds to the ASSR stimuli were measured first in all subjects while their cochlear implant was turned off. Subjects were included in the ASSR EP study only if they did not produce a behavioral response both to pure-tone stimuli at audiometer limits and to the ASSR stimuli at the limits of the ASSR equipment. This means that the EP results, described below, were obtained in subjects who produced no behavioral responses to sound at the limits of our instrumentation. For the EP measurements, subjects were divided into two groups of five subjects each. For one group, ASSR measurements at each frequency were repeated within a single session, whereas for the other group, these measurements were repeated at separate sessions scheduled on different days.
In the EP experiment, surface electrodes were placed at the high forehead and the nape of the neck, with a third electrode placed at the side of the forehead serving as ground. Electrode impedances were always within the acceptable range internally set by the ASSR system. Following instructions, subjects sat in a recliner in a sound-isolated, electrically shielded booth. Subjects were asked to relax and sleep if possible. Subjects were not sedated. The ASSR measurements were performed by presenting a single carrier frequency that was amplitude modulated at the frequency determined by the manufacturer for that carrier (within the range from about 70 to 90 Hz). Typically, measurements were initiated with a stimulus level of 80 dB HL. This system averages the response waveforms from many presentations, performs a fast Fourier transform on the averaged waveform and then compares the amplitude in the frequency bin associated with the modulation frequency (signal) with the amplitude in several bins adjacent to this frequency (noise). It then uses these data to derive an estimate of the signal-to-noise ratio. Next, it evaluates the extent to which the level in the signal bin exceeded the level in the noise bins using F-ratio statistics. In its default condition (which was used in the present study since it was assumed that this is how the system will be used by clinicians), a response was considered present if the F-ratio probability was < 0.05. Testing stopped if the F-ratio criterion was achieved, if the noise level was reduced to a minimum level (set in the software), or after 32 sweeps, whichever occurred first. Approximately 4 hr of data collection time were required to obtain the behavioral and EP data for each subject.
Notably, we made no adjustment for the number of statistical comparisons that were made for each stimulus condition. This may be problematic in that the F-ratio was evaluated after each sweep, and there could be as many as 32 sweeps. That is, there could be as many as 32 individual comparisons for which a significance test was performed. Perhaps the nominal probability (0.05) should have been divided by the number of sweeps, which was not done in this study. (On average, there were 15.4 sweeps per condition in the present study.) Rather, testing was done in exactly the manner in which clinicians would make these measurements. To our knowledge, the software does not adjust the F-ratio probabilities in relation to the number of sweeps. In any case, the data reported below were obtained under conditions that would be in use in the clinic.
Results
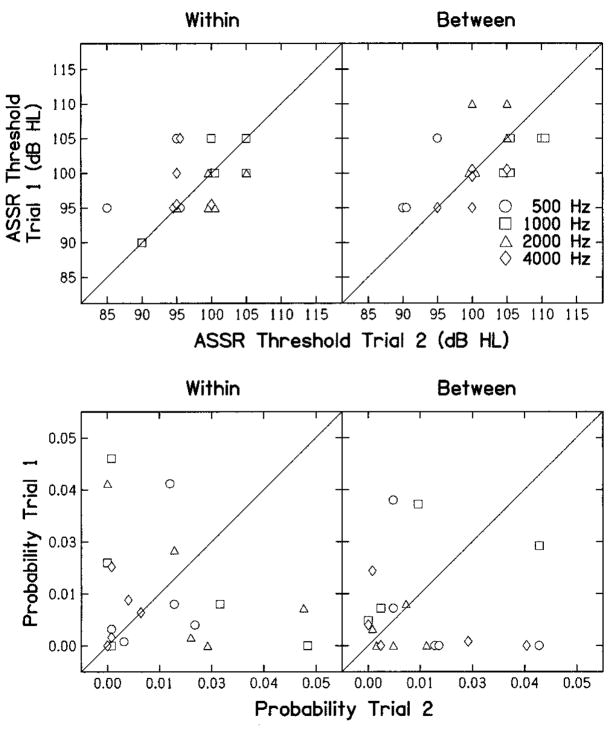
The top row of Figure 1 plots ASSR thresholds from the first measurement as a function of ASSR thresholds from the repeated measurement. The bottom row plots the F-ratio probabilities in the same way. In both cases, left and right columns represent data for within- and between-session repeated measures, respectively. Different symbols represent different frequencies, and each data point represents a pair of ASSR measurements from one subject. The diagonal line is included to provide a point of reference. Data points falling on the line represent cases when the same results were observed for initial and repeated measurements. The extent to which the data points deviate from this line represents the variability from trial to trial. There are 20 data points in all four panels of this figure; however, in the case of ASSR measurements, thresholds occurred at one of 5 discrete levels (85 to 105 dB HL for within-session repeated measurements; 90 to 110 dB HL for between-session repeated measurements). To make each data point visible, overlapping points were offset slightly. Data for several subjects and frequencies fell on the diagonal line, whereas others did not. The largest threshold difference between trials was 10 dB, which occurred for 3 of 20 comparisons in the within-session conditions and in 2 of 20 comparisons in the between-session conditions, suggesting that these “responses” were reproducible within a subject. Even though the thresholds were not identical in all trials, ASSR thresholds were measured at all four frequencies, on both trials in all 10 subjects. As expected, given the stopping criterion, the F-ratio probabilities, plotted in the bottom row of panels, always were less than the criterion probability of 0.05. In 49 of the total of 80 conditions, the F-ratio probability was less than 0.01; thus, it is likely that significance would have been achieved in many conditions even if adjustments were made for the number of sweeps.
Fig. 1.
Auditory steady-state response (ASSR) thresholds (dB HL) and F-ratio probabilities in top and bottom rows, respectively. In both cases, results from trial 1 are plotted as a function of the results in trial 2. Within-session and between-session repeated measurements are shown in left and right columns, respectively. Data points were offset slightly in the top row to improve the visualization of overlapping points.
Table 2 lists the instrumentation limits at which no behavioral response was observed for each subject. It also lists their ASSR EP thresholds. Instrumentation limits are provided for both pure-tone audiometric measurements and for behavioral measurements using the ASSR stimuli. ASSR EP thresholds from repeated measurements are shown, with half of these repeated measurements taken within the same session and half in sessions separated by several days. In addition to providing another summary of the results shown in Figure 1, Table 1 shows the maximum levels produced by the instrumentation used for both sets of behavioral measurements. The instrumentation limits (for which no behavioral responses were obtained) exceeded ASSR EP “thresholds” for each subject. Although no subject produced a behavioral response to any pure-tone or ASSR stimuli, every subject produced an ASSR at each of the four test frequencies in both the initial and repeated measurements.
TABLE 2.
Instrumentation limits at which individual subjects did not produce a behavioral response and their auditory steady-state response thresholds
| Within-session
| |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Behavioral
|
ASSR-Behavioral
|
ASSR
|
|||||||||||
| Subject | 500 | 1000 | 2000 | 4000 | 500 | 1000 | 2000 | 4000 | Trial | 500 | 1000 | 2000 | 4000 |
| W1 | 110* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 100 | 105 | 100 | 95 |
| 2 | 100 | 105 | 95 | 105 | |||||||||
| W2 | 100* | 115* | 115* | 110* | 118* | 122* | 120* | 119* | 1 | 95 | 100 | 100 | 95 |
| 2 | 95 | 100 | 95 | 95 | |||||||||
| W3 | 100* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 95 | 105 | 95 | 95 |
| 2 | 95 | 100 | 95 | 95 | |||||||||
| W4 | 110* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 85 | 100 | 100 | 95 |
| 2 | 95 | 105 | 100 | 100 | |||||||||
| W5 | 105* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 100 | 90 | 105 | 100 |
| 2 | 95 | 90 | 100 | 95 | |||||||||
| Between-session
| |||||||||||||
| Behavioral
|
ASSR-Behavioral
|
ASSR
|
|||||||||||
| Subject | 500 | 1000 | 2000 | 4000 | 500 | 1000 | 2000 | 4000 | Trial | 500 | 1000 | 2000 | 4000 |
|
| |||||||||||||
| B1 | 110* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 100 | 110 | 105 | 105 |
| 2 | 100 | 105 | 105 | 100 | |||||||||
| B2 | 100* | 105* | 115* | 105* | 118* | 122* | 120* | 119* | 1 | 95 | 105 | 105 | 100 |
| 2 | 105 | 100 | 110 | 100 | |||||||||
| B3 | 105* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 90 | 105 | 100 | 100 |
| 2 | 95 | 105 | 100 | 95 | |||||||||
| B4 | 110* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 105 | 110 | 100 | 100 |
| 2 | 100 | 105 | 110 | 100 | |||||||||
| B5 | 110* | 115* | 115* | 115* | 118* | 122* | 120* | 119* | 1 | 90 | 105 | 100 | 95 |
| 2 | 95 | 100 | 100 | 95 | |||||||||
Results are shown for pure-tone stimuli (behavioral thresholds), the instrumentation limits for which no behavioral response was measured for the auditory steady-state response (ASSR) stimuli, and the levels at which the ASSR criterion (F-ratio probability < 0.05) was observed. ASSR thresholds are shown for both the within-session and between-session repeated measures.
No response at level indicated.
Discussion
To summarize, mean ASSR thresholds were observed at 100 dB HL (averaged across the four test frequencies) in a group of subjects for whom there was no behavioral response, either for pure-tone audiometric stimuli or for the amplitude-modulated stimuli produced by the ASSR system. This observation suggests that it is unlikely that the measured ASSRs reflect peripheral auditory function. In turn, this suggests that these responses were the result of a stimulus or equipment artifact. Similar observations were made by Small and Stapells (2003a, 2003b), who also observed artifactual responses to bone-conducted stimuli. In addition, they observed that alternating the phase of the modulation frequency either eliminated the artifact or increased the level at which the artifact was observed. However, it is unclear how alternating phase would reduce artifact at the modulation frequency because the stimulus presumably does not include energy at that frequency. Small and Stapells (2003b) and Stapells (2003) noted that changes in the sampling rate resulted in a reduction in the artifact and suggested that the problem might be associated with an aliasing effect. This latter point was demonstrated in recent work by Picton and John (2003), who also described several solutions to the problem, including using an analog-to-digital sampling rate that was unrelated to the carrier frequency and/or selecting stimuli such that the aliasing frequencies do not occur at the same frequencies at which the response is being measured.
Given that the F-ratio probability was less than 0.01 for 49/80 conditions and that the average number of sweeps was 15.4, it is unlikely that the ASSR threshold observations in the present study occurred by chance, even if the probability was adjusted to take into account the number of comparisons for each condition. The distribution of F-ratio probabilities, as shown in Figure 1, also is surprising. One would predict that these distributions would be more uniformly distributed from 0.00 to 0.05 for both trials if only random noise was present in the measured response. The observation that this was not the case suggests the presence of a nonrandom artifact.
These data suggest that reliable ASSR measurements, as currently implemented by this system, cannot be made for stimulus levels (on average) at and above 100 dB HL. At this level, responses were measured in ears in which there were no behavioral responses to sound at the limits of the instrumentation, which, depending on frequency, ranged from 118 to 122 dB HL. The present measurements were obtained under conditions that presumably are the same as those used in the clinic. If the ASSR thresholds were used to predict behavioral thresholds, on average, behavioral thresholds would have been underestimated by at least 18 to 22 dB. These data bring into question the view that, as currently implemented, there is a wider dynamic range for ASSR measurements, compared with ABR measurements. Clinically, this may be of little consequence because of the likelihood that a patient will receive a cochlear implant when “responses” are only observed at such high levels. At the present time, caution should be exercised in the interpretation of high-level ASSR threshold measurements because they may not be providing information about peripheral hearing loss, which is what we seek to rehabilitate when fitting amplification or providing a co-chlear implant. Fortunately, the manufacturer is aware of this problem and is currently investigating potential solutions that might, in fact, extend the dynamic range of these measurements. As a result, data may become available soon that demonstrate that the level at which reliable ASSR measurements can be made has been extended. Indeed, a solution may be provided in an implementation similar to the one recently described by Picton and John (2003).
Acknowledgments
We would like to thank Bio-Logic System Corp., Inc., for providing the instrumentation that was used for the ASSR measurements. We also thank Carolyn Brown, Dave Stapells, and Gabe Raviv for helpful discussions of these data, Stapells for sharing some preliminary data related to solving the dynamic range problem, and Terry Picton and Sasha John for sharing a recent manuscript (before publication), in which a solution to the problem was described. Tiffany Johnson made suggestions that were helpful in the revision of this paper. This work was supported in part by a grant from the NIH (NIDCD R01 2251). Subject recruitment was supported by a core grant from the NIH (NIDCD P30 4662).
References
- American National Standards Institute. Specification for Audiometers, S3.2 1996 [Google Scholar]
- Cone-Wesson B, Dowell RC, Tomlin D, Rance G, Ming WJ. The auditory steady-state response: Comparison with the auditory brainstem response. Journal of the American Academy of Audiology. 2002;13:173–187. [PubMed] [Google Scholar]
- Dimitrijevic A, John MS, Van Roon P, Purcell DW, Adamonis J, Ostroff J, Nedslski JM, Picton TW. Estimating the audiogram using multiple auditory steady-state responses. Journal of the American Academy of Audiology. 2002;13:205–224. [PubMed] [Google Scholar]
- Don M, Eggermont JJ, Brackmann DE. Reconstruction of the audiogram using brainstem responses and high-pass noise masking. Annals of Otology, Rhinology, and Laryngology. 1979;83, Suppl, 57:1–20. doi: 10.1177/00034894790880s301. [DOI] [PubMed] [Google Scholar]
- Don M, Elberling C. Use of quantitative measures of auditory brain-stem response peak amplitude and residual background noise in the decision to stop averaging. Journal of the Acoustical Society of America. 1996;99:491–499. doi: 10.1121/1.414560. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KL, Bergman BM. A comparison of ABR thresholds and latencies elicited by air and bone conducted stimuli. Ear and Hearing. 1993;14:85–94. doi: 10.1097/00003446-199304000-00003. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Reiland JK, Beauchaine KA, Jesteadt W. Auditory brainstem responses to tone bursts in normal-hearing subjects. Journal of Speech and Hearing Research. 1988;31:87–97. doi: 10.1044/jshr.3101.87. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Neely ST. Some factors that may influence the accuracy of auditory brainstem response estimates of hearing loss. In: Seewald RC, Gravel JS, Phonak AG, editors. A Sound Foundation Through Early Amplification 2001. Chicago, IL: 2002. pp. 49–61. [Google Scholar]
- Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106(3) doi: 10.1542/peds.106.3.e43. URL: http//www.pediatrics.org/cgi/content/full/106/3/e43. [DOI] [PubMed]
- Picton TW, John MS. Avoiding electromagnetic artifacts when recording auditory steady-state responses. Journal of the American Academy of Audiology. 2003 doi: 10.3766/jaaa.15.8.2. in press. [DOI] [PubMed] [Google Scholar]
- Picton TW, Ouellette J, Hamel G, Smith AD. Brainstem evoked potentials to tonepips in notched noise. Journal of Otolaryngology. 1979;8:289–314. [PubMed] [Google Scholar]
- Rance G, Dowell RC, Rickards FW, Beer DE, Clark GM. Steady-state evoked potential and behavioral hearing thresholds in a group of children with absent click-evoked auditory brain stem response. Ear and Hearing. 1998;19:48–61. doi: 10.1097/00003446-199802000-00003. [DOI] [PubMed] [Google Scholar]
- Rance G, Rickards F. Prediction of hearing thresholds in infants using steady-state evoked potentials. Journal of the American Academy of Audiology. 2002;13:236–245. [PubMed] [Google Scholar]
- Small SA, Stapells DR. Auditory steady-state responses: Stimulus artifact issues. paper presented at the 2003 Meeting of the American Auditory Society; Scottsdale, AZ. 2003a. [Google Scholar]
- Small SA, Stapells DR. Artifactual responses when recording auditory steady-state responses. XVIII Biennial Symposium of the International Electric Response Audiometry Study Group; Puerto de la Cruz, Tenerife, Canary Islands, Spain. 2003b. [Google Scholar]
- Stapells DR. Personal communication 2003 [Google Scholar]
- Stapells DR, Gravel JS, Martin BA. Thresholds for auditory brainstem responses to tones in notched noise from infants and young children with normal hearing or sensorineural hearing loss. Ear and Hearing. 1995;12:361–371. doi: 10.1097/00003446-199508000-00003. [DOI] [PubMed] [Google Scholar]
- Vander Werff KR, Brown CJ, Gienapp B, Schmidt-Clay KM. Comparison of auditory steady-state response and auditory brainstem response thresholds in children. Journal of the American Academy of Audiology. 2002;13:227–235. [PubMed] [Google Scholar]
- Yoshinaga-Itano C, Sedey AL, Coulter DK, Mehl AL. Language of early- and late-identified children with hearing loss. Pediatrics. 1998;102:1161–1171. doi: 10.1542/peds.102.5.1161. [DOI] [PubMed] [Google Scholar]