Abstract
Purpose
To determine the effects of inhibition of protein geranylgeranyltransferase type I (GGTase-I), which isoprenylates so-called CaaX proteins, including the GTP-binding proteins such as Rho GTPases and the βγ subunits of heterotrimeric G-proteins, on aqueous humor outflow and trabecular meshwork cytoskeletal integrity.
Methods
A selective small molecular inhibitor of GGTase-I, GGTI-DU40, was tested in this study to investigate its effects on actin cytoskeletal integrity, cell adhesions, cell-cell junctions, myosin II phosphosphorylation, and membrane localization of GTP-binding proteins in trabecular meshwork (TM) cells, using immunofluorescence detection and immunoblotting analysis. The effects of GGTI-DU40 on aqueous humor outflow were determined using organ-cultured, perfused anterior segments of porcine eyes.
Results
In the TM cell lysates, GGTI-DU40 was confirmed to inhibit GGTase-I activity in a dose-dependent manner. TM cells treated with GGTI-DU40 displayed dose-dependent changes in cell morphology and reversible decreases in actin stress fibers, focal adhesions, and adherens junctions. Myosin light chain phosphorylation was decreased significantly, and membrane localization of isoprenylated small GTPases and Gβγ was impaired in drug-treated TM cells. Aqueous outflow facility was increased significantly in eyes perfused with GGTI-DU40.
Conclusions
These data demonstrate that inhibition of geranylgeranyl isoprenylation of CaaX proteins in the aqueous outflow pathway increases aqueous humor outflow, possibly through altered cell adhesive interactions and actin cytoskeletal organization in cells of the outflow pathway. This study indicates that the GGTase-I enzyme is a promising molecular target for lowering increased ocular pressure in glaucoma patients.
Glaucoma, a leading cause of blindness characterized by optic nerve degeneration and progressive visual field loss, is commonly associated with elevated intraocular pressure (IOP). In primary open-angle glaucoma (POAG), the most common form of the disease, elevated IOP occurs as a result of pathologically increased resistance to drainage of aqueous humor through the pressure-dependent trabecular or conventional outflow system.1 Therefore, elevated IOP is considered a major risk factor for glaucoma, and lowering IOP is the only modality available for the treatment of POAG.1 The conventional outflow pathway is composed of the trabecular mesh-work (TM), juxtacanalicular region (JCT), and Schlemm canal. In humans this pathway represents a predominant route of aqueous humor drainage.2,3 Aqueous humor is secreted by nonpigmented epithelial cells that line the iris and ciliary body and flows into the anterior chamber, which then drains through the TM into Schlemm canal and the episcleral veins on a continuous basis.4 Glaucoma is an age-related disease, and increased contractile activity and cell adhesive interactions of the cells of aqueous outflow pathway are believed to be partly responsible for the elevated IOP and POAG.4-7
Perfusion studies using cytoskeleton-interfering agents such as actin-depolymerizing agents, inhibitors of myosin light chain kinase, myosin II, protein kinase C, Rho GTPase, and Rho kinase, in different model systems, have indicated a link between cytoskeletal integrity within the TM and aqueous outflow through the TM.2,4,5,7,8 Agents that increase actin depolymerization and decrease cell– extracellular matrix interactions and myosin II phosphorylation in the TM increase aqueous outflow, presumably by cellular relaxation, altering the geometry of outflow pathway and fluid flow through the inner wall of the Schlemm canal.2,7 In contrast to the effects of inhibiting Rho, Rho-kinase, myosin II, and myosin light-chain kinase, physiological agonists including endothelin-1, lysophosphatidic acid, thrombin, and TGF-β (which are known to activate Rho/Rho kinase signaling) have been shown to reduce aqueous humor outflow in the perfused model systems.6,9-11 These different observations collectively implicate the activation status of the Rho/Rho-kinase pathway, heterotrimeric G-proteins, and the contractile force of the TM in the regulation of aqueous humor outflow and homeostasis of IOP.
GTP-binding proteins, including small GTP-binding proteins such as Rho and Rac and the heterotrimeric G-proteins G12/13 and Gq, regulate tissue contractile and relaxation properties and actin cytoskeletal organization and cell adhesive interactions in smooth muscle and nonmuscle cells.12-15 The activity of these G proteins (both monotrimeric and heterotrimeric) requires the addition of an isoprenoid lipid at the carboxyl terminal through enzyme-mediated isoprenylation posttranslational modification. This lipid modification aids the G-proteins and Rho GTPases to localize to the plasma membrane, where they interact with other regulatory proteins and participate in signaling activity to control various cellular processes.16,17 The CaaX prenyltransferases protein farnesyltransferase and protein geranylgeranyltransferase type I (GGTase-I) attach either a 15-carbon farnesyl group or a 20-carbon geranylgeranyl group, respectively, to the cysteine residue found within the CaaX motif at the C-terminus of protein substrates.16 GGTase-I substrates include Rho and Rac GTPases and the gamma subunit of most heterotrimeric G-proteins, and the inhibition of isoprenylation has been considered one of the rational and promising approaches to modulate the activity of oncogenic small GTP-binding proteins and heterotrimeric G-proteins with therapeutic importance for the treatment of cancers.16,18 Recently, a novel small molecular pyrazole-derived compound, GGTI-DU40 (Fig. 1), has been identified and characterized as a specific and potent inhibitor of GGTase-I. GGTI-DU40 has been shown to impair membrane localization of various geranylgeranylated G proteins, including Rho and Rap GTPases, by inhibiting the isoprenylation modification in different cell types.19
Figure 1.
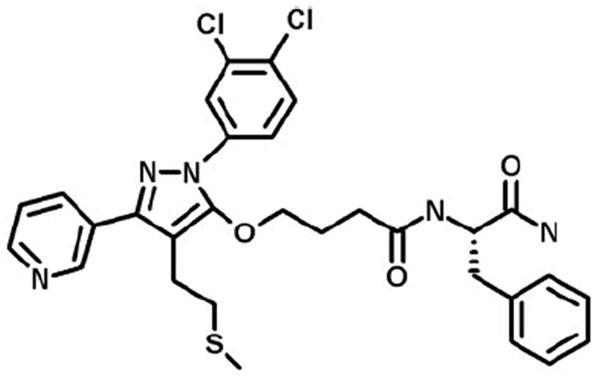
Structure of GGTI-DU40. Reprinted with permission from Peterson YK, Kelly P, Weinbaum CA, Casey PJ. A novel protein geranylgeranyltransferase-I inhibitor with high potency, selectivity, and cellular activity. J Biol Chem. 2006;281:12445–12450. © American Society for Biochemistry and Molecular Biology, Inc.
Rho GTPases and G-protein–coupled receptors regulate cell shape, actin cytoskeletal organization, cell adhesive interactions, and contractile activity, which have now been well recognized to participate in the modulation of aqueous humor outflow.2,6,7 Indeed, experiments using a peptidomimetic GGTI, GGTI-298, showed blocking geranylgeranylation can decrease leukocyte adhesion.20 In this study, we have explored the effects of GGTI-DU40 on aqueous humor outflow and TM cell cytoskeletal and adhesive characteristics. The results of this study reveal the promising effect of GGTI-DU40 on aqueous outflow through the TM pathway and identify GGTase-I as a potential molecular target for lowering IOP in glaucoma patients.
Materials and Methods
Materials
GGTI-DU40 was synthesized at the Duke Small Molecule Synthesis Facility. Geranylgeranyl diphosphate (GGPP) was purchased from Biomol, Inc. (Plymouth Meeting, PA). [3H]GGPP was purchased from PerkinElmer Life Sciences (Waltham, MA). Rhodamine-phalloidin, monoclonal antibodies of vinculin, and β-catenin were purchased from Sigma-Aldrich (St. Louis, MO). Phosphospecific MLC polyclonal antibody and monoclonal anti-Rap1GTPase antibody were from Cell Signaling Technology, Inc. (Beverly, MA) and Santa Cruz Biotechnology (Santa Cruz, CA), respectively. Anti-Gβ antibody (985) was a gift from Thomas Gettys (Pennington Biomedical Research Center, Baton Rouge, LA). All other chemicals were of analytical grade.
Cell Culture
Porcine TM (PTM) cells were isolated from freshly obtained cadaver eyes by collagenase IV digestion, as we described previously.21 Cells were cultured at 37°C under 5% CO2 in Dulbecco modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) and penicillin (100 U/mL)/streptomycin (100 μg/mL). All experiments were conducted using confluent cell cultures between passages 3 and 5.
Immunostaining
Porcine TM cells were grown to confluence on 2% gelatin-coated, glass coverslips. Cells were treated with GGTI-DU40 (1–40 μM dissolved in dimethyl sulfoxide) for 24 hours in the presence of 0.1% serum. Changes in cell shape were recorded with a phase-contrast microscope (IM 35; Carl Zeiss Meditec, Dublin, CA). After treatment with GGTI-DU40, cells were fixed with 3.7% formaldehyde and actin; focal adhesions and adherens junctions were stained with rhodamine-phalloidin, anti-vinculin antibody, and anti-β-catenin antibody in combination with TRITC-conjugated secondary antibody, respectively, as described previously.21 Representative fluorescence micrographs were recorded using a fluorescence microscope (Axioplan-II; Carl Zeiss Meditec).
RT-PCR
Human TM cells were isolated as we described earlier22 and were cultured at 37°C under 5% CO2 in DMEM containing 10% FBS and penicillin (100 U/mL)/streptomycin (100 μg/mL)/glutamine (292 μg/mL). Expression of GGTase-I in human TM cells (passage 4) was confirmed by RT-PCR analysis. Total RNA extracted (RNeasy Mini kit; Qiagen, Valencia, CA) from the human TM cells was treated with DNaseI to eliminate contamination from genomic DNA. RNA was then reverse transcribed (Advantage RT-for-PCR kit; Clontech, Mountain View, CA) according to the manufacturer’s instructions. Controls lacking reverse transcriptase (RT) were also set up to confirm the absence of genomic DNA-generated signals. PCR amplification was then performed on the resultant TM RT-derived complementary DNA libraries (Advantage RT-for-PCR kit), according to the manufacturer’s instructions, with sequence-specific forward and reverse oligonucleotide primers for the GGTase-I. RT-PCR–amplified DNA fragments were sequenced to confirm gene identity.
MLC Phosphorylation
To determine the effects of GGTI-DU40 on MLC phosphorylation in PTM cell cultures, cells were grown to confluence and treated with GGTI-DU40 for 24 hours in the absence of serum. The status of MLC phosphorylation in PTM cells was determined by urea-glycerol gel electrophoresis and immunoblot analysis, as we described previously,21 using phosphospecific anti-MLC (Thr18/Ser19) polyclonal antibody. Equal protein loading was verified by probing for actin with antisera for β-actin in the same cell lysates.
Cell Viability and Toxicity
To evaluate the effects of GGTI-DU40 on the viability of PTM cells, cells treated with drug for 24 hours were subsequently incubated with fluorescein diacetate and propidium iodide, as described in our previous studies.21 Viable cells and dead or damaged cells, which stained green and red, respectively, were evaluated by fluorescence microscopy.
Gβγ and Rap1 Membrane Partitioning and Immunoblotting
PTM cells were grown to confluence in Petri dishes and treated with GGTI-DU40 in the absence of serum for 24 hours. After drug treatment, cells were rinsed with cold PBS and lysed with ice-cold hypotonic lysis buffer (70 mM HEPES, pH 7.4, 2 mM EDTA, 1 mM dithiothreitol, and protease inhibitors), as described by Peterson et al.19 After sonication, the whole cell lysate was centrifuged at 100,000g for 45 minutes at 4°C. After protein concentration determination, the supernatant and the pellet fractions were subjected to SDS-PAGE electrophoresis with 4% to 20% gradient acrylamide gels. Proteins were transferred to nitrocellulose membranes and immunoblotted for Gβγ. Rap1 was immunoblotted using antisera specific for the unprenylated form of Rap1, and sample loading was verified by reprobing the blots with antisera specific for total Rap1.
GGTase-I Assay
GGTase-I activity was determined by the following incorporation of radiolabeled isoprenoid from 3H-GGPP into Ras protein (Ras-CVLL), as described previously.19 Briefly, the 100,000g supernatant of PTM cell lysates (25 μg protein) was used to initiate reactions containing 0.5 μM GGPP, and a concentration of 1 μM appropriately purified His-tagged Ras substrates (Ras-CVLL). As indicated in the figure legend (Fig. 2), GGTI-DU40 inhibitor was added at the indicated concentrations. Final dimethyl sulfoxide concentration was 2% for all samples. Reactions were carried out for 10 minutes at 30°C before precipitation and product determination.19
Figure 2.
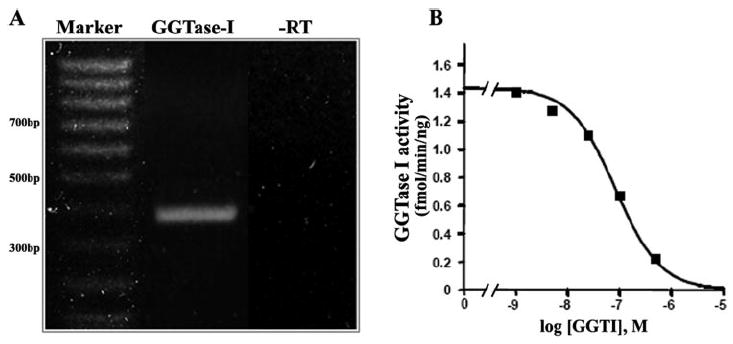
Geranylgeranyltransferase I expression and activity profiles in cultured TM cells. (A) Expression of GGTase-I in TM cells. RT-PCR analysis was performed using sequence-specific oligonucleotide primers corresponding to human GGTase-I and RNA extracted from human TM cells. Reactions lacking reverse transcriptase (−RT) were also set up to confirm lack of contamination of RNA with DNA. The PCR product was sequenced to confirm the identity of GGTase-I. (B) GGTase-I activity in TM cells. Soluble extracts (25 μg protein from 100,000g supernatant) of PTM cells were prepared, and GGTase-I activity was measured by quantifying the incorporation of radiolabeled isoprenoid from 3H-GGPP into Ras protein (Ras-CVLL) in the absence and presence of GGTI-DU40, as described in Materials and Methods. A representative analysis is illustrated to show the inhibition of GGTase-I activity in TM cell lysates by GGTI-DU40.
Perfusion
Porcine eyes (obtained fresh from a local abattoir) were placed in culture according to published techniques.23 Anterior segments from paired eyes were prepared by dissecting the eyes at the equator; removing the lens, iris, and vitreous; and rinsing thoroughly with culture medium before clamping them to a two-cannulae, modified Petri dish, as we described earlier.23 To avoid disturbing the TM tissue, anterior segments were left with some ciliary body and iris. Anterior segments were perfused with high-glucose DMEM containing 0.1% FBS, penicillin (100 U/mL), streptomycin (100 μg/mL), gentamicin (36 μg/mL), and amphotericin (0.25 μg/mL) at a constant flow (3 μL/min), using microinfusion pumps (Harvard Bioscience, South Natick, MA), under 5% CO2 at 37°C. Perfusion pressure was monitored continuously with a pressure transducer connected to the second cannula and was recorded with an automated computerized system. After the initial baseline aqueous outflow facility was recorded for 36 hours, the anterior segments of the test eyes were exchanged into, and perfused with, medium containing GGTI-DU40 (10 or 25 μM) for 6 days. Contralateral fellow eyes were perfused with medium containing vehicle alone. Data were recorded continuously at 1-minute intervals over 6 days. The outflow facility at the initial 36-hour perfusion before exposure of eyes to drug was taken as baseline facility. The percentage of change in the aqueous outflow facility from the baseline values was computed for drug- and sham-perfused eyes. At each 12-hour interval, the significance of differences in median percentage of change in outflow facility was assessed with the paired t-test.
Results
Expression of GGTase-1 in Human TM Cells
To confirm the expression of the GGTI-DU40 drug target GGTase-I, total RNA extracted from the cultured human TM cells was subjected to RT-PCR analysis using the sequence-specific oligonucleotide primers. Figure 2A shows the expression of GGTase-I in HTM cells. In addition to detecting the GGTase-I transcripts, we also determined the GGTase-I enzyme activity in the TM cell lysates derived from the porcine species in the absence and presence of GGTI-DU40. As shown in Figure 2B, GGTase-I activity was readily detectable in the TM cell lysates and was inhibited by GGTI-DU40 in a concentration-dependent manner. The IC50 (half-maximal inhibitory concentration) for GGTI-DU40 inhibition of TM cell GGTase-I activity was roughly 70 nM, and complete inhibition was observed at 5 μM. Data in Figure 2B depict representative results of two independent analyses.
GGTI-DU40–Induced Changes in TM Cell Shape, Actin Cytoskeletal Organization, and Cell Adhesive Interactions
To determine the influence of inhibition of protein isoprenylation mediated by GGTase-I on TM cell morphology, the confluent cultures of porcine PTM cells maintained in 0.1% fetal bovine serum for 24 hours were treated with different concentrations (5–40 μM) of GGTI-DU40, and the changes in cell morphology were monitored by viewing under a phase-contrast microscope. GGTI-DU40 treatment of TM cells resulted in dose- and time-dependent influences on cell morphology. TM cells exhibited cell separation and cell rounding with GGTI-DU40 treatment starting from 6 hours, and by 24 hours cells treated with 10 μM GGTI-DU40 appeared completely round (Fig. 3).
Figure 3.
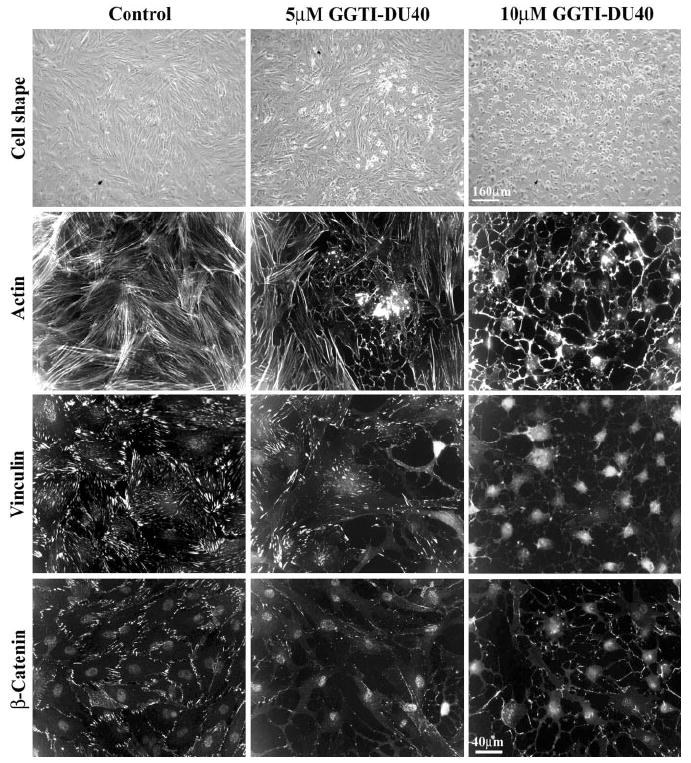
GGTI-DU40 –induced changes in cell shape, actin cytoskeletal organization, focal adhesions, and cell– cell junctions in cultured PTM cells. The effects of GGTI-DU40 on TM cell morphology, actin cytoskeletal organization, focal adhesions, and adherens junctions were evaluated by treating cells for 24 hours with a 5-μM or 10-μM concentration of drug. Drug-treated TM cells exhibit dose-dependent changes in morphologic and histologic attributes, such as cell rounding and disorganized actin filaments, together with loss of actin stress fibers, decreased focal adhesions (stained with vinculin antibody), and decreased cell– cell junctions (stained with β-catenin antibody).
The effects of GGTI-DU40 on TM cell morphology were associated with decreased actin stress fibers, as determined by recording the actin filament staining with rhodamine phalloidin. TM cells treated with 10 μM GGTI-DU40 for 24 hours showed almost no detectable actin stress fibers (Fig. 3). Similarly, the drug-treated cells exhibited a dose-dependent decrease in focal adhesions and cell– cell junctions in a time-dependent manner. TM cells treated with GGTI-DU40 (5 and 10 μM) for 24 hours and immunostained for focal adhesions and adherens junctions with antibodies specific for vinculin and β-catenin, respectively, showed marked decreases in their staining, as shown in Figure 3. GGTI-DU40–induced changes in cell morphology, actin filament depolymerization, and decreased focal adhesions were found to be reversible within 24 to 36 hours after drug withdrawal from the cell culture media (data not shown). Further, under drug treatment with 10 and 25 μM GGTI-DU40 for 24 hours, TM cells did not exhibit any significant increase in cell toxicity over the control cells based on live cell imaging of fluorescein diacetate fluorescence and propidium iodide incorporation (not shown).
Decreased Myosin II Phosphorylation by GGTI-DU40
Because many of the small GTP-binding proteins, including Rho and Rac, and the βγ subunits of heterotrimeric G-proteins such as Gq and G12/13, which are known to control tissue contractile activity by regulating the myosin II phosphorylation, are isoprenylated through GGTase-I,12,16,19 we determined the effects of GGTI-DU40 on TM cell myosin II phosphorylation. The contractile and relaxation characteristics of many tissues are regulated by the status of myosin light chain (MLC), the regulatory subunit of myosin II.12 Therefore, confluent cultures of PTM cells maintained in 0.1% FBS for 24 hours were treated with GGTI-DU40 (5 and 10 μM) for 24 hours and evaluated for changes in the status of MLC phosphorylation by immunoblot analysis using anti-phospho-MLC–specific antibody. Drug-treated TM cells showed a dose-dependent, significant decrease in the levels of phosphorylated MLC. A representative immunoblot of MLC phosphorylation derived from the drug-treated cell lysates and its quantitative differences between the control and drug-treated samples is shown in Figures 4A and 4B, respectively.
Figure 4.
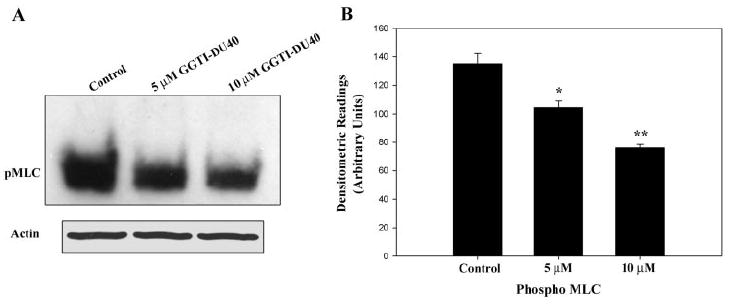
GGTI-DU40–induced decreases in myosin light-chain phosphorylation in TM cells. To determine the effects of GGTI-DU40 on MLC phosphorylation in TM cells, PTM cells cultured under 0.1% serum for 24 hours were treated with different concentrations of GGTI-DU40 (24 hours). Cell lysates were prepared and evaluated for changes in MLC phosphorylation using immunoblot analysis in conjunction with a phosphospecific anti-MLC polyclonal antibody. (A) Representative immunoblot of MLC phosphorylation in drug-treated samples. (B) Quantitative and significant differences in the MLC phosphorylation between the drug-treated and control samples.
GGTI-DU40–Induced Inhibition of Isoprenylation of G Proteins and Small GTP-Binding Proteins in TM Cells
To determine the impact of GGTI-DU40 treatment on the modification of geranylgeranylated proteins in TM cells, the drug (5 and 10 μM for 24 hours)-treated TM cells, as described earlier, were examined for the partitioning of the Gβγ subunit between the soluble and membrane fraction (100,000g supernatants and pellets) by immunoblot analysis. PTM cells treated with 10 μM GGTI-DU40 for 24 hours revealed increased levels of Gβγ in the soluble fraction compared with control cells in which the Gβγ was partitioned primarily to the insoluble or membrane fraction, indicating impaired isoprenylation of Gβγ in the drug-treated cells and leading to its accumulation in the soluble fraction (Fig. 5A). In addition to Gβγ, we examined the isoprenylation status of Rap1, a small GTP-binding protein involved in integrin-mediated cell adhesion signaling.24 Here we used Rap1 as a representative of geranylgeranylated small GTP-binding proteins and examined its isoprenylation status in the GGTI-DU40–treated TM cells by immunoblot analysis using an antibody specific to the nonisoprenylated Rap1. As shown in Figure 5B, TM cells treated with 5 and 10 μM GGTI-DU40 for 24 hours showed increased levels of nonisoprenylated Rap1 compared with control cells. In these same cell lysates, the levels of total Rap1 were determined using the anti-Rap1 antibody by immunoblot analysis and were found to be the same between the control and the drug-treated samples (Fig. 5B).
Figure 5.
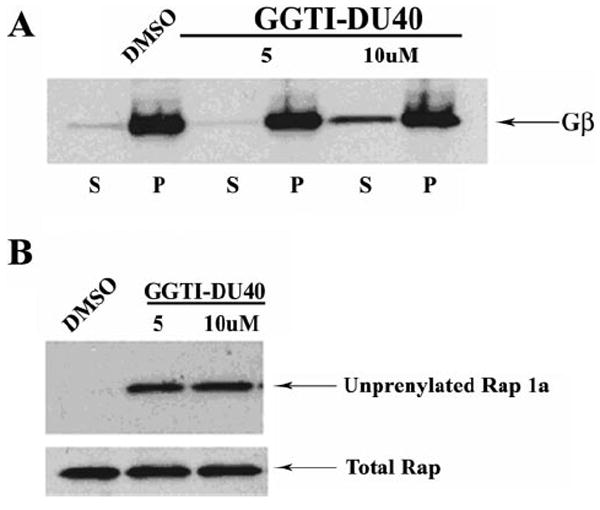
Effects of GGTI-DU40 treatment on isoprenylation of Gβγ and Rap1 in TM cells. (A) Impact of GGTI-DU40 treatment on Gβγ membrane association. TM cells were treated with the indicated concentrations of drug, lysed, and separated into soluble and insoluble fractions (100,000g pellet). Soluble (S) and insoluble (P) fractions were monitored for changes in the levels of cytosolic versus membrane-associated forms of Gβγ by immunoblot analysis. Accumulation of Gβγ in the soluble fractions of the drug-treated (10 μM) samples was observed, indicating impaired membrane targeting because of altered isoprenylation. (B) Impact of GGTI-DU40 treatment on Rap1 processing. Accumulation of unprenylated Rap1a in the drug-treated TM cells was assessed using an antibody that selectively recognizes unprenylated Rap1. Total Rap1 levels are shown for comparison and were found to be the same between control and drug-treated samples.
Increased Aqueous Humor Outflow Facility with Perfusion of GGTI-DU40
Organ-cultured porcine eye anterior segments (n = 8) perfused with GGTI-DU40 (10 and 25 μM) at a constant flow demonstrated a time-dependent increase in aqueous outflow facility (Figs. 6A, 6B). This increase in facility was progressive, starting from approximately 5% (at 6 hours) and reaching 40% to 45% (at 66 hours) after drug perfusion with GGTI-DU40. In the case of 10 μM drug-perfused samples, the change in outflow facility was maintained at approximately 40% from baseline until 108 hours, and later it started to drop to 30% (Fig. 6A). With 25 μM drug, the change in outflow facility reached up to 50% at 66 hours after drug perfusion and was maintained at that level until the end of the 144-hour perfusion (Fig. 6B). Sham-treated eyes exhibited a lower increase in facility, starting with almost no change from the baseline facility (±5%) at 6 hours and reaching a maximum of 15% at 66 hours of perfusion that was maintained until the end of the 144-hour perfusion. Although there was a noticeable variance in each sample, especially in the 25-μM drug-perfused samples, the difference in aqueous outflow facility between sham- and drug-treated samples (n = 8) was statistically significant (*P < 0.05 and **P < 0.01) after 10 and 25 μM GGTI-DU40 perfusion.
Figure 6.
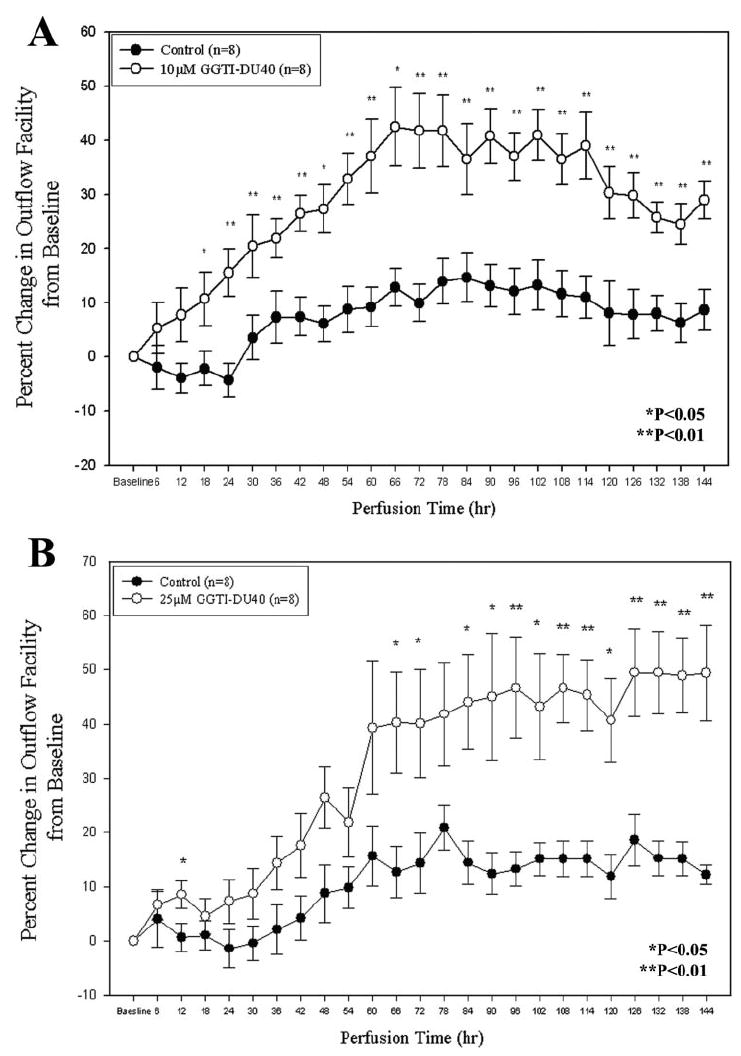
GGTI-DU40–induced increases in aqueous humor outflow facility in organ-cultured porcine eye anterior segments. To determine the effects of GGTI-DU40 on aqueous humor outflow, organ cultured anterior segments of porcine eyes were perfused with drug, followed by time-dependent measurements of changes in aqueous outflow. Perfusions carried out with two different concentrations (10 and 25 μM) of drug exhibited significant and time-dependent increases in aqueous outflow facility compared with sham-treated specimens.
Discussion
This study provides evidence for a critical role of GGTase-I in the modulation of aqueous humor outflow and documents the effects of GGTI-DU40, a specific inhibitor of GGTase-1 activity, on aqueous humor outflow in the organ-cultured anterior eye segment-based perfusion model. Perfusion of organ cultured porcine eye anterior segments with GGTI-DU40, a potent and specific inhibitor of GGTase-1, leads to increases in aqueous humor outflow, a response that appears to be linked to impaired activity of monomeric GTPases including the Rho GTPases and of heterotrimeric G-proteins. GGTI-DU40 treatment was also accompanied by alterations in cell morphology and cytoskeletal integrity in TM cells. Furthermore, the concentration(s) of GGTI-DU40 that elicited changes in aqueous humor outflow in perfused eyes were also effective at triggering biochemical and cellular/morphologic changes in cultured TM cells. These observations identify GGTase-I as a novel and promising molecular target for lowering increased IOP in glaucoma patients.
Based on data acquired from the effects of various pharmacologic modulators of contractile function, cytoskeletal integrity, and cell adhesions in TM cells, inhibitors of TM tissue contractility and actin/myosin interactions have been identified as having potential therapeutic significance for lowering IOP in patients with glaucoma.4,6-8 Members of the Rho GTPase subfamily of monomeric GTPases, including Rho, Rac, and Cdc42, Rap1, and heterotrimeric G-proteins including G12/13 and Gq, which are isoprenylated proteins, play critical role(s) in smooth muscle contraction, maintenance of cell morphology, cytoskeletal organization, trafficking, and cell adhesive interactions.12-16,24,25 Specific inhibitors of Rho GTPase, Rho kinase, myosin light chain kinase, and myosin II have been demonstrated to increase aqueous humor outflow and to lower IOP in various perfusion and live animal models.4,8,21,26-29 In addition to this class of pharmacologic agents, cholesterol-lowering statins, which are inhibitors of HMG-CoA reductase (the rate-limiting enzyme of the cholesterol biosynthetic pathway) have also been documented to increase aqueous outflow in perfused eye anterior segments.23 Anecdotally, human patients on a statin treatment regimen are reported to have a lowered incidence of glaucoma.30 Although the precise mechanism behind the lowered incidence of glaucoma in this group of patients is not completely understood, supplementation with geranylgeranyl pyrophosphate has been shown to reverse the statin-induced increases in aqueous humor outflow facility in perfused eyes, indicating a potential significance for protein geranylgeranyl isoprenylation in the modulation of aqueous humor outflow.23 Furthermore, statins have been demonstrated to impair Rho GTPase activity in various cell types because of their ability to lower the levels of isoprenoids.23,31
Based on the widely documented observations supporting a role for the Rho GTPases in the regulation of aqueous humor outflow and the importance of the isoprenylation modification for the activity of Rho GTPases, we assessed in this study the role of GGTase-I on aqueous outflow using a specific inhibitor, GGTI-DU40. GGTI-40 is a potent inhibitor of GGTase-I and has been demonstrated to impair the processing of various geranylgeranyl isoprenylated proteins, including Rho and Rap1.19 Mechanistically, GGTI-DU40 has been shown to compete for the CaaX peptide-binding site on GGTase-I.19 Lysates derived from PTM cells treated with 5 μM GGTI-DU40 exhibited a complete inhibition of GGTase-I activity (Fig. 2B). Further, GGTI-DU40–treated TM cells also revealed decreased processing of Rap1 and Gβγ, indicating impaired isoprenylation of Rho GTPases and heterotrimeric G-proteins in response to drug treatment. Additionally, the GGTI-DU40–induced effects on TM cell morphology, cytoskeletal organization, and myosin light chain phosphorylation appeared similar to those triggered by statins and Rho kinase inhibitors.21,23 The drug concentration required for these effects, however, was much lower for GGTI-DU40 (5–10 μM) than for the statins.23 Similarly, perfusion with 10 μM GGTI-DU40 also induced a significant increase in aqueous outflow facility.
Taken together, these observations suggest that, unlike statins, which indirectly impair not only the activity and function of the Rho GTPases but also those of all other isoprenylated proteins including Ras and Rab GTPases, the GGTase-I inhibitor GGTI-DU40 exhibits higher selectivity to inhibit geranylgeranyl modification of the Rho GTPases.19 Moreover, unlike the Rho GTPase and Rho kinase inhibitors, the use of GGTase-I inhibitors impairs Rho and Rac GTPase activities.19 Rac GTPase is recognized to play a critical role in regulating cell– cell junctions and thereby influences para-cellular permeability barrier function.32,33 Enhanced permeability barrier activity of the inner wall of the Schlemm canal is presumed to increase resistance to aqueous humor drainage through the conventional route.8,34 Therefore, it is possible that the ability of GGTI-DU40 to target the activity of both Rac GTPase and Rho GTPase underlies the increased efficacy this inhibitor exerts on increasing aqueous drainage, as opposed to inhibitors of either Rho GTPase or Rho kinase. Both Rho GTPase and Rho kinase have been reported to influence retinal ganglion cell survival, and statins and inhibitors of these proteins have been documented to have neuroprotective effects.35-38 Therefore, it is reasonable to speculate that GGTI-DU40 might also exhibit a similar neuroprotective activity. Further studies are warranted to explore and confirm this hypothesis and to assess the in vivo effects of GGTI-DU40 on IOP.
In conclusion, findings from this study reveal a critical role for GGTase-I in the modulation of aqueous outflow facility, with the inhibition of GGTase-I by GGTI-DU40 leading to increases in aqueous outflow facility. Thus, the direct inhibition of GGTase-I in the aqueous outflow pathway may be of therapeutic significance in lowering intraocular pressure in patients with glaucoma.
Acknowledgments
The authors thank Peifeng Deng and Missy Infante for technical assistance.
Supported by National Institutes of Health Grants EY12201 and EY013573 (PVR), Research to Prevent Blindness Wasserman Merit Award (PVR), and Grants F32-GM073420 (YKP) and GM46372 (PJC).
Footnotes
Disclosure: P.V. Rao, P; Y.K. Peterson, P; T. Inoue, None; P.J. Casey, P
References
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363(9422):1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Tan JC, Peters DM, Kaufman PL. Recent developments in understanding the pathophysiology of elevated intraocular pressure. Curr Opin Ophthalmol. 2006;17(2):168–174. doi: 10.1097/01.icu.0000193079.55240.18. [DOI] [PubMed] [Google Scholar]
- 3.Lutjen-Drecoll E. Functional morphology of the trabecular mesh-work in primate eyes. Prog Retin Eye Res. 1999;18(1):91–119. doi: 10.1016/s1350-9462(98)00011-1. [DOI] [PubMed] [Google Scholar]
- 4.Gabelt BT, Kaufman PL. Changes in aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res. 2005;24(5):612–637. doi: 10.1016/j.preteyeres.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Tian B, Geiger B, Epstein DL, Kaufman PL. Cytoskeletal involvement in the regulation of aqueous humor outflow. Invest Ophthalmol Vis Sci. 2000;41(3):619–623. [PubMed] [Google Scholar]
- 6.Wiederholt M, Thieme H, Stumpff F. The regulation of trabecular meshwork and ciliary muscle contractility. Prog Retin Eye Res. 2000;19(3):271–295. doi: 10.1016/s1350-9462(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 7.Rao PV, Epstein DL. Rho GTPase/Rho kinase inhibition as a novel target for the treatment of glaucoma. BioDrugs. 2007;21:167–177. doi: 10.2165/00063030-200721030-00004. [DOI] [PubMed] [Google Scholar]
- 8.Epstein DL, Rowlette LL, Roberts BC. Acto-myosin drug effects and aqueous outflow function. Invest Ophthalmol Vis Sci. 1999;40(1):74–81. [PubMed] [Google Scholar]
- 9.Lutjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFβ2 for the pathogenesis of the disease. Exp Eye Res. 2005;81(1):1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Mettu PS, Deng PF, Misra UK, Gawdi G, Epstein DL, Rao PV. Role of lysophospholipid growth factors in the modulation of aqueous humor outflow facility. Invest Ophthalmol Vis Sci. 2004;45(7):2263–2271. doi: 10.1167/iovs.03-0960. [DOI] [PubMed] [Google Scholar]
- 11.Rao PV, Deng P, Sasaki Y, Epstein DL. Regulation of myosin light chain phosphorylation in the trabecular meshwork: role in aqueous humour outflow facility. Exp Eye Res. 2005;80(2):197–206. doi: 10.1016/j.exer.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 12.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 13.Wettschureck N, Offermanns S. Rho/Rho-kinase mediated signaling in physiology and pathophysiology. J Mol Med. 2002;80(10):629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- 14.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420(6916):629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 15.Burridge K, Wennerberg K. Rho and Rac take center stage. Cell. 2004;116(2):167–179. doi: 10.1016/s0092-8674(04)00003-0. [DOI] [PubMed] [Google Scholar]
- 16.Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 17.Cox AD, Der CJ. Farnesyltransferase inhibitors: promises and realities. Curr Opin Pharmacol. 2002;2(4):388–393. doi: 10.1016/s1471-4892(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 18.Philips MR, Cox AD. Geranylgeranyltransferase I as a target for anti-cancer drugs. J Clin Invest. 2007;117(5):1223–1225. doi: 10.1172/JCI32108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peterson YK, Kelly P, Weinbaum CA, Casey PJ. A novel protein geranylgeranyltransferase-I inhibitor with high potency, selectivity, and cellular activity. J Biol Chem. 2006;281(18):12445–12450. doi: 10.1074/jbc.M600168200. [DOI] [PubMed] [Google Scholar]
- 20.Liu L, Moesner P, Kovach NL, et al. Integrin-dependent leukocyte adhesion involves geranylgeranylated protein(s) J Biol Chem. 1999;274(47):33334–33340. doi: 10.1074/jbc.274.47.33334. [DOI] [PubMed] [Google Scholar]
- 21.Rao PV, Deng PF, Kumar J, Epstein DL. Modulation of aqueous humor outflow facility by the Rho kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci. 2001;42(5):1029–1037. [PubMed] [Google Scholar]
- 22.Sanka K, Maddala R, Epstein DL, Rao PV. Influence of actin cytoskeletal integrity on matrix metalloproteinase-2 activation in cultured human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48(5):2105–2114. doi: 10.1167/iovs.06-1089. [DOI] [PubMed] [Google Scholar]
- 23.Song J, Deng PF, Stinnett SS, Epstein DL, Rao PV. Effects of cholesterol-lowering statins on the aqueous humor outflow pathway. Invest Ophthalmol Vis Sci. 2005;46(7):2424–2432. doi: 10.1167/iovs.04-0776. [DOI] [PubMed] [Google Scholar]
- 24.Kooistra MR, Dube N, Bos JL. Rap1: a key regulator in cell-cell junction formation. J Cell Sci. 2007;120(pt 1):17–22. doi: 10.1242/jcs.03306. [DOI] [PubMed] [Google Scholar]
- 25.Cox AD, Der CJ. Protein prenylation: more than just glue? Curr Opin Cell Biol. 1992;4(6):1008–1016. doi: 10.1016/0955-0674(92)90133-w. [DOI] [PubMed] [Google Scholar]
- 26.Honjo M, Inatani M, Kido N, et al. Effects of protein kinase inhibitor, HA1077, on intraocular pressure and outflow facility in rabbit eyes. Arch Ophthalmol. 2001;119(8):1171–1178. doi: 10.1001/archopht.119.8.1171. [DOI] [PubMed] [Google Scholar]
- 27.Liu X, Hu Y, Filla MS, et al. The effect of C3 transgene expression on actin and cellular adhesions in cultured human trabecular meshwork cells and on outflow facility in organ cultured monkey eyes. Mol Vis. 2005;11:1112–1121. [PubMed] [Google Scholar]
- 28.Zhang M, Rao PV. Blebbistatin, a novel inhibitor of myosin II ATPase activity, increases aqueous humor outflow facility in perfused enucleated porcine eyes. Invest Ophthalmol Vis Sci. 2005;46(11):4130–4138. doi: 10.1167/iovs.05-0164. [DOI] [PubMed] [Google Scholar]
- 29.Vittitow JL, Garg R, Rowlette LL, Epstein DL, O’Brien ET, Borras T. Gene transfer of dominant-negative RhoA increases outflow facility in perfused human anterior segment cultures. Mol Vis. 2002;8:32–44. [PubMed] [Google Scholar]
- 30.McGwin G, Jr, McNeal S, Owsley C, Girkin C, Epstein D, Lee PP. Statins and other cholesterol-lowering medications and the presence of glaucoma. Arch Ophthalmol. 2004;122(6):822–826. doi: 10.1001/archopht.122.6.822. [DOI] [PubMed] [Google Scholar]
- 31.Liao JK, Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wojciak-Stothard B, Potempa S, Eichholtz T, Ridley AJ. Rho and Rac but not Cdc42 regulate endothelial cell permeability. J Cell Sci. 2001;114(pt 7):1343–1355. doi: 10.1242/jcs.114.7.1343. [DOI] [PubMed] [Google Scholar]
- 33.Braga VM, Machesky LM, Hall A, Hotchin NA. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137(6):1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alvarado JA, Betanzos A, Franse-Carman L, Chen J, Gonzalez-Mariscal L. Endothelia of Schlemm’s canal and trabecular meshwork: distinct molecular, functional, and anatomic features. Am J Physiol Cell Physiol. 2004;286(3):C621–C634. doi: 10.1152/ajpcell.00108.2003. [DOI] [PubMed] [Google Scholar]
- 35.Bertrand J, Winton MJ, Rodriguez-Hernandez N, Campenot RB, McKerracher L. Application of Rho antagonist to neuronal cell bodies promotes neurite growth in compartmented cultures and regeneration of retinal ganglion cell axons in the optic nerve of adult rats. J Neurosci. 2005;25(5):1113–1121. doi: 10.1523/JNEUROSCI.3931-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amarenco P, Moskowitz MA. The dynamics of statins: from event prevention to neuroprotection. Stroke. 2006;37(2):294–296. doi: 10.1161/01.STR.0000201856.90105.ab. [DOI] [PubMed] [Google Scholar]
- 37.Delanty N, Vaughan CJ, Sheehy N. Statins and neuroprotection. Exp Opin Investig Drugs. 2001;10(10):1847–1853. doi: 10.1517/13543784.10.10.1847. [DOI] [PubMed] [Google Scholar]
- 38.Lingor P, Tonges L, Pieper N, et al. ROCK inhibition and CNTF interact on intrinsic signalling pathways and differentially regulate survival and regeneration in retinal ganglion cells. Brain. 2008;131:250–263. doi: 10.1093/brain/awm284. [DOI] [PubMed] [Google Scholar]


