Abstract
Expression of the human epidermal growth factor receptor (EGFR) in murine Schwann cells results in loss of axon–Schwann cell interactions and collagen deposition, modeling peripheral nerve response to injury and tumorigenesis. Mast cells infiltrate nerves in all three situations. We show that mast cells are present in normal mouse peripheral nerve beginning at 4 weeks of age, and that the number of mast-cells in EGFR+ nerves increases abruptly at 5–6 weeks of age as axons and Schwann cells dissociate. The increase in mast cell number is preceded and accompanied by elevated levels of mRNAs encoding the mast-cell chemoattractants Rantes, SCF and VEGF. Genetic ablation of mast cells and bone marrow reconstitution in W41 × EGFR+ mice indicate a role for mast cells in loss of axon – Schwann cell interactions and collagen deposition. Pharmacological stabilization of mast cells by disodium cromoglycate administration to EGFR+ mice also diminished loss of axon – Schwann cell interaction. Together these three lines of evidence support the hypothesis that mast cells can contribute to alterations in peripheral nerves.
Keywords: Schwann cell, NF1, EGFR, Remak bundle
INTRODUCTION
The peripheral nerve is a complex organ containing neuronal axons, Schwann cells, fibroblasts, blood vessels and mast cells. In addition to their well-characterized role in allergic inflammation (Galli et al., 2005), mast cells are implicated in arthritis and multiple sclerosis (Lee et al., 2002; Tanzola et al., 2003). Although mast cells were identified in peripheral nerve over 100 years ago (Gamble and Goldby, 1961) their function in this tissue is unknown. In the normal peripheral nerve, large axons are surrounded by a Schwann cell myelin sheath to allow for efficient transmission of electric current. Small axons are grouped together in Remak bundles, that are organized and ensheathed by the membrane of a single non-myelinating Schwann cell (Corfas et al., 2004). Thus, axons are in intimate contact with Schwann cell processes that wrap them, and the two cell types are co-dependent for proper development, morphology and function.
Axon – Schwann cell interactions are disrupted in pathological conditions, including after nerve injury and in tumors of the peripheral nervous system. Under these conditions mast-cell numbers increase but a possible role of mast cells in nerve homeostasis has not been studied.
Peripheral nerve tumors highly infiltrated by mast cells are the hallmark of the disorder neurofibromatosis type 1 (NF1) (Pineda, 1965; Olsson, 1971), and patients often complain of pain and itchiness localized to regions of their tumors, neurofibromas (Riccardi, 1987), which is a classic indicator of mast-cell activation. NF1 affects 1:3000 live births and results from mutations in the NF1 gene. Neurofibromas are composed of elements of normal peripheral nerve: mainly Schwann cells and fibroblasts, as well as mast cells and neurons. The tumors can be highly disfiguring and are a major source of patient morbidity (Rasmussen and Friedman, 2000). Schwann cells are the crucial pathogenic cell type in neurofibroma formation because mutations in both NF1 alleles are present only in neurofibroma Schwann cells (Kluwe et al., 1999; Serra et al., 2001). Treatment of NF1 patients with Ketotifen, a mast-cell stabilizer, minimizes neurofibroma-associated pain, although tumor growth was not quantified (Riccardi, 1987; Riccardi, 1993).
Schwann cell survival, proliferation and differentiation are regulated via the ErbB2/ErbB3 receptor signaling complex (Jessen et al., 1994; Leimeroth et al., 2002). The related ErbB1 receptor, epidermal growth factor receptor (EGFR), is not expressed by Schwann cells but is expressed in Schwann cells with Nf1 loss in vitro, in cells derived from mouse and human NF1 malignant nerve tumors, and in a few S100+ cells from primary neurofibromas (DeClue et al., 2001; Li et al., 2002; Carroll and Stonecypher, 2005). We previously generated mice that express human EGFR in Schwann cells (EGFR+ mice) through the use of the CNPase promoter, which serves as a promoter throughout the Schwann cell lineage (Ling et al., 2005; Gravel et al., 1994). A striking peripheral nerve phenotype develops in EGFR+ mice, which is similar to pathological changes found in injured peripheral nerve and in human neurofibromas, with mast-cell infiltration, collagen deposition and disrupted axon – Schwann cell interactions. Chen et al. showed that blocking ErbB signaling in non-myelinating Schwann cell results in a progressive peripheral neuropathy (Chen et al., 2003). This pathology is distinct from changes that manifest in the EGFR+ model, but both point to a crucial role for ErbB-family signaling in proper maintenance of axon – Schwann cell interactions in nerve homeostasis, especially in non-myelin forming Schwann cells. The EGFR+ mouse affords a straightforward system in which factors affecting nerve homeostasis can be assessed.
We used the EGFR+ model to directly test the role of mast cells in nerve pathology. By time-course analysis, we show that arrival of increased mast cells to EGFR+ nerve correlates with phenotype onset. By ablation and reconstitution we show that bone marrow-derived cells play a causative role in the development of pathologies observed in EGFR+ nerves. Pharmacological stabilization of mast cells by disodium cromoglycate (cromolyn) administration also blocks EGFR+-induced pathology. These studies offer new insights into nerve homeostasis and describe a novel role for mast cells in nerve pathology.
OBJECTIVE
The goal of this study was to test the hypothesis that mast cells play a causative role in the development of peripheral nerve pathology observed in mice that aberrantly express EGFR in Schwann cells. To this end, we correlated mast-cell influx into nerve with disruption of axon–glial interactions, and utilized the mast cell-deficient mouse strain W41 to genetically ablate mast cells in EGFR+ mice. We also pharmacologically stabilized mast cells by cromolyn administration to define the role of mast cells in initiation and maintenance of nerve pathology.
METHODS
Mice and genotyping
C57Bl/6 CNPase-EGFR transgenic mice, which express wild type, human EGFR in Schwann cells, had been backcrossed onto the C57Bl/6 background for more than seven generations at the initiation of this study. Mice were genotyped as described (Ling et al., 2005). W41 mice were purchased from Jackson Laboratories (Bar Harbor). They were on the C57Bl/6 strain and were intercrossed at least two generations with the CNPase-EGFR C57Bl/6 transgenic mice. The W41 mice are genotyped based on coat color; W41/W41 mice are white with gray spots, and W41/+ mice are black with a single white belly spot. Mice were housed in a temperature- and humidity-controlled viaticum that was kept on a 12-hour dark/light cycle with free access to food and water. The animal care and use committees of the University of Cincinnati and the Cincinnati Children’s Hospital Research Foundation approved all animal use.
Histology
We sacrificed mice by perfusion fixation with 4% paraformaldehyde and harvested sciatic and saphenous nerves. We embedded nerves in paraffin and cut 5–6 µm thin sections proximal to the sciatic bifurcation. We stained sections with either toluidine blue or Giemsa for mast cells and Masson’s trichrome for collagen. Mast cells were identified in tissue sections from their characteristic granular, metachromatic appearance.
Quantitative real-time PCR
Sciatic nerves were harvested from wild-type and EGFR+ mice at designated ages. cDNA was generated and triplicate PCR reactions per primer set were performed as described (Ling et al., 2005). Primer sequences are published (Ling et al., 2005).
Examination of nerve ultrastructure
Disruption of axon–Schwann interaction was quantified as a percentage of grouped unmyelinated axons in electron micrographs as described in Ling et al. (Ling et al., 2005). An axon bundle was considered grouped if three or more small axons were wrapped by a single Schwann cell, and ungrouped if two or fewer. At least 200 axon bundles were counted in each experimental group using micrographs from 3–4 mice per genotype.
BrdU incorporation
Proliferation was assessed in tissue sections as described (Wu et al., 2006).
Bone marrow engraftment
Congenic wild-type adult C57Bl/6 mice from the W41 × EGFR+ intercrosses were sacrificed and long leg bones and iliac crest removed and placed in sterile RPMI. In a sterile hood, marrow was flushed from bones, triturated and spun down at 1000 RPM for 10 min. Cells were then resuspended in 4 ml red blood cell (RBC) lysis buffer for 4 min at 25°C. RBC lysis buffer is an ammonium chloride solution containing 4.15 g NH4Cl and 0.65 g Tris (0.01 M final concentration) in 500 ml ddH2O. The solution was brought to a pH of 7.5 using 1 M HCl and then sterile filtered. The lysis reaction was stopped by adding 6 ml of RMPI + 10% FBS. Cells were spun at 1000 RPM for 10 min, washed in RPMI + 10% FBS. The spin and wash steps were repeated and cells reconstituted in sterile 0.1 M PBS for injections. Four-week-old mice were injected via tail vein with 1.8 × 107 cells in ~150 µl. Non-engrafted control mice were injected with 150 µl of sterile 0.1 M PBS. Mice were sacrificed 12 weeks after injection and analyzed.
Cromolyn administration
A solution of Cromolyn (Sigma) was made fresh daily in sterile 1 × PBS. For short-term studies, mice were injected i.p. with 10 mg kg−1 cromolyn solution or sterile 1 × PBS daily for 21 days, beginning at 6 weeks of age. Mice were sacrificed at 9 weeks of age and analyzed. For long-term studies, mice were injected daily with the same dose/volume of cromolyn or PBS for 8 weeks, beginning at 4 months of age. Mice were sacrificed at 6 months of age and analyzed.
Statistical analysis
All statistical analysis was performed using ANOVA and Kruskall-Wallis.
RESULTS
Time course of mast-cell recruitment to nerve and EGFR+ nerve pathology
Although the presence of mast cells in normal peripheral nerve is well documented their function and temporal arrival to nerves has not been analyzed. Therefore, we monitored the timing of mast-cell recruitment to normal nerves. We used total mast cells per nine high-powered fields (400 ×) in sciatic nerve to monitor mast cell numbers. Mast cells arrived at the same time and in similar numbers in wild-type and EGFR+-transgenic nerves at 4 weeks of age; mast cells began to increase in the EGFR+ animals at 5–6 weeks (Fig. 1A). Nerve mast-cell number and the extent of axon – Schwann cell disruption were assessed at weekly intervals between 3 and 7 weeks of age to elucidate the sequence of events leading to nerve disruption in the EGFR model (n = 3 for each genotype at each time point).
Fig. 1. The arrival of mast cells at peripheral nerves correlates with disruption of axon–Schwann cell interactions in EGFR+ mice.
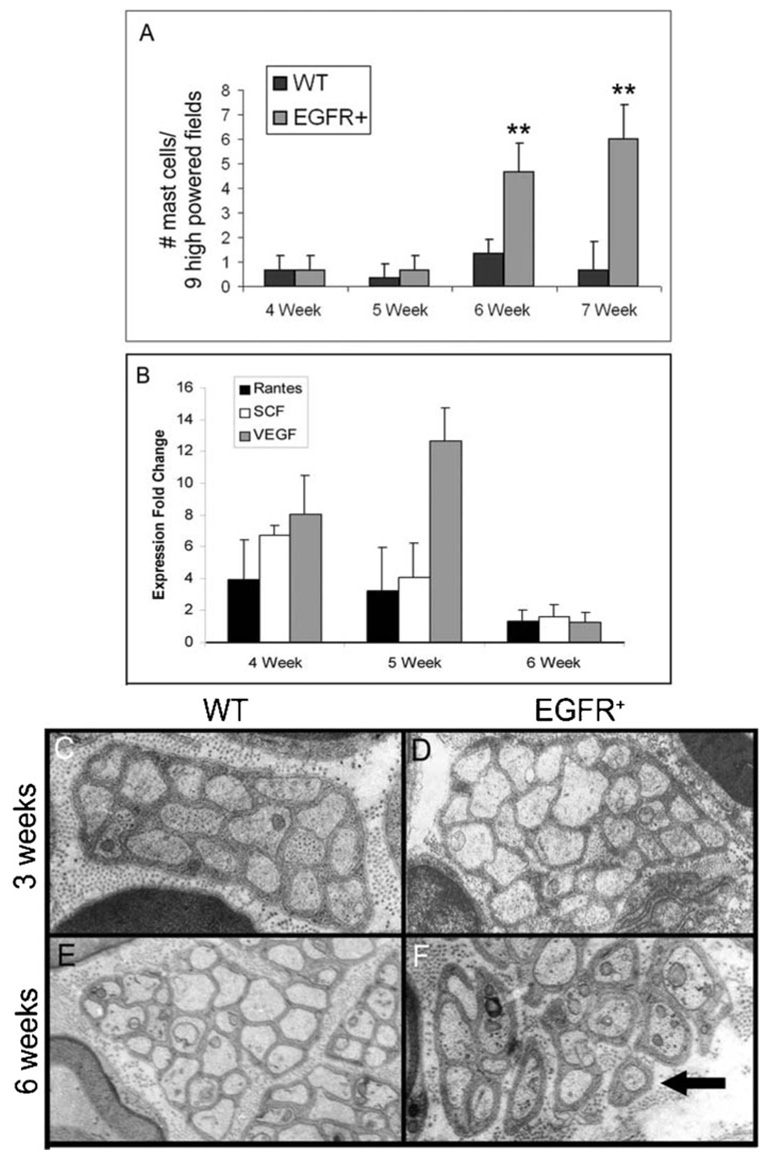
(A) Average number of mast cells per nine high-powered fields (400 ×) in wild-type (WT) and EGFR+ sciatic nerves at designated weeks of age (**, P ≤ 0.02). (B) Fold change in the expression of mast-cell chemoattractants in EGFR+ nerves compared to wild-type nerves at designated weeks of age. (C–F) Representative electron micrographs of saphenous nerves showing progressive disruption of the ultrastructure of Remak bundles in an EGFR+ nerve (D,F) compared to Remak bundles in a wild-type nerve (C,E). Arrow indicates disrupted Remak bundle. C,D, 3 weeks of age; E,F, 6 weeks of age. Electron micrographs are at 10 000 × magnification. For A and C–F, n = 3 for each genotype at each time point.
Because of the abrupt increase in mast-cell number in transgenic nerve, we investigated the expression level of mRNAs encoding mast-cell chemoattractants in wild-type and EGFR+ nerve at 4, 5 and 6 weeks of age. We examined Rantes, stem cell factor (SCF), transforming growth factor-β1 (TGF-β1) and vascular endothelial growth factor (VEGF) by quantitative real-time PCR (QRT-PCR) because we have shown previously that mRNAs encoding these proteins are either present or upregulated in EGFR+ adult nerve (Ling et al., 2005). TGF-β1 mRNA was not expressed in either wild-type or EGFR+ nerve at these early ages (data not shown). Compared to wild-type nerves, mRNA encoding Rantes was upregulated in EGFR+ nerve 3.94-fold (2.43–6.40) at 4 weeks of age and 3.2-fold (1.74–5.91) at 5 weeks of age. SCF mRNA was upregulated 6.73-fold (6.17–7.36) at 4 weeks of age and 4.08-fold (3.16–5.28) at 5 weeks of age. VEGF mRNA was upregulated 8.05-fold (6.18–10.50) at 4 weeks of age and 12.64-fold (10.85–14.72) at 5 weeks of age. The concentration of mRNAs encoding these three chemoattractants was not upregulated in EGFR+ nerve compared to wild-type nerve at 6 weeks of age (Fig. 1B). This data shows that there is a surge in expression of mast-cell chemoattractant mRNAs at 4–5 weeks of age and indicates that Rantes, SCF and/or VEGF might contribute to the accumulation of mast cells in EGFR+ nerves at 6 weeks of age.
The ultrastructure of transgenic nerves was analyzed by electron microscopy at weekly intervals between 3 and 6 weeks of age. Axon–Schwann cell interactions were normal until mast-cell numbers increased in EGFR+ nerves (Fig. 1C–F). Wild-type and EGFR+ nerves showed no differences in ultrastructure at 3 weeks of age (Fig. 1C,D) or at 5 weeks of age (data not shown). Only at 6 weeks (Fig. 1E,F), when mast cells were significantly increased (P = 0.01) in EGFR+ nerve did non-myelinating Schwann cells associated with Remak bundles show diminished ability to wrap small axons (<1 µM). As shown previously, the nerve pathology worsens with age (Ling et al., 2005).
Genetic ablation of mast cells inhibits pathology
The correlation between mast-cell infiltration and the initiation of nerve pathology led us to investigate the role of mast cells in the EGFR+ phenotype. We mated C57Bl/6 EGFR+ mice to the fertile, mast-cell deficient, dominant white spotting W41 strain, also on the C57Bl/6 background. W41 mice are Kit hypomorphs, with a point mutation in the C-terminal domain of the Kit receptor tyrosine kinase (RTK) that is necessary for migration and maturation of mast cells. This point mutation reduces RTK activity by ~85% (Nocka et al., 1990; Reith et al., 1990). In addition to mast-cell deficiency, W41 mutants have mild, macrocytic anemia and a slightly elevated white cell count (Geissler and Russell, 1983). Importantly, these hematological abnormalities do not compromise either fertility or survival because the lifespan of these animals is normal. Therefore, these mice represent a unique, fertile, mast-cell deficient animal model. Intercrosses between the two strains resulted in six genotypes: wild type, EGFR+, W41/W41/EGFR+, W41/+/EGFR+, W41/W41 and W41/+. Offspring were aged and analyzed at 6 months of age when the EGFR+ phenotype is pronounced (n = 3 for each genotype except for W41/W41/EGFR+, where n = 5).
First, we analyzed mast-cell numbers in peripheral nerve for all six genotypes. Adult EGFR+ nerves harbored ~10-fold more mast cells than wild type (Ling et al., 2005). W41/W41/EGFR+ nerves contained fewer mast cells than wild type, and W41/+/EGFR+ nerves contained an intermediate number of mast cells compared to EGFR+ and wild-type nerves (Fig. 2A). Therefore, crossing EGFR+ mice onto the W41 strain dramatically reduces mast-cell numbers in peripheral nerves.
Fig. 2. Mast-cell ablation prevents collagen accumulation.
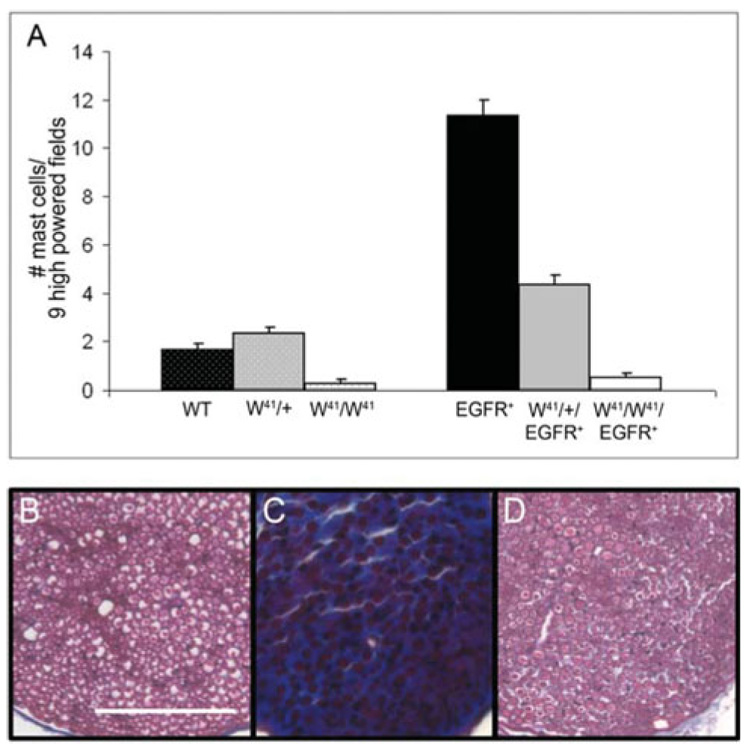
(A) Average number of mast cells per nine high-powered fields (400 ×) in wild-type (WT), W41/ +, W41/W41, EGFR+, W41/+/EGFR+ and W41/W41/EGFR+ sciatic nerves at 6 months of age. (B–D) Photomicrographs of sciatic nerve tissue sections stained with Masson’s trichrome for collagen (blue) from (B) wild-type, (C) EGFR+ and (D) W41/W41/EGFR+ animals at 6 months of age. Note lack of collagen deposition in W41/W41/EGFR+ animal (D). Scale bar, 50 µm. n = 3 for wild type, W41/+, W41/W41, EGFR+ and W41/+/EGFR+; n = 5 for W41/W41/EGFR+.
Second, we examined fibrosis at 6 months of age by staining sciatic nerve sections with Masson’s trichrome for collagen (Fig. 2B–D). Wild-type nerves had very little endoneurial collagen (Fig. 2B) whereas EGFR+ nerves stained heavily (Fig. 2C; blue). W41/W41/EGFR+ nerves had wild-type levels of collagen (Fig. 2D).
Third, we examined nerve ultrastructure at 6 months by electron microscopy. Wild-type nerves showed characteristically well-organized small axons ensheathed by non-myelinating Schwann cells (Remak bundles) (n = 3) (Fig. 3A) whereas Remak bundles from EGFR+ nerves were highly disrupted (n = 3) (Fig. 3B), as described previously (Ling et al., 2005). In many instances Schwann cell processes appear to have no association with an axon, although is possible that association with one or more axons exists out of the plane of section. Nerve ultrastructure in W41/W41/EGFR+ animals was either partially or completely restored (n = 5). Representative electron micrographs from two W41/W41/EGFR+ animals are shown (Fig. 3C,D). Disruption of axon–Schwann cell interaction was quantified (Fig. 3E) as a percentage of grouped axons. An axon bundle was considered grouped if three or more axons were wrapped by a single Schwann cell, and ungrouped if two or fewer. At least 200 axon bundles were counted for each genotype. The organization of wild-type nerves differed significantly from EGFR+ nerves (P = 0.001). W41/W41/EGFR+ nerves differed significantly from EGFR+ nerves (P = 0.002). W41/+/EGFR+ nerves did not differ significantly from EGFR+ nerves (P = 0.13). Counts of myelinated and unmyelinated axons did not identify differences in total axon number in EGFR+ saphenous nerves compared to wild-type nerves (data not shown). Detailed analysis of axon numbers in the dorsal root and saphenous nerve are necessary to definitively exclude axonal loss.
Fig. 3. Genetic ablation of mast cells prevents disruption of nerve ultrastructure.
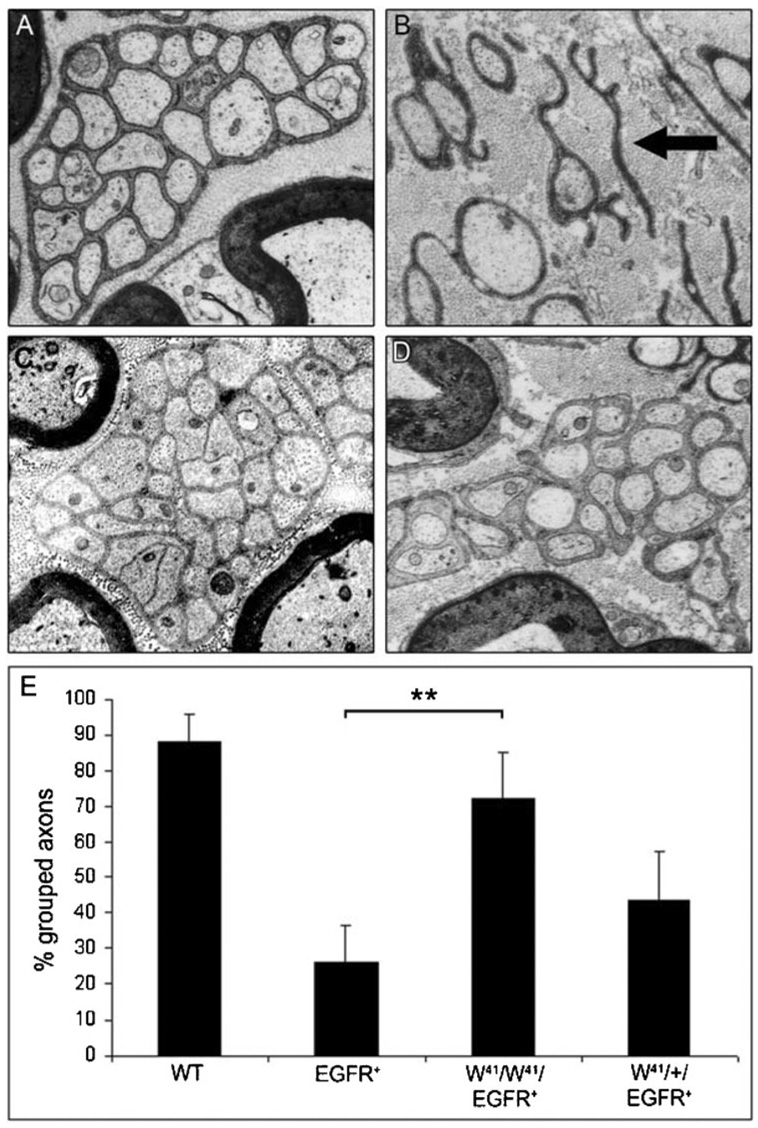
(A) Representative electron micrograph of a wild-type saphenous nerve at 6 months of age. (B) Representative electron micrograph of an EGFR+ saphenous nerve at 6 months of age, showing disrupted organization of small axon bundles. Note non-myelinating Schwann cell process that in this plane of section is not associated with an axon (black arrow). (C,D) Representative electron micrographs of W41/W41/EGFR+ saphenous nerves at 6 months of age, showing restoration of axon–Schwann cell bundles. All micrographs are 10 000 ×. (E) Quantification of axon bundles. Graph shows percentage small axons considered ‘grouped’ (3 or more small axons/bundle; **, P = 0.002).
We also examined BrdU incorporation in adult W41/W41/EGFR+ mouse nerve (n = 3), and found that mast-cell ablation had no effect on Schwann cell proliferation. W41/W41/EGFR+ nerves displayed a proliferation rate of 0.13%, which is characteristic of Schwann cells in normal adult mouse nerve (Brown and Asbury, 1981), and did not differ significantly from wild-type, adult nerve (P = 0.53). We previously showed that the percentage of proliferating cells in adult EGFR+ nerves does not differ from wild-type nerve (Ling et al., 2005); rather, a burst of Schwann cell proliferation occurs in EGFR+ nerves between birth and 2 weeks of age (Wu et al., 2006). Mast cells do not arrive at peripheral nerves until 4 weeks of age (Fig. 1A), and so are not expected to have an effect on Schwann cell proliferation in this model.
Bone marrow reconstitution restores pathology
To test whether phenotypic reversal in W41 matings is caused by loss of blood-derived mast cells, rather than an unrelated effect of the Kit receptor mutation, we engrafted wild-type bone marrow into W41/W41/EGFR+ animals. We chose this approach because purified mast cells do not cross the blood – brain barrier (Tanzola et al., 2003), and so are not expected to cross the blood – nerve barrier. Bone marrow is the source of all hematopoietic progenitors including mast-cell precursors. Mast-cell precursors from wild-type marrow harbor functional Kit, allowing proper migration and maturation in mutant hosts. We injected mice (via the tail vein) with wild-type bone marrow cells at 4 weeks of age to recapitulate temporal arrival of mast cells to peripheral nerves.
We examined animals 12 weeks after injection (at 4 months of age) and quantified mast cells in nerve tissue sections after Giemsa and toluidine blue staining. Mast cells were evident in engrafted W41/W41/EGFR+ peripheral nerves (Fig. 4A). Engrafted W41/W41/EGFR+ nerves had significantly more mast cells than non-engrafted W41/W41/EGFR+ controls at 4 months (P = 0.007), but did not differ from 6-month-old EGFR+ nerve controls (P = 0.92) (Fig. 4B). We examined the effects of mast-cell reconstitution on collagen accumulation by Masson’s trichrome stain. Non-engrafted W41/W41/EGFR+ nerve (Fig. 4C) showed little endoneurial collagen, while engrafted W41/W41/EGFR+ nerve (Fig. 4D) stained heavily (blue).
Fig. 4. Engraftment of wild-type marrow into W41/W41/EGFR+ mice causes mast-cell accumulation in peripheral nerve and EGFR+ nerve pathology.
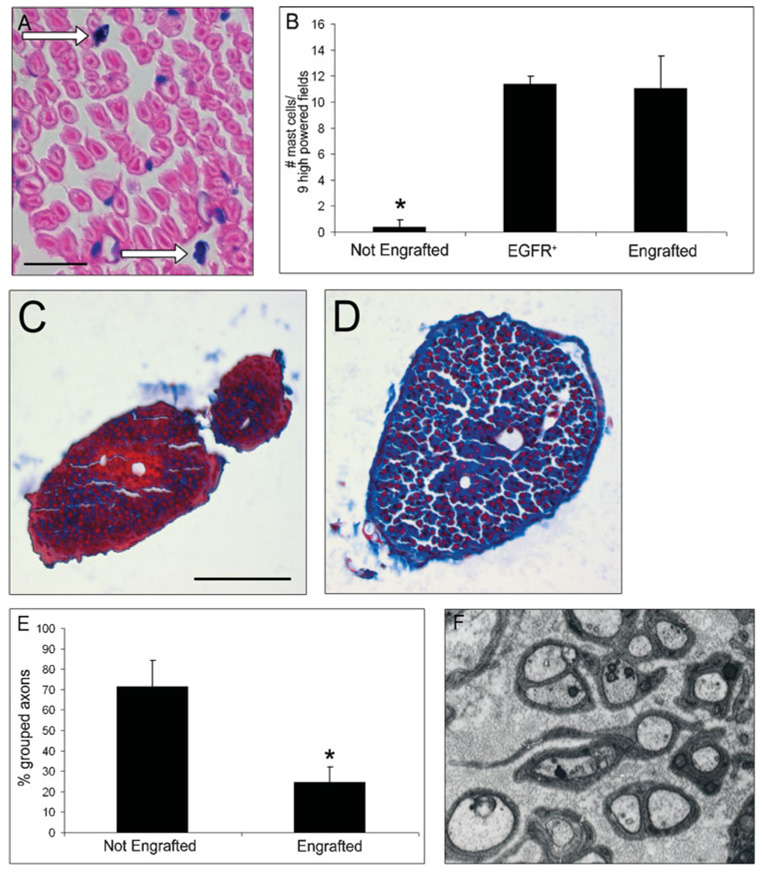
(A) Section through marrow-engrafted W41/W41/EGFR+ sciatic nerve at 4 months of age stained with Giemsa shows mast-cell reconstitution (white arrows). Scale bar 100 µm. (B) Average number of mast cells per nine high-powered fields (400 ×) in non-engrafted W41/W41/EGFR+ sciatic nerves (Not Engrafted, n = 3), EGFR+ sciatic nerve (EGFR+; n = 3), and marrow-engrafted W41/W41/EGFR+ sciatic nerve (Engrafted; n 4 animals) (*, P = 0.03). (C) Photomicrograph of non-engrafted W41/W41/EGFR+ nerve stained with Masson’s trichrome showing relative paucity of collagen staining, 4 months of age. (D) Photomicrograph of marrow-engrafted W41/W41/EGFR+ nerve stained with Masson’s trichrome showing collagen accumulation (blue) at 4 months of age. Scale bar in C and D, 50 µm. (E) Quantification of small axon bundles in non-engrafted W41/W41/EGFR+ (n = 3) nerves and engrafted W41/W41/EGFR+ nerves (n = 3; *, P = 0.04). (F) Electron micrograph of representative marrow-engrafted W41/W41/EGFR+ animal, showing ultrastructure disruption, 10 000 ×.
We examined nerve ultrastructure and found that disruption was significantly more severe in engrafted than non-engrafted W41/W41/EGFR+ nerve (P = 0.03). Quantification of axon bundles in engrafted versus non-engrafted W41/W41/EGFR+ nerves and a representative electron micrograph of an engrafted W41/W41/EGFR+ nerve are shown (Fig. 4E,F). Mast cells, thus, reconstitute to peripheral nerve after bone-marrow transfer, and bone-marrow transfer results in nerve pathology.
We also examined EGFR+ peripheral nerves for increases in other hematopoietic cells: neutrophils, eosinophils and basophils, and found no influx of these cell types to peripheral nerves (data not shown). This observation, and the marrow engraftment experiments, are consistent with the hypothesis that EGFR signaling and mast cells are relevant for the pathology observed in EGFR+ nerves.
Early cromolyn treatment ablates mast-cell accumulation and prevents pathology
We sought a pharmacological approach to test if mast cells, rather than other hematopoetic cells, were responsible for nerve pathology in EGFR+ mice. The release of granules from mast cells is blocked by cromolyn (Ginsburg and Baldwin, 2004; Goldgraber, 1976). We injected mice i.p. daily from 6–9 weeks of age with 10 mg kg−1 cromolyn. Cromolyn also blocks granular release from other cell types, such as eosinophils and basophils but, as noted above, we did not observe an influx of these cells in EGFR+ peripheral nerves.
We examined nerve ultrastructure and found that early exposure of EGFR+ animals to cromolyn maintained nerve axon – Schwann cell interactions at wild-type levels. The percentage of grouped axons was quantified (Fig. 5A). Even at this young age, there is a significant disruption of Remak bundles; EGFR+ + carrier differs from wild-type nerve + carrier (P = 0.01). Nerves from EGFR+ mice treated with cromolyn, in contrast, showed wild-type nerve organization (P = 0.10). An interpretation of this result is that mediators released from mast cells contribute to EGFR+ pathology.
Fig. 5. Cromolyn treatment prevents the development of pathology and mast-cell recruitment.
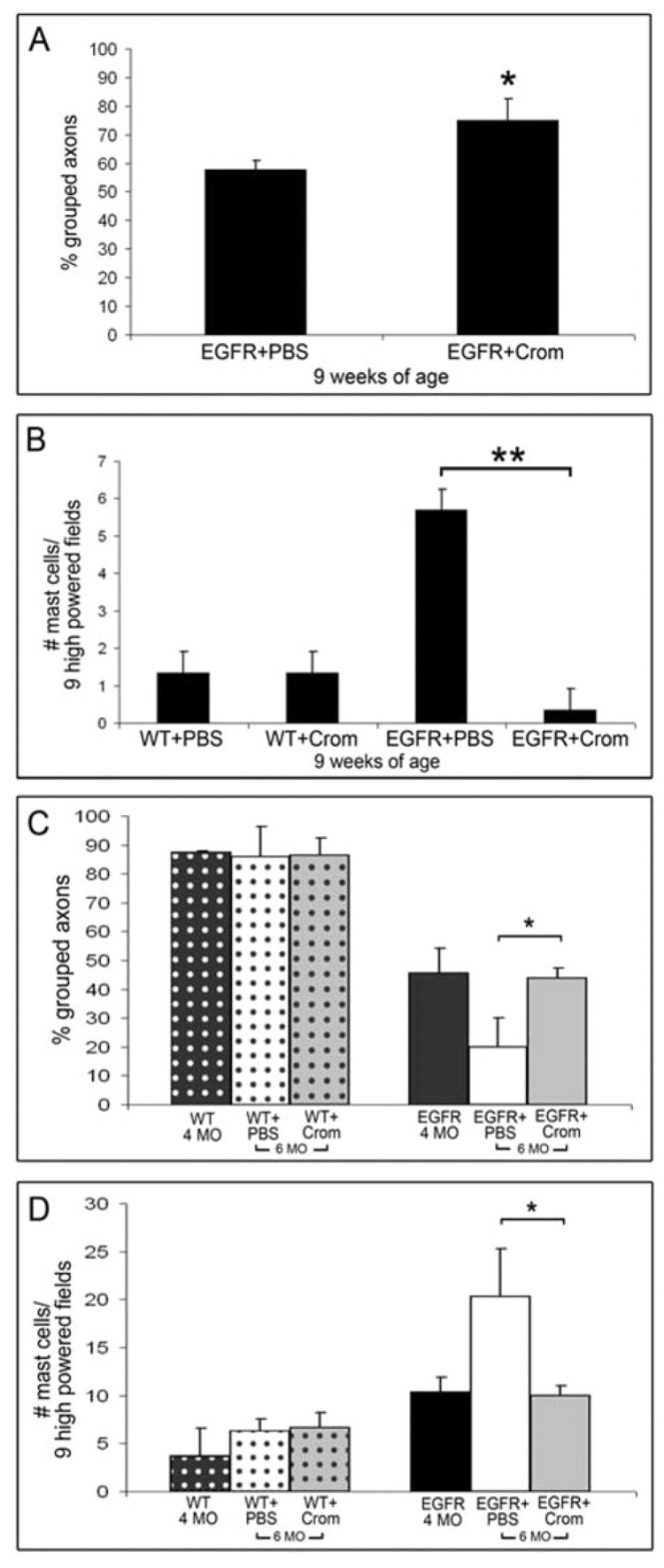
(A–D) Pathological quantification of nerves following early (A,B) and late (C,D) treatment with cromolyn. (A) Small axon bundle quantification of nerves from EGFR+ + carrier (EGFR+PBS) and EGFR+ + cromolyn (EGFR+Crom) animals. Mice were treated for 21 days, beginning at 6 weeks of age and ending at 9 weeks of age. Graph shows percentage small axons considered ‘grouped’ (≥3 small axons/bundle; *, P = 0.01). (B) Graph showing average number of mast cells per nine high-powered fields (400X) in wild type + carrier (WT+PBS; n = 3), wild-type + cromolyn (WT+Crom; n = 4), EGFR+ + carrier (EGFR+PBS; n = 4), and EGFR+ + cromolyn (EGFR+Crom; n = 3) sciatic nerve at 9 weeks of age (**, P = 0.0003). (C) Quantification of small axon bundles in nerves from 4-month-old wild-type animals, no treatment (WT 4 MO; n = 3), wild type + carrier (WT+PBS; n = 3), wild type + cromolyn (WT+Crom; n = 4), 4-month-old EGFR+ animals, no treatment (EGFR 4 MO; n = 4), EGFR+ + carrier (EGFR+PBS; n = 4) and EGFR+ + cromolyn (EGFR+Crom; n = 3). 6 MO: 6 months of age, injected mice were treated with carrier or cromolyn for 8 weeks, beginning at 4 months of age and ending at 6 months of age. Graph shows percentage of small axons considered ‘grouped’ (3 or more small axons/bundle; *, P = 0.02). (D) Graph showing average number of mast cells per nine high-powered fields (400 ×) in nerves from 4-month-old (4 MO) wild-type animals, no treatment (WT 4 MO), wild type + carrier (WT+PBS), wild type + cromolyn (WT+CROM), 4 MO EGFR+ animals, no treatment (EGFR 4 MO), EGFR+ + carrier (EGFR+PBS), and EGFR+ + cromolyn (EGFR+Crom), *, P = 0.02.
We quantified mast-cell number in peripheral nerves. In wild-type peripheral nerves, mast-cell number was unaffected by treatment of mice with either carrier (n = 3) or cromolyn (n = 4) (Fig. 5B). EGFR+ nerves from 9-week-old mice were unaffected by carrier and retained 5-fold more mast cells than wild-type nerves following 21 days of exposure. In contrast, cromolyn treated EGFR+ nerve (n = 3) had significantly reduced numbers of mast cells compared to EGFR+ + carrier (n = 4; P = 0.0003), and similar levels to wild type (P = 0.01). Thus, cromolyn blocks mast-cell recruitment to nerves in EGFR+ mice. One interpretation of this is that mast-cell degranulation products are necessary for continued recruitment of mast-cell progenitors to peripheral nerves, and for promoting disruption of EGFR+ nerves.
Late cromolyn treatment arrests pathology from time of administration
To test if later treatment with cromolyn reversed pathology once it had begun to manifest, we treated mice daily for 8 weeks with 10 mg kg−1 cromolyn. We began treatment at 4 months of age, when pathology in EGFR+ nerves is pronounced and ended treatment at 6 months of age, when pathology is severe. Control mice were injected daily with saline. We also included untreated, 4-month-old mice in the analysis of this experiment.
We examined nerve ultrastructure and found that cromolyn exposure from 4–6 months of age in EGFR+ animals maintained nerve axon – Schwann cell interactions at 4-month-old pathological levels. The percentage of grouped axons was quantified (Fig. 5C); organization in treated EGFR+ mice treated with cromolyn between 4–6 months of age was not significantly different to untreated, 4-month-old nerves (P = 0.78), but was significantly different to EGFR+ + carrier (P = 0.02).
We quantified nerve mast-cell number and found that treatment of mice with carrier (n = 3) or cromolyn (n = 4) had no effect on mast-cell number in wild-type peripheral nerves (Fig. 5D). In contrast, cromolyn treated EGFR+ nerves (n = 3) had significantly fewer mast cells compared to EGFR+ + carrier (n = 4; P = 0.02), and similar numbers to untreated 4-month-old EGFR+ nerves (n = 4; P = 0.77).
Based on this experiment we conclude that late exposure cromolyn (starting after pathologies are manifest) does not restore normal morphology to EGFR+ nerves. However, late administration of cromolyn prevents further pathological developments, and maintains the nerve in an earlier state of disruption.
CONCLUSIONS
The onset of EGFR+ peripheral nerve pathology correlates with an influx of mast cells, which is preceded by an increase in expression of mRNA encoding VEGF, Rantes and SCF in EGFR+ peripheral nerves.
Genetic ablation of mast cells prevents the development of pathology in EGFR+ peripheral nerves.
Bone marrow engraftment restores mast-cell influx and peripheral nerve pathology in mast cell-ablated EGFR+ animals.
Pharmacological stabilization of mast cells by cromolyn prevents the development of EGFR+ peripheral nerve pathology when administered from 6–9 weeks of age, and prevents progression of pathology when administered from 4–6 months of age.
DISCUSSION
In this work we provide several lines of evidence that support a role for mast cells in the pathology of EGFR+ peripheral nerves. First, the number of mast cells increases in EGFR+ nerve at the same time as axon – Schwann cell interactions are disrupted and fibrosis develops. Second, genetic ablation of mast cells through W41 mating prevents pathologies associated with transgenic expression of EGFR. Third, reconstitution of blood-derived cells, including mast cells, to W41/W41/EGFR+ peripheral nerve via marrow engraftment restores pathologies. Fourth, treatment of EGFR+ mice with cromolyn inhibits mast-cell recruitment to peripheral nerves and pathological progression. Based on these experiments, we hypothesize that mast-cells that are normally resident in peripheral nerve are activated in response to the EGFR+ environment, possibly in response to increased Rantes, SCF and VEGF present at 4 and 5 weeks of age, and recruit additional mast-cell progenitors from bone marrow, which exacerbates EGFR+-initiated pathologies (Fig. 6).
Fig. 6. Model for the development of EGFR+ pathology.
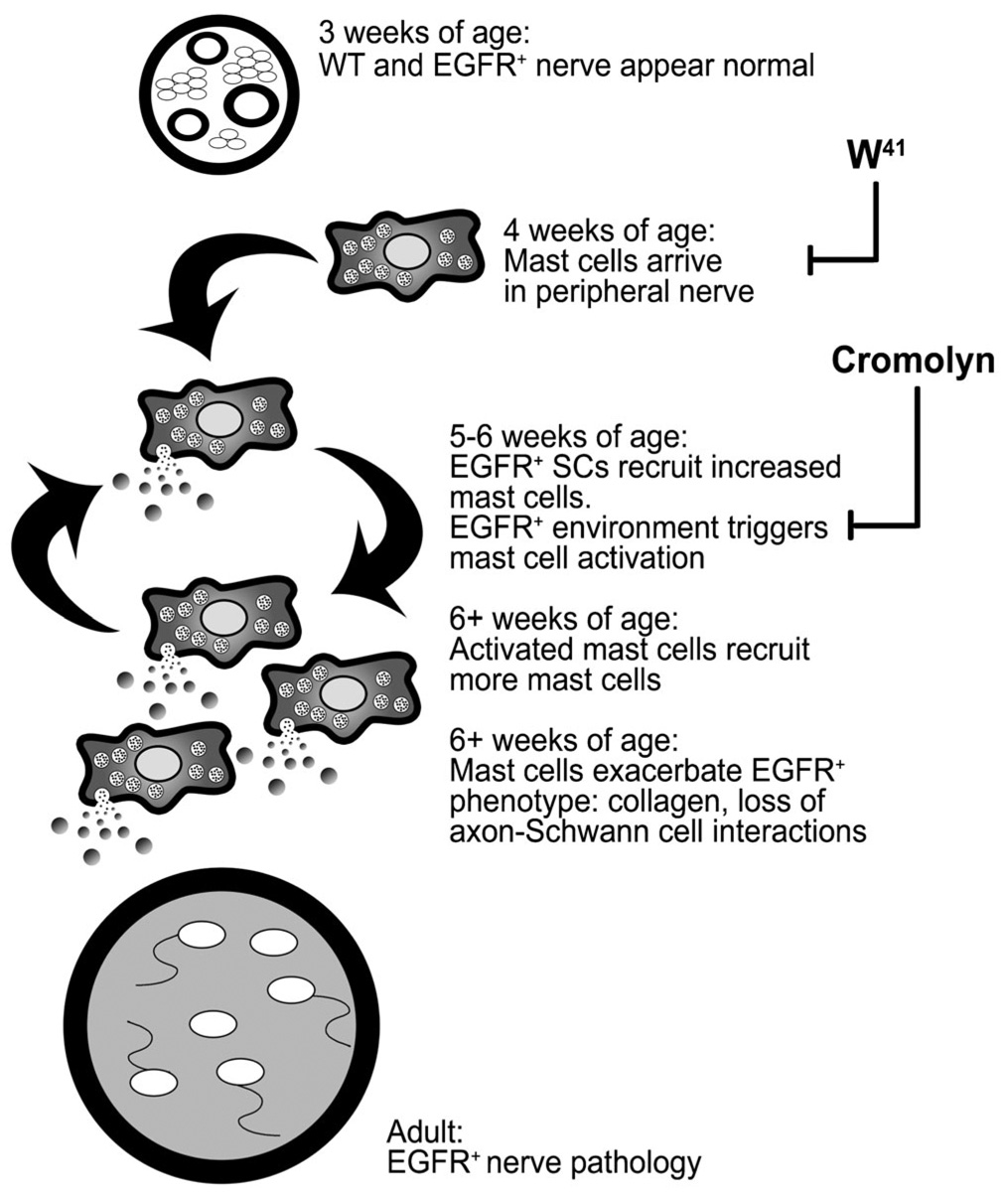
EGFR+ nerves are indistinguishable ultrastructurally from wild-type nerves at 3 weeks of age. Normally mast cells arrive at the nerve at 4 weeks of age and are activated by the EGFR+ environment. Cytokines from EGFR+ Schwann cells and/or mediators from activated mast cells recruit increased numbers of mast-cell progenitors from blood. These mast cells are also activated by the EGFR+ environment and/or previous degranulation products, forming a paracrine loop. In addition to recruiting more mast cells, degranulation products also alter nerve homeostasis, which exacerbates both collagen deposition and progressive loss of axon–Schwann cell interactions that were initiated by EGFR+ Schwann cells. These pathologies are blocked by genetic ablation of mast cells (W41) and by cromolyn administration.
W41 mice are Kit hypomorphs, and Ryan et al. showed that Kit is expressed in an MPNST cell line derived from an NF1 patient, but not in normal human Schwann cells (Ryan et al., 1994). Therefore the loss of the nerve pathology phenotype in W41/W41/EGFR+ might result from effects on Schwann cells. Restoration of the phenotype by bone marrow reconstitution, which restores normal mast cells but not Schwann cells, makes this explanation unlikely.
Mast cells arrive in wild-type and EGFR+ nerve at the same time in development (4 weeks) (Fig. 1A); although mast cells are known constituents of peripheral nerve, to our knowledge, this is the first study that has documented when they arrive at this tissue. Mast-cell numbers do not increase in EGFR+ nerves until 6 weeks of age. We have shown previously that mRNAs encoding several potential mast-cell chemoattractants are upregulated in adult EGFR+ nerve (Ling et al., 2005), and show in this study that mRNAs encoding Rantes, SCF and VEGF are transiently upregulated in EGFR+ nerve at 4 and 5 weeks of age. Yang et al. showed that SCF is secreted by Nf1−/− Schwann cells and that it is a potent migratory stimulus for Nf1 +/− mast cells (Yang et al., 2003). SCF also inhibits mast cell chemotaxis, thereby contributing to their accumulation and enhancement of function (Sawada et al., 2005). A role for SCF in recruitment of mast cells to EGFR+ nerve is consistent with our data, but other factors might also play a role.
SCF was reported to directly trigger mast-cell degranulation (Taylor et al., 1995), although recent studies call this result into question (Gilfillan and Tkaczyk, 2006). Our data showing that cromolyn treatment prevents nerve pathology supports the idea that mast-cell degranulation, mediated by SCF or other factors, is crucial in the EGFR+ phenotype. Cromolyn also caused a profound decrease in the number of mast cells recruited to EGFR+ peripheral nerves. Because cromolyn is thought to block mast-cell degranulation, we suggest that mast cells in EGFR+ nerves degranulate in response to the EGFR+ environment, and that the mast-cell mediators that are released contribute to increased recruitment of mast cells to nerves (Fig. 6). However, additional work is necessary to definitely demonstrate mast-cell degranulation in this model and identify factors that might trigger degranulation.
We can exclude a cromolyn effect on other bone marrow-derived cells that contain granules because eosinophils, neutrophils and basophils were not observed in nerves. It is also unlikely that cromolyn affects nerve cells directly because the nerves of wild-type, control animals treated with cromolyn were morphologically similar to the nerves of either untreated or carrier-injected wild-type animals. However, because the exact mechanism of action of cromolyn is not known, we can not completely rule out effects on other cell types. It is also possible that other cell types are necessary intermediates between Schwann cells and mast cells.
Mast cells can release mediators from preformed granules by exocytosis; these mediators include proteases, granule amines, proteoglycans, heparin, NGF and serotonin (Leon et al., 1994; Gurish and Austin, 2001; Maurer et al., 2003; Crivellato et al., 2004). Any of the >20 molecules released from mast-cell granules might be responsible for disruption of EGFR+ nerves. Although NGF and serotonin are attractive candidates because Schwann cells express their receptors (Taniuchi et al., 1986; Gaietta et al., 2003), in preliminary experiments we found that blocking serotonin receptors did not affect EGFR+ nerve pathology (not shown). The local environment can regulate the composition of mast-cell granules (Galli et al., 2005), and it is possible that the Schwann cell environment in EGFR+ mice results in the de novo synthesis of mast-cell mediators that are responsible for the pathological progression in EGFR+ nerves. We also cannot exclude piecemeal degranulation in the EGFR+ nerve phenotype.
This work describes novel roles for mast cells in nerve homeostasis and pathology. A pivotal link between inflammation and carcinogenesis is being unraveled (Coussens et al., 1999; Balkwill and Coussens, 2004; de Visser et al., 2005), and the classical view of mast cells as effectors in allergy and IgE-mediated immune response is becoming outmoded as essential, non-immunological roles for mast cells are identified (Crivellato and Ribatti, 2005; Galli et al., 2005). Mast cells might have roles in the development of other nerve pathologies in which Schwann cells lose contact with axons, including peripheral nerve tumor formation in NF1, Guillain-Barré syndrome and Wallerian degeneration (Tanzola et al., 2003; Dines and Powell, 1997). Our findings contribute to this newly emerging body of work and support a vital role for mast cells in the development of Schwann cell-mediated nerve pathology.
ACKNOWLEDGEMENTS
We gratefully acknowledge helpful suggestions of D. Wade Clapp (Indiana University), Ann Dvorak (Beth Israel Deaconess Medical Center), Greg Gregory (Northwestern University), Michael Gurish (Harvard University), Todd Nick (Cincinnati Children’s Hospital), Anat Stemmer-Rachamimov (Mass. General Hospital) and Mindy Tsai (Stanford University). We also thank Peter Ackerman, Edward Brown and Jennifer Kordich for outstanding technical support. This work was supported by grants NIH NS28840 and DAMD 17-02-1-0679 to N.R. K.R.M. was supported by T32-CA-59268 and the Albert J. Ryan Foundation. J.W. was a DAMD Neurofibromatosis Fellow.
REFERENCES
- Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405–406. doi: 10.1038/431405a. [DOI] [PubMed] [Google Scholar]
- Brown MJ, Asbury AK. Schwann cell proliferation in the postnatal mouse: timing and topography. Experimental Neurology. 1981;74:170–186. doi: 10.1016/0014-4886(81)90157-6. [DOI] [PubMed] [Google Scholar]
- Carroll SL, Stonecypher MS. Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling. Journal of Neuropathology Experimental Neurology. 2005;64:1–9. doi: 10.1093/jnen/64.1.1. [DOI] [PubMed] [Google Scholar]
- Chen S, Rio C, Ji RR, Dikkes P, Coggeshall RE, Woolf CJ, Corfas G. Disruption of ErbB receptor signaling in adult non-myelinating Schwann cells causes progressive sensory loss. Nature Neuroscience. 2003;6:1186–1193. doi: 10.1038/nn1139. [DOI] [PubMed] [Google Scholar]
- Corfas G, Velardez MO, Ko CP, Ratner N, Peles E. Mechanisms and roles of axon-Schwann cell interactions. Journal of Neuroscience. 2004;24:9250–9260. doi: 10.1523/JNEUROSCI.3649-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendsten O, Werb Z, et al. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes and Development. 1999;13:1382–1397. doi: 10.1101/gad.13.11.1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crivellato E, Beltrami CA, Mallardi F, Ribatti D. The mast cell: an active participant or an innocent bystander? Histology and Histopathology. 2004;19:259–270. doi: 10.14670/HH-19.259. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Ribatti D. Involvement of mast cells in angiogenesis and chronic inflammation. Current Drug Targets. Inflammation and Allergy. 2005;4:9–11. doi: 10.2174/1568010053622876. [DOI] [PubMed] [Google Scholar]
- DeClue JE, Heffelfinger S, Benvenuto G, Ling B, Li S, Rui W, et al. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. Journal of Clinical Investigation. 2001:1233–1241. doi: 10.1172/JCI7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Dines KC, Powell HC. Mast cell interactions with the nervous system: relationship to mechanisms of disease. Journal of Neuropathology Experimental Neurology. 1997;56:627–640. [PubMed] [Google Scholar]
- Gaietta GM, Yoder EJ, Deerinck T, Kinder K, Hanono A, Han A, et al. 5-HT2a receptors in rat sciatic nerves and Schwann cell cultures. Journal of Neurocytology. 2003;32:373–380. doi: 10.1023/B:NEUR.0000011331.58835.fd. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Kalesnikoff J, Grimbaldeston MA, Piliponsky AM, Williams CM, Tsai M. Mast cells as “tunable” effector and immunoregulatory cells: recent advances. Annual Review of Immunology. 2005;23:749–786. doi: 10.1146/annurev.immunol.21.120601.141025. [DOI] [PubMed] [Google Scholar]
- Gamble HJ, Goldby S. Mast cells in peripheral nerve trunks. Nature. 1961;189:766–767. doi: 10.1038/189766a0. [DOI] [PubMed] [Google Scholar]
- Geisseler EN, Russell ES. Analysis of the hematopoietic effects of new dominant spotting (W) mutations of the mouse. I. Influence upon hematopoietic stem cells. Experimantal Hematology. 1983;11:452–460. [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signaling pathways for mast-cell activation. Nature Reviews Immunology. 2006;6:218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Ginsburg MI, Baldwin AL. Disodium cromoglycate stabilizes mast cell degranulation while reducing the number of hemoglobin-induced microvascular leaks in rat mesentery. American Journal of Physiology. Heart and Circulatory Physiology. 2004;286:H1750–H1756. doi: 10.1152/ajpheart.00605.2003. [DOI] [PubMed] [Google Scholar]
- Goldgraber MB. Disodium cromoglycate in immunological mast cell degranulation of mice: an experimental study. Annals of Allergy. 1976;37:123–125. [PubMed] [Google Scholar]
- Gravel M, DeAngelis D, Braun PE. Molecular cloning and characterization of rat brain 2′,3′-cyclic nucleotide 3′-phosphodiesterase isoform 2. Journal of Neuroscience Research. 1994;53:243–247. doi: 10.1002/jnr.490380302. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Austen KF. The diverse roles of mast cells. Journal of Experimental Medicine. 2001;194:F1–F5. doi: 10.1084/jem.194.1.f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen KR, Brennan A, Morgan L, Mirsky R, Kent A, Hashimoto Y, et al. The Schwann cell precursor and its fate: a study of cell death and differentiation during gliogenesis in rat embryonic nerves. Neuron. 1994;12:509–527. doi: 10.1016/0896-6273(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Kluwe L, Friedrich R, Mautner VF. Loss of NF1 allele in Schwann cells but not in fibroblasts derived from an NF1-associated neurofibroma. Genes Chromosomes Cancer. 1999;24:283–285. doi: 10.1002/(sici)1098-2264(199903)24:3<283::aid-gcc15>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Lee DM, Friend DS, Gurish MF, Benoist C, Mathis D, Brenner MB. Mast cells: a cellular link between autoantibodies and inflammatory arthritis. Science. 2002;297:1689–1692. doi: 10.1126/science.1073176. [DOI] [PubMed] [Google Scholar]
- Leimeroth R, Lobsiger C, Lussi A, Taylor V, Suter U, Sommer L. Membrane-bound neuregulin1 type III actively promotes Schwann cell differentiation of multipotent Progenitor cells. Developmental Biology. 2002;246:245–258. doi: 10.1006/dbio.2002.0670. [DOI] [PubMed] [Google Scholar]
- Leon A, Buriani A, Dal Toso R, Fabris M, Romanello S, Aloe L, et al. Mast cells synthesize, store, and release nerve growth factor. Proceedings of the National Academy of Science of the U.S.A. 1994;91:3739–3743. doi: 10.1073/pnas.91.9.3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Velasco-Miguel S, Vass WC, Parada LF, DeClue JE. Epidermal growth factor receptor signaling pathways are associated with tumorigenesis in the Nf1:p53 mouse tumor model. Cancer Research. 2002;62:4507–4513. [PubMed] [Google Scholar]
- Ling BC, Wu J, Miller SJ, Monk KR, Shamekh R, Rizvi TA, et al. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer Cell. 2005;7:65–75. doi: 10.1016/j.ccr.2004.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, Theoharides T, Granstein RD, Bischoff SC, Bienenstock J, Henz B, et al. What is the physiological function of mast cells? Experimental Dermatology. 2003;12:886–910. doi: 10.1111/j.0906-6705.2003.0109a.x. [DOI] [PubMed] [Google Scholar]
- Nocka K, Tan JC, Chiu E, Chu TY, Ray P, Traktman P, et al. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO Journal. 1990;9:1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson Y. Mast cells in human peripheral nerve. Acta Neurologica Scandinavica. 1971;47:357–368. doi: 10.1111/j.1600-0404.1971.tb07490.x. [DOI] [PubMed] [Google Scholar]
- Pineda A. Mast cells – their presence and ultrastructural characteristics in peripheral nerve tumors. Archives of Neurology. 1965;13:372–382. doi: 10.1001/archneur.1965.00470040038006. [DOI] [PubMed] [Google Scholar]
- Rasmussen SA, Friedman JM. NF1 gene and neurofibromatosis 1. American Journal of Epidemiology. 2000;151:33–40. doi: 10.1093/oxfordjournals.aje.a010118. [DOI] [PubMed] [Google Scholar]
- Reith AD, Rottapel R, Giddens E, Brady C, Forrester L, Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes and Development. 1990;4:390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Riccardi VM. A controlled multiphase trial of ketotifen to minimize neurofibroma-associated pain and itching. Archives of Dermatology. 1987;123:1011–1016. [PubMed] [Google Scholar]
- Riccardi VM. Mast-cell stabilization to decrease neurofibroma growth. Preliminary experience with ketotifen. Archives of Dermatology. 1993;129:577–581. [PubMed] [Google Scholar]
- Ryan JJ, Klein KA, Neuberger TJ, Leftwich JA, Westin EH, Kauma S, et al. Role for the stem cell factor/KIT complex in Schwann cell neoplasia and mast cell proliferation associated with neurofibromatosis. Journal of Neuroscience Research. 1994;37:415–432. doi: 10.1002/jnr.490370314. [DOI] [PubMed] [Google Scholar]
- Sawada J, Shimizu S, Tamatani T, Kanegasaki S, Saito H, Tanaka A, et al. Stem cell factor has a suppressive activity to IgE-mediated chemotaxis of mast cells. Journal of Immunology. 2005;174:3626–3632. doi: 10.4049/jimmunol.174.6.3626. [DOI] [PubMed] [Google Scholar]
- Serra E, Rosenbaum T, Nadal M, Winner U, Ars E, Estivill X, et al. Mitotic recombination effects homozygosity for NF1 germline mutations in neurofibromas. Nature Genetics. 2001;28:294–296. doi: 10.1038/90148. [DOI] [PubMed] [Google Scholar]
- Taniuchi M, Clark HB, Johnson EM., Jr Induction of nerve growth factor receptor in Schwann cells after axotomy. Proceedings of the National Academy of Science of the U.S.A. 1986;83:4094–4098. doi: 10.1073/pnas.83.11.4094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzola MB, Robbie-Ryan M, Gutekunst CA, Brown MA. Mast cells exert effects outside the central nervous system to influence experimental allergic encephalomyelitis disease course. Journal of Immunology. 2003;171:4385–4391. doi: 10.4049/jimmunol.171.8.4385. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Galli SJ, Coleman JW. Stem-cell factor, the kit ligand, induces direct degranulation of rat peritoneal mast cells in vitro and in vivo: dependence of the in vitro effect on period of culture and comparisons of stem-cell factor with other mast cell-activating agents. Immunology. 1995;86:427–433. [PMC free article] [PubMed] [Google Scholar]
- Wu J, Crimmins JT, Monk KR, Williams JP, Fitzgerald ME, Tedesco S, et al. Perinatal epidermal growth factor receptor blockade prevents peripheral nerve disruption in a mouse model reminiscent of benign world health organization grade I neurofibroma. American Journal of Pathology. 2006;168:1686–1696. doi: 10.2353/ajpath.2006.050859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang FC, Ingram DA, Chen S, Hingtgen CM, Ratner N, Monk KR, et al. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. Journal of Clinical Investigation. 2003;112:1851–1861. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]


