Abstract
Purpose
To describe the clinical course of a patient with multiple recurrences of primary intraocular lymphoma (PIOL).
Design
Interventional case report,
Methods
Retrospective chart review.
Results
A 57-year-old female treated with multiple intravitreal methotrexate injections became refractory to intravitreal methotrexate after a year. Lymphoma cells evaluated using immunocytochemistry and confocal microscopy showed aberrant multidrug resistance-related protein (MRP) and decreased reduced folate carrier (RFC) and folate binding protein (FBP) expression compared to PIOL cells from another patient clinically responsive to methotrexate.
Conclusions
This case suggests that alterations in the transport of methotrexate across the cell membrane might contribute to resistance following repeated intravitreal injections.
Keywords: CNS lymphoma, cytokine, drug resistance, methotrexate, multidrug resistance protein
Primary central nervous system lymphoma (PCNSL) is a rare, diffuse, large B-cell, non-Hodgkin lymphoma.1 PIOL is a subset of PCNSL that occurs in the eye.2 Chemotherapy regimens with high-dose methotrexate are recommended for PCNSL. Intraocular chemotherapy has the advantage of limiting the side effects and is shown to be effective in PIOL patients.3 Drug resistance, a well-recognized obstacle in the management of lymphoma, has not been studied in PIOL.
CASE REPORT
A 57-year-old- female with PCNSL and PIOL in the left eye was treated with high-dose methotrexate, vincristine, and procarbazine with complete resolution of the ocular and CNS disease.4 Eight months later she developed subretinal infiltrates in the right eye without evidence of recurrent lymphoma in the left eye or CNS (MRI and CSF cytology negative). A diagnostic vitrectomy demonstrated large B-cell lymphoma and elevated interleukin-10 (IL-10/IL-6 > 96.1). As the recurrence was limited to only the right eye (Figure 1A), the patient was treated with 3 intravitreal methotrexate injections (250 μg/0.1 mL qod) with complete resolution clinically (Figure 1B).
FIGURE 1.
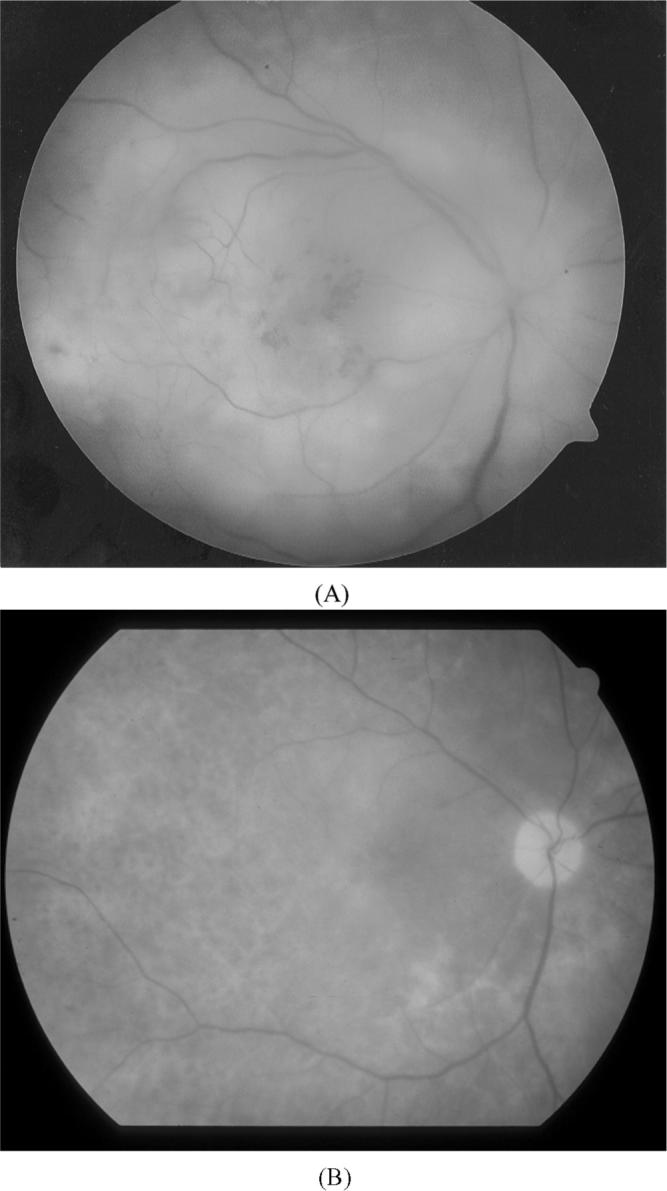
Fundus photo of the OD shows active recurrent PIOL (A) before and (B) after treatment with intravitreal methotrexate. A cytokine analysis from the AC revealed an IL-10/IL-6 of > 16.5. The IL-10 and IL-6 from the AC following treatment were below detectable levels.
Over the next 18 months she had multiple PIOL recurrences in the right eye with gradually diminished response to intravitreal methotrexate (250−450 μg/0.1 mL), raising the possibility that the PIOL cells had become resistant to methotrexate (Table 1).
TABLE 1.
Clinical course and intraocular cytokine levels in recurrent PIOL
| Examination date | Clinical activity | Cytology | IL10/IL6 before Rx | Intravitreal MTX* | IL 10/IL 6 after treatment | Vision before-after treatment | Clinical impression |
|---|---|---|---|---|---|---|---|
| February 2001 | Active | PIOL | >96.1 (v†) | 0.25 mg/0.1 mL (×3)+ in 7 days | Not done | 20/25−20/25 | |
| Early-April 2001 | Quiet | Rare atypical lymphocytes | Below detectable (v) | None | Not done | 20/25 | Stable |
| Late-April 2001 | Active (disc edema) | Atypical cells | Not done | 0.25 mg/0.1 mL (×1) | Not done | 20/25−20/25 | |
| May 2001 | Active (disc edema, retinal hemorrhage) | Not done | Not done | 0.65 mg/0.1 mL (×1), 0.25 mg/0.1 mL (×2) in 8 days | Not done | 20/40−20/25 | |
| June 2001 to February 2002 | Active at each visit (every 4−8 weeks) | Not done | >8.01 to >21.7 on 4 recurrences (ac††) | 0.45 mg/0.1 mL (series of 3 in 8 days, 5 times) | Following 2nd treatment: 9.3−38.1 Following 3rd treatment: 19.4−20.7 | 20/25—CF++ | |
| March to April 2002 | Active (subretinal lesion) | Not done | >7.3 (v) | 0.45 mg/0.1 mL (×2 per week for 2 weeks, weekly for 2 weeks) | Below detectable (ac) | CF—20/640 | PLAN: induction-consolidation-maintenance |
| Mid-April 2002 | Quiet | Not done | Not done | None | Not done | 20/640 | Corneal toxicity, injections held |
| Late-April 2002 | Quiet | Not done | >4.1 | None | Not done | 20/640 | Cornea recovered |
| Mid-May to August 2002 | Continuously active | PIOL | 62.3 (ac) | 0.45 mg/0.1 mL (×7) in 10 weeks | Following last treatment: 41.6 (Range: 2.7−44.4) | 20/640−20/500 | Persistent PIOL, possible MTX resistance: start RT‡‡ |
| September 2002 | Quiet | Not done | Before RT: >46.9 (ac) | None | <1 (ac) | 20/800 | Stable |
| Late-October 2002 | Quiet | Not done | Not done | After RT (15 d): Below detectable (ac) | 20/800 | Stable | |
| Mid-November 2002 | Quiet | Not done | Not done | After RT (1mo): Not done | 20/800 | Stable | |
| January 2003 | Quiet | Not done | Not done | After RT (2.5 mo): Not done | CF | Cataract progression OD | |
| February 2003 | Quiet | Not done | Not done | After RT (3.5 mo):Below detectable (ac) | CF | Cataract surgery planned | |
| May 2003-June 2005 | Quiet | Not done | Not done | After RT (2.5 yrs): Not done | CF | Pseudophakic, OD |
Note.
Methotrexate; ,vitreous
indicates how many times the injections were given
anterior chamber
count fingers
ocular radiotherapy.
PIOL cells from the anterior chamber were evaluated. Normal lymphocytes, malignant cells known to express multidrug resistance protein (MRP), folate binding protein (FBP) and reduced folate carrier (RFC), and lymphoma cells from another PIOL patient who was clinically responsive to methotrexate were studied as control specimens. The PIOL patient whose lymphoma cells were used as control was a 51-year-old male who had had CNS and intraocular lymphoma for 4.2 years and had multiple recurrences in his right eye (13 recurrences in 4 years) requiring 37 intravitreal injections (250−450 μg/0.1 mL).
To investigate the mechanism of the methotrexate resistance, PIOL cells from the anterior chamber of the phakic eye were collected and stained using an immunofluorescent technique with primary monoclonal antibodies against the multidrug resistance protein (MRP), folate binding protein (FBP), or rabbit polyclonal antibody against reduced folate carrier (RFC) protein. The secondary antibodies were fluorescent goat anti-mouse Alexa 488 (Molecular Probes, OR), goat anti-rabbit Alexa 555 (Molecular Probes, OR), or goat anti-rat Cy5 (Jackson Immunoresearch Laboratories, PA), respectively. The slides were viewed under a confocal microscope (Leica Microsystems, Exton, PA). Normal lymphocytes and intraocular lymphoma cells from the above described PIOL patient who responded to intravitreal methotrexate were used as control cells.
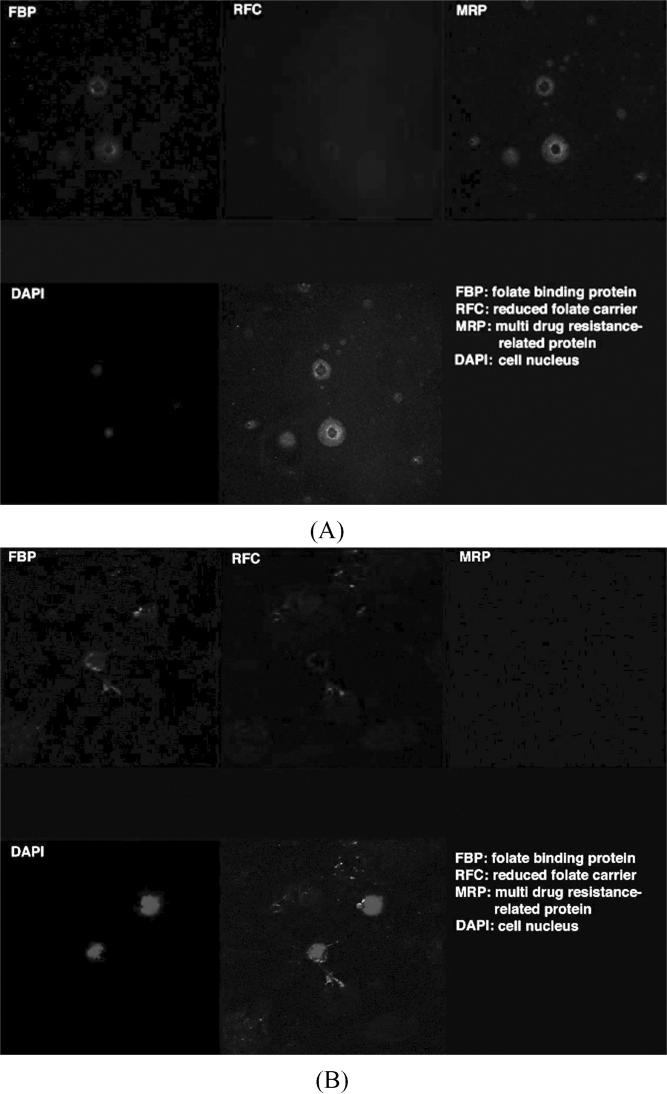
Most PIOL cells from our patient showed decreased expression of RFC and FBP, while fewer cells showed the aberrant expression of MRP (Figure 2A). Normal lymphocytes showed expression of RFC, FBP, and minimal MRP (data not shown). In contrast, lymphoma cells from the PIOL patient who responded to methotrexate showed absent MRP and relatively increased expression of FBP and RFC compared to our patient (Figure 2B).
FIGURE 2.
Confocal microscopy images illustrate (A) the positive staining for MRP, decreased staining for FBP and RFC in our patient compared to (B) negative staining for MRP, positive staining for RFC and FBP in the other PIOL patient clinically responsive to methotrexate (indirect immunocytochemistry; blue, DAPI staining for nuclei; green for FBP, red for RFC, and cyan for MRP; × 1000). The patterns of FBP on the PIOL cells were different between the two cases, methotrexate resistant and responsive patients; the former mainly inside the cytoplasm and the latter mainly on the cell membrane.
Ultimately, the PIOL resolved following radiotherapy (OD) and the IL-10 reached below detectable levels. The patient remains tumor-free 4 years postradiation.
DISCUSSION
Detection of monoclonality by molecular techniques and cytokine analysis is shown to facilitate the diagnosis of PIOL. Vitreous IL-10/ IL-6 >1.0 was reported to correctly distinguish PIOL from uveitis with approximately 75% sensitivity and specificity.5 The correlation between the ophthalmic findings and the intraocular IL-10/IL-6 levels in this patient suggests that cytokine analysis may be used as a biomarker to assess the PIOL in the eye. Recently elevation of aqueous IL-10 has been reported in PIOL patients.6
Resistance of chemotherapeutic drugs is a well-recognized obstacle in the treatment of lymphoma. Resistance to intravitreal methotrexate in PIOL has not been well documented previously. The most common mechanism of resistance to methotrexate is decreased intracellular accumulation due to a net reduction in its transport across the cell membrane. Influx of folate and methotrexate into the target cells is mediated by several systems, including FBP and RFC.7 Efflux of methotrexate is mainly mediated by MRP. Hence, the intracellular level of methotrexate is determined by influx (FBP and RFC) and efflux (RFC and MRP) transport pathways. Well-documented multiple mechanisms of antifolate resistance in tumor cells in vitro and in vivo include impaired antifolate uptake due to loss of RFC function, increased antifolate efflux due to overexpression of ATP-driven MDR efflux transporters, overexpression of dihydrofolate reductase (DHFR) and mutations that decrease its affinity for antifolates, over-expression of thymidylate synthase and mutations that decrease its affinity for antifolates, defective antifo-late polyglutamylation due to decreased folylpoly-γ-glutamate synthetase (FPGS) expression and/or inactivating mutations, increased expression of γ-glutamyl hydrolase (GGH), and finally expansion of intracellular tetrahydrofolate (THF) cofactor pools.8
Methotrexate has played an important role in cancer chemotherapy since late 1940s as a better DHFR inhibitor. More newer-generation antifolates, such as pemetrexed, have been introduced. The unique properties of this new agent include (1) rapid conversion to active polyglutamate derivatives in the cell and potent prolonged inhibition of its major target enzyme thymidylate synthase, (2) high affinity for three folate transporters, and (3) marked sensitivity to the level of intracellular physiologic folates, resulting in the unique and paradoxical finding that pemetrexed activity is preserved when transport mediated by the reduced folate carrier is impaired. Neither amplification of DHFR nor loss of RFC function seems to be associated with acquired pemetrexed resistance in vitro. Currently approved for the treatment of only few solid tumors, pemetrexed with its unique properties may be an alternative in the treatment of lymphoma in the future.9
Decreased expression of RFC and FBP and increased expression of MRP in our patient's PIOL cells suggest that these cells may have escaped the cytotoxicity of methotrexate by an acquired ability to reduce intracellular accumulation and metabolism of the drug. The findings provide a plausible explanation at the cellular level for the resistance to intravitreal methotrexate. Further studies in more patients are needed to determine the correlation between clinical drug resistance and cellular membrane transport system in PIOL. Identification and characterization of the molecular mechanisms of antifolate resistance may prove helpful for the development of novel antifolates and strategies that could conceivably overcome drug-resistance phenomena.
Footnotes
Primary antibodies of anti-human rat monoclonal antibody against the multidrug resistance protein (MRP), anti-human mouse monoclonal antibody against folate binding protein (FBP), and anti-human rabbit polyclonal antibody against reduced folate carrier (RFC) protein were kindly provided by Michael Gottesman of the National Cancer Institute of the National Institutes of Health.
Contributor Information
Gordon Byrnes, Retina Section, Ophthalmology Department, National Naval Medical Center, Bethesda, Maryland, USA.
Robert N. Fariss, Laboratory of Mechanisms of Ocular Disease, National Eye Institute, National Institutes of Health, Bethesda, Maryland, USA
REFERENCES
- 1.Schabet M. Epidemiology of primary CNS lymphoma. J Neurooncol. 1999;43:199–201. doi: 10.1023/a:1006290032052. [DOI] [PubMed] [Google Scholar]
- 2.Chan CC, Buggage RR, Nussenblatt RB. Intraocular lymphoma. Curr Opin Ophthalmol. 2002;13:411–418. doi: 10.1097/00055735-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Smith JR, Rosenbaum JT, Wilson DJ, et al. Role of intravitreal methotrexate in the management of primary central nervous system lymphoma with ocular involvement. Ophthalmology. 2002;109:1709–1716. doi: 10.1016/s0161-6420(02)01125-9. [DOI] [PubMed] [Google Scholar]
- 4.Levy-Clarke GA, Byrnes GA, Buggage RR, et al. Primary intraocular lymphoma diagnosed by fine needle aspiration biopsy of a subretinal lesion. Retina. 2001;21:281–284. doi: 10.1097/00006982-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 5.Chan CC, Wallace Intraocular lymphoma: update on diagnosis and management. Cancer Control. 2004;11:285–295. doi: 10.1177/107327480401100502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassoux N, Giron A, Bodaghi B, et al. IL-10 measurement in aqueous humor for screening patients with suspicion of primary intraocular lymphoma. Invest Ophthalmol Vis Sci. 2007;48:3253–3259. doi: 10.1167/iovs.06-0031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assaraf YG, Rothem L, Hooijberg JH, et al. Loss of multidrug resistance protein 1 expression and folate efflux activity results in a highly concentrative folate transport in human leukemia cells. J Biol Chem. 2003;278:6680–6686. doi: 10.1074/jbc.M209186200. [DOI] [PubMed] [Google Scholar]
- 8.Assaraf YG. Molecular basis of antifolate resistance. Cancer Metastasis Rev. 2007;26(1):153–81. doi: 10.1007/s10555-007-9049-z. [DOI] [PubMed] [Google Scholar]
- 9.Chattopadhyay S, Moran RG, Goldman ID. Pemetrexed: biochemical and cellular pharmacology, mechanisms, and clinical applications. Mol Cancer Ther. 2007;6:404–417. doi: 10.1158/1535-7163.MCT-06-0343. [DOI] [PubMed] [Google Scholar]