Abstract
KCNE transmembrane peptides are a family of modulatory β-subunits that assemble with voltage-gated K+ channels, producing the diversity of potassium currents needed for proper function in a variety of tissues. Although all five KCNE transcripts have been found in cardiac and other tissues, it is unclear whether two different KCNE peptides can assemble with the same K+ channel to form a functional complex. Here, we describe the derivatization of a scorpion toxin that irreversibly inhibits KCNQ1 K+ channel complexes that contain a specific KCNE peptide. Using this KCNE sensor, we show that heteromeric complexes form and the functional output from these complexes reveals a hierarchy in KCNE modulation of KCNQ1 channels: KCNE3 > KCNE1 ≫ KCNE4. Furthermore, our results demonstrate that KCNQ1/KCNE1/KCNE4 complexes also generate a slowly activating current that has been previously attributed to homomeric KCNQ1/KCNE1 complexes, providing a potential functional role for KCNE4 peptides in the heart.
Cellular potassium efflux is controlled by the orchestrated openings and closings of K+channel proteins. To meet the potassium conduction requirements for a wide variety of tissues, K+ channels co-assemble with different β-subunits to afford membrane-embedded complexes with distinctive gating properties. The KCNE type I transmembrane peptides are a class of β-subunits that assemble with and modulate the electrical output of voltage-gated K+ channels (1). KCNE peptides are particularly fond of KCNQ1 (Q1) channels, as all five members (E1 – E5) form complexes with this voltage-gated K+ channel (1). The number of KCNE peptides in a K+ channel complex is still debated. Studies have proposed 2 or 4 KCNE peptides per K+ channel tetramer, however, alternative subunit compositions could not be ruled out (2–4). Q1 channels must assemble with KCNE peptides for proper physiological function, as mutations that disrupt complex formation give rise to congenital deafness and inherited cardiac arrhythmias (5, 6). In the heart, the Q1/E1 K+ channel complex has long been thought to generate the slowly activating cardiac IKs current (7, 8). In contrast, E2 and E3 subunits have been shown to assemble with Q1 channel subunits in epithelial cells where they maintain salt and water homeostasis (9, 10). The biological roles of Q1/E4 and Q1/E5 complexes have yet to be defined.
Since the functional output of Q1 channels is controlled by KCNE peptides, their tissue distribution has been assumed to underlie Q1-KCNE complex function in vivo. Recently all five KCNE transcripts have been detected in cardiac (11, 12) and other tissues (13), raising the possibility that two different KCNE peptides can assemble with the same KCNQ1 channel to form a heteromeric complex. Determining the functional output of heteromeric Q1-KCNE complexes has been hampered by the fact that macroscopic electrical recordings measure the total current from a cell making it difficult to deconvolute the contribution of heteromeric complexes (e.g. Q1/E1/E4) from two populations of homomeric complexes (e.g. Q1/E1 and Q1/E4) functioning at the cell surface. Co-immunoprecipitation of one KCNE peptide by another KCNE peptide has indirectly hinted at heteromeric complex formation (14). However, these qualitative biochemical experiments are unsatisfying since it is unclear whether these precipitated complexes are functional. Therefore, we utilized a tethered blocker approach in combination with electrophysiological recordings to determine the KCNE composition and functional consequences of heteromeric Q1-KCNE K+ channel complex formation.
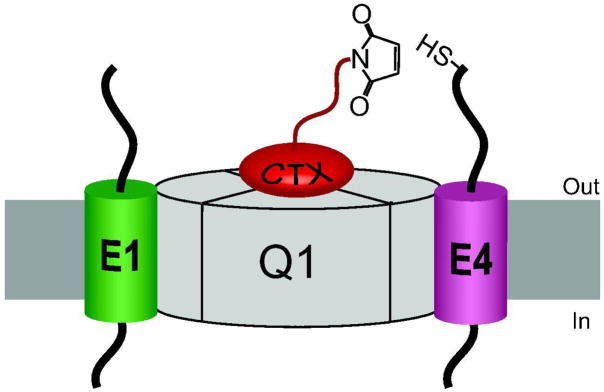
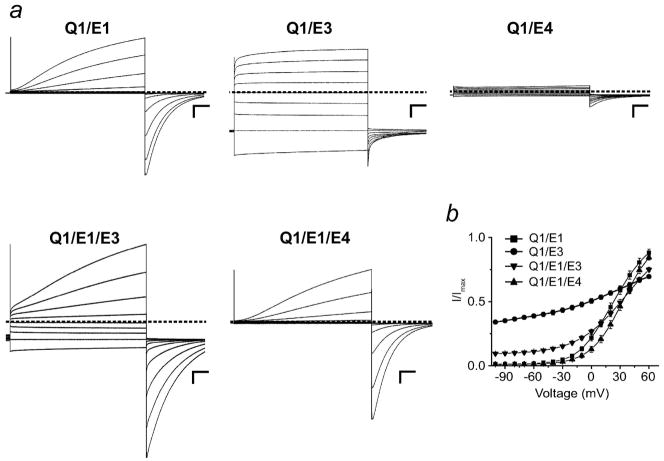
KCNE peptides have a striking effect on the opening and closing of Q1 channels and these gating differences have been traditionally used to identify the composition of homomeric Q1-KCNE complexes (Figure 1). Q1/E1 complexes are strongly voltage-dependent and upon depolarization give rise to a slowly activating K+ current (7, 8), whereas Q1/E3 complexes are open at both negative and positive voltages and have nearly instantaneous gating kinetics (Figure 1a) (10). In contrast, when Q1 assembles with E4, the complexes are essentially non-conducting at the plasma membrane (Figure 1a) (15). Co-expression of Q1 and E1 with E3 or E4 results in an amalgam of currents and activation curves (Figure 1a and 1b), which upon visual inspection does not reveal whether there are heteromeric Q1-KCNE complexes functioning at the plasma membrane. To detect KCNE peptides in functioning Q1 channel complexes, we synthetically modified charybdotoxin (CTX) to specifically react with cysteine residues within the N-termini of KCNE peptides (Figure 2a). CTX is a peptide scorpion toxin that reversibly binds K+ channels with high affinity (16, 17). Previous studies with CTX has shown that an arginine on the backside of the toxin can be mutated to cysteine for derivatization without affecting its affinity for K+ channels (18, 19). To convert CTX into a cysteine reactive inhibitor (CTX-Mal), the mixed disulfide protected R19C charybdotoxin mutant was treated with DTT followed by the addition of 100-fold molar excess bis-maleimide (Figure 2b).
Figure 1.
Co-expression of KCNQ1 channels with two different KCNE peptides gives rise to an amalgam of voltage-dependent and -independent currents. a) Two-electrode voltage clamp recordings for homo- and heteromeric Q1-KCNE complexes. Currents were elicited by a family of command voltages from −100 to 60 mV at 20-mV increments and recorded in KD50 solution. Dashed line indicates zero current. Scale bars represent 1 μA and 0.5 s. b) Voltage-activation curves of homo- and heteromeric Q1-KCNE complexes. The data were fit to a Boltzmann and normalized (21). Data were averaged from 3–5 oocytes each and error bars are ± SEM.
Figure 2.
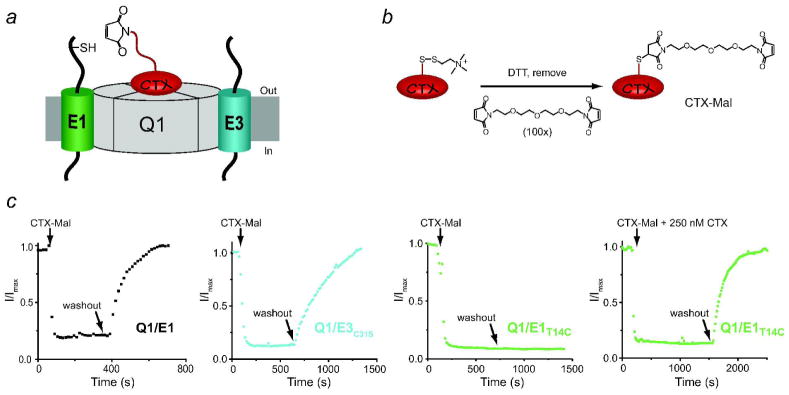
Strategy, synthesis and utilization of CTX-Mal for identifying KCNE peptides in functioning Q1-KCNE complexes. a) Cartoon depiction of the toxin tethering reaction between CTX-Mal and a cysteine residue in E1. b) Synthetic scheme for CTX-Mal. c) Inhibition of Q1-KCNE complexes with (10 nM) CTX-Mal in ND96 solution. Current was monitored at 20 mV every 15 s. Q1/E1 and Q1/E3C31S complexes lack extracellular cysteine residues. Single cysteine containing Q1/E1T14C complexes were irreversibly inhibited by CTX-Mal after washout; inhibition was completely prevented in the presence of excess (250 nM) CTX.
Since CTX does not inhibit native Q1/E1 complexes, we utilized a variant of Q1 that is blocked by nanomolar concentrations of toxin (2). CTX-sensitive Q1/E1 complexes do not possess any extracellular cysteines and thus CTX-Mal should reversibly bind to these complexes. When cells expressing CTX-sensitive Q1/E1 complexes were treated with 10 nM CTX-Mal, the current was blocked, and then upon washout it fully recovered to pretreated levels, demonstrating that inhibition was reversible (Figure 2c). Q1/E3 complexes were also reversibly blocked by CTX-Mal when the lone cysteine in E3 (C31S) was removed by mutagenesis (Figure 2c, upper right panel). These control experiments showed that chemical derivatization of CTX did not disrupt binding to the Q1 channel pore. We next cysteine-scanned the N-terminus of E1 to find residues that were covalently modifiable by CTX-Mal. The N-termini of KCNE peptides are ideal for cysteine mutagenesis since these regions in E1 and E3 are not required for assembly with or modulation of Q1 channels (20, 21). Mutation of three E1 residues (T14, Q22, and S34) to cysteine resulted in Q1/E1 complexes with wild-type gating kinetics that were irreversibly blocked by CTX-Mal. Q1/E1T14C complexes were the most reactive, showing no relief of inhibition upon washout of CTX-Mal (Figure 2c). Covalent tethering of CTX-Mal to Q1/E1T14C required binding to the channel pore since the reaction was completely prevented by competitive inhibition with 250 nM CTX (Figure 2c). In all experiments, only ~ 85% of the total current could be inhibited (reversibly or irreversibly); even in the presence of 250 nM wild-type CTX, which based on the calculated dissociation constant for CTX-sensitive Q1/E1 complexes should afford > 99.9% inhibition (Figure 1c, lower right panel) (2). We did not expect 100% block since Xenopus oocytes possess low levels of endogenous, CTX-insensitive Q1 channels that migrate to the cell surface with the injection of KCNE mRNAs (Supplementary Figure 1) (2, 7).
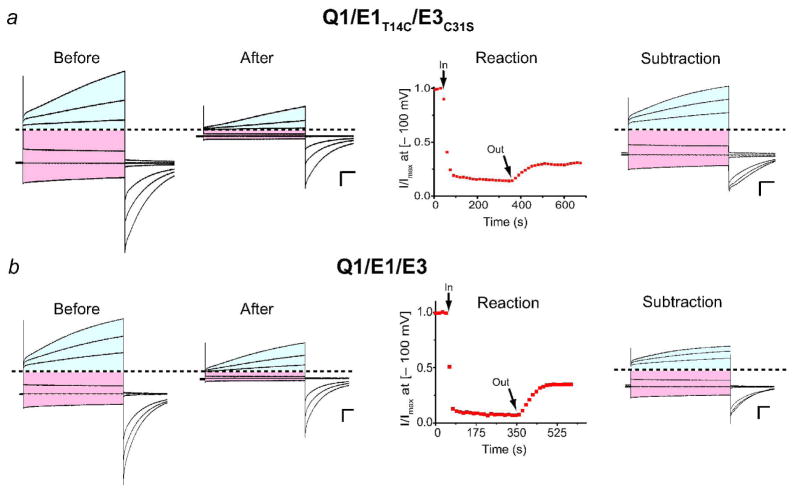
Armed with a reagent to detect KCNE peptides in K+ channel complexes, we determined whether Q1/E1/E3 complexes assemble and are functional. We first placed the target cysteine in E1. Co-expression of Q1/E1T14C/E3C31S resulted in both outward and inward currents in response to a family of depolarizations in 50 mM external K+ (Figure 3a, Before). This external solution (KD50) allows for the visualization of the inward currents (shaded red); however, this concentration of external K+ noticeably slows the activation/deactivation kinetics of some Q1-KCNE complexes (Figures 1 & 3) compared to ND96 solution (Figure 4). Treatment of these cells with CTX-Mal covalently modifies and irreversibly blocks Q1 channel complexes that have assembled with E1T14C peptides. Upon washout, ~ 80% of the CTX-sensitive current generated by a − 100 mV test potential was irreversibly inhibited (Figure 3a, Reaction). Examination of the current-voltage traces after CTX-Mal washout (Figure 3a, After) shows a mixture of E1 and E3 modulated Q1 currents. Since CTX-Mal irreversibly blocks all of the CTX-sensitive current in exogenously expressed Q1/E1T14C complexes (Figure 2c, lower left panel), we postulate that the remaining Q1/E1 current is due to endogenous (CTX-insensitive) Q1 channels that have assembled with E1T14C peptides (Supplementary Figure 1). To visualize the currents irreversibly blocked by CTX-Mal, we mathematically subtracted the remaining current after block from the before block currents. The subtracted traces are a mixture of currents from Q1/E1 homomers and Q1/E1/E3 heteromers. However, since homomeric Q1/E1 complexes are closed and non-conducting with test potentials less than − 30 mV (Figure 1a), the inward currents in the subtracted traces in Figure 3a are entirely from Q1/E1T14C/E3C31S complexes. Unlike the inward currents, the outward currents (shaded blue) in the subtracted traces are contaminated withslowly activating homomeric Q1/E1T14C currents. To determine whether Q1/E1/E3 complexes possess any time dependent activation at positive potentials, we utilized the native cysteine in E3 and looked at irreversible block of outward currents in wild type Q1/E1/E3 complexes. The blocked outward currents in this configuration will still be a mixture of homo- and heteromeric complexes; however, the homomeric complexes are Q1/E3, which are devoid of slow gating (Figure 1a). Thus, any slow gating observed would be due to heteromeric Q1/E1/E3 complexes. CTX-Mal treatment and subsequent washout of cells expressing Q1/E1/E3 resulted in a reduction of both inward and outward currents as before (Figure 3b). Mathematical subtraction reveals that heteromeric Q1/E1/E3 complexes slowly activate at positive voltages and slowly deactivate upon repolarization (Figure 3b, Subtraction). In total, these results show that the Q1/E1/E3 complex generates current with the combined properties of homomeric Q1/E1 and Q1/E3 complexes: it is a heteromeric complex that is open at negative potentials, but slowly activates with positive depolarizations.
Figure 3.
Detection of heteromeric Q1/E1/E3 complexes with CTX-Mal. a) Q1/E1T14C/E3C31S b) Q1/E1/E3 (using native cysteine (C31) in E3). Current traces shown are pre-treatment (Before) and after washout (After) of 10 nM CTX-Mal. Currents were measured in KD50 solution and were from command voltages (− 100, − 80, − 60, 0, 20, and 40 mV). Subtraction traces are a mathematical difference of the After and Before traces. Outward currents are shaded blue; inward are shaded red. Dashed line indicates zero current. Scale bars represent 1 μA and 0.5 s. Reaction profiles (Reaction) with 10 nM CTX-Mal were monitored at − 100 mV every 15 s in KD50 solution. For Q1/E1T14C/E3C31S, 78 ± 2% of the CTX-sensitive current was irreversibly inhibited after washout; for Q1/E1/E3, 74 ± 4%. Data were averaged from 3–5oocytes ± SEM.
Figure 4.
KCNE4 peptides form heteromeric complexes with KCNQ1 and KCNE1 or KCNE3. a) Q1/E1/E4L2C b) Q1/E3C31S/E4L2C. Current traces shown are pre-treatment (Before) and after washout (After) of 10 nM CTX-Mal. Currents were measured in ND96 and elicited by a family of command voltages from − 100 to 40 mV with 20-mV steps. Subtraction traces are a mathematical difference of the After and Before traces. Dashed line indicates zero current. Scale bars represent 1 μA and 0.5 s. Reaction profiles (Reaction) with 10 nM CTX-Mal were monitored at 20 mV every 15 s in ND96 solution. For Q1/E1/E4L2C, 52 ± 3% of the CTX- sensitive current was irreversibly inhibited after washout; for Q1/E3/E4L2C, 80 ± 1%. Data were averaged from 2–5 oocytes ± SEM.
We next examined whether Q1/E1/E4 complexes were functional. E4 is the most abundant KCNE transcript in heart (11, 12), yet when the peptide assembles with Q1 it produces a non-conducting channel complex (Figure 1a) (15). Therefore, to detect functional Q1/E1/E4 complexes, the cysteine residue was placed in E4. When E4L2C was co-expressed with Q1/E1, the resultant CTX-sensitive currents in ND96 solution were irreversibly blocked (52 ± 3%) by CTX-Mal upon washout (Figure 4a). Irreversible blockade of any current indicates Q1/E1/E4 complex assembly since homomeric Q1/E4L2C complexes are like wild type Q1/E4 and non-conducting (data not shown). The amount of irreversible inhibition measured was predictably dependent on the ratio of E1:E4L2C expressed. Injection ratios that favored the formation of Q1/E1 homomers resulted in a reduction of irreversible block whereas injection of more E4L2C mRNA resulted in an increase of irreversible block (Supplementary Figure 2). Subtraction of the after traces from the before traces revealed the gating kinetics of Q1/E1/E4 complexes (Figure 4a, Subtraction), which resemble the cardiac IKs current that has been attributed to Q1/E1 complexes. E4 also forms functional heteromeric Q1-complexes with E3. Figure 4b shows that Q1/E3C31S/E4L2C complexes were irreversibly blocked by CTX-Mal in ND96 solution. Although E4 is present in both heteromeric complexes studied, the peptide has little effect on the gating kinetics. Thus for Q1 channels, E3 and E1 dictate gating in heteromeric complexes with E4 peptides.
Our results using CTX-Mal demonstrate that a tetrameric Q1 channel can simultaneously assemble with two different KCNE peptides. Although we show that heteromeric Q1-KCNE complexes form, we cannot determine the assembly preference for KCNE peptides with Q1 subunits since we are looking at an endpoint (current expression) and not the biogenesis and stability of the protein subunits. The formation of heteromeric complexes requires that there are at least two KCNE peptides in the K+ channel complex; however, irreversible inhibition with CTX-Mal cannot distinguish between two or more peptides within the complex, which is still a surprisingly unsettled issue (2–4). Our results also indicate that the N-termini of KCNE peptides are near the Q1 channel outer vestibule since the linker connecting CTX to the maleimide is too short to reach beyond the S3–S4 loop of the voltage sensor (22). Based on a recent NMR structure of CTX bound to a bacterial K+ channel (23), we tentatively position the KCNE N-terminus within ~ 20 Å of the ion conduction pathway. CTX-Mal enabled us to determine that certain KCNE peptides govern Q1 modulation in heteromeric complexes: E3 > E1 ≫ E4, where the dominant peptide has substantial control over the opening and closing of the channel. This hierarchy of KCNE modulation of Q1 channels has several implications on KCNE physiology. In the heart, it has been assumed that homomeric Q1/E1 complexes produce the unmistakably slowly activating/deactivating current involved in repolarization (7, 8). E4 may also generate the cardiac IKs current since Q1/E1/E4 and Q1/E1 complexes have similar gating kinetics. Likewise, in most epithelial cells where the resting membrane potential is negative, the assembly of E1, E4 or E5 with Q1 has been questioned since these complexes would be essentially closed. However, E1 and E4 peptides can act as structural surrogates in Q1/E3 heteromeric complexes to produce channels that are open at negative potentials and thus may partake in salt and water homeostasis. In either case, these heteromeric complexes can substitute for homomeric complexes; however, they require the presence of the dominant KCNE peptide, consistent with KCNE knockout mice studies (9, 24). In contrast, over expression of constitutively conducting Q1/E1/E3 complexes could be detrimental in maintaining the cardiac action and resting potentials, suggesting that cells regulate Q1-KCNE assembly. Simple binary transcriptional regulation of functionally opposed KCNE peptides could be used to control the potassium efflux of a cell. Alternatively, KCNE assembly with Q1 channel subunits may be regulated at the protein folding level. If Q1-KCNE assembly is chaperone-mediated, these proteins must reside in either the ER or cis-Golgi since KCNE peptides assemble with Q1 channels early in the secretory pathway (25).
We have used a synthetically modified scorpion toxin to detect Q1 subunit assembly with a mixed KCNE population. CTX-Mal can also be used to disentangle the phenotypic effects of KCNE mutations on assembly from those on modulation by deconvolving the functional contributions of unpartnered Q1 channels and mutant Q1-KCNE complexes. The discovery that heteromeric Q1-KCNE complexes form implies that other voltage-gated K+ channels will be amenable to co-assembly with two different KCNE peptides. Given the cadre of scorpion and spider toxins available to specifically inhibit a wide-range of ion channels, our approach can be readily applied to study other membrane-embedded β-subunits in functioning ion channel complexes.
METHODS
Mutagenesis and In Vitro Transcription
The CTX sensitive Q1 construct (2), E1, E3, and E4 were subcloned into a vector containing the 5′ and 3′ UTRs from the Xenopus β-globin gene for optimal expression in Xenopus oocytes. Single point mutations in KCNE peptides were introduced using Quikchange site-directed mutagenesis (Stratagene) and confirmed by DNA sequencing of the entire gene. E1 and E3 constructs possessed the hemagglutinin A (HA) tag, YPYDVPDYA, in the N-terminus between residues 22 and 23, and 11 and 12, respectively, which has been shown to increase toxin affinity (2). The cDNA plasmids were linearized and cRNA synthesized by run-off transcription using SP6 or T7 RNA polymerase (Promega).
Electrophysiology
Standard techniques were used for preparation of and recording from Xenopus oocytes by two-electrode voltage clamp 2 to 4 days (5–6 days for E4) after cRNA injection (26). Oocytes expressing KCNE extracellular cysteine residues were stored in ND96 bathing solution (mM): 96 NaCl, 2 KCl, 1.8 CaCl2, 5 HEPES, pH 7.4, 50 μg/mL gentamicin containing 1 mM L-glutathione (Sigma) and incubated in ND96 recording (mM): 96 NaCl, 2 KCl, 0.3 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4 containing 1 mM TCEP (CalBioChem) for 10 min prior to recording. cRNA injection ratios were as follows: Q1/E1, Q1/E1T14C, Q1/E3, Q1/E3C31S, and Q1/E4L2C, 3:1; Q1/E1T14C/E3C31S, Q1/E1/E3, Q1/E4L2C/E3C31S, 4:1:0.2; Q1/E1/E4L2C, 4:1:1. Current-voltage relationships were measured in ND96 or KD50 (mM) 50 KCl, 48 NaCl, 0.3 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4 by holding at − 80 mV and pulsing for 2 or 4 s to potentials between − 100 and + 60 mV in 10-mV increments. Activation curves were generated from tail currents and measured 6 ms after repolarization to − 80 mV in KD50, as was previously described for channels with basal activation (21). For CTX experiments, bath solutions also contained 50 μg mL−1 bovine serum albumin. Solution exchanges were performed by gravity-fed perfusion system with a chamber clearing time of ~ 10 s. Families of currents before and after block were recorded in the appropriate bath solution by holding at − 80 mV and pulsing from −100 to 60 mV in 20 mV increments. Currents from heteromeric Q1-KCNE complexes were revealed by subtracting post-block family traces from pre-block families (Clampfit 9.0 Axon Instruments).
CTX Derivatization
Recombinant CTX R19C was purified and the protected CTX-MTSET adduct was prepared according to Shimony and Miller (18). CTX-Mal was synthesized as follows: 16 nmols of CTX-MTSET in 2 mL Buffer C: (mM) 10 NaCl, 10 KPi, pH 7.4 was reduced with 1 mM DTT for 30 mins and bound to a SP Sephedex (Sigma) cation exchange column. DTT was washed from the column using 150 mL Buffer C and the resin bound CTX was labeled with 3 mL of 5 mM BM[PEO]3 (Pierce) in Buffer C. After 10 min, the column was washed with Buffer C to remove excess label, and CTX-Mal was eluted with 1M NaCl in 10 mM KPi, pH 7.4. CTX-Mal was desalted and HPLC purified using a C18 column (4.6 mm × 250 mm) eluting with solvent A: 0.1% TFA; solvent B: acetonitrile; gradient 10 to 40% B over 30 min. The concentration of purified CTX-Mal was determined by UV spectrometry (OD280 of 1.0 = 100 uM CTX (18)) and labeling efficiency of CTX-R19C was determined to be 35% ± 5% (n = 10). The purified product was confirmed by ESI mass spectrometry (Supplementary Material) and was aliquoted, lyophilized, and stored at − 20°C. Individual aliquots were resuspended in recording solutions immediately prior to use.
Figure 5.
Acknowledgments
We thank A. George (Vanderbilt University) for the E4 clone. W. Kobertz was supported in part by a Burroughs-Wellcome Foundation for a Career Award in the Biomedical Sciences. This work was supported by the NIH GM0707650 and DC007669.
Footnotes
Supporting Information Available: This material is available free of charge via the Internet.
References
- 1.McCrossan ZA, Abbott GW. The MinK-related peptides. Neuropharmacology. 2004;47:787–821. doi: 10.1016/j.neuropharm.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 2.Chen H, Kim LA, Rajan S, Xu S, Goldstein SA. Charybdotoxin binding in the IKs pore demonstrates two MinK subunits in each channel complex. Neuron. 2003;40:15–23. doi: 10.1016/s0896-6273(03)00570-1. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Xia J, Kass RS. MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel. J Biol Chem. 1998;273:34069–74. doi: 10.1074/jbc.273.51.34069. [DOI] [PubMed] [Google Scholar]
- 4.Wang KW, Goldstein SA. Subunit composition of minK potassium channels. Neuron. 1995;14:1303–9. doi: 10.1016/0896-6273(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 5.Splawski I, Shen J, Timothy KW, Lehmann MH, Priori S, Robinson JL, Moss AJ, Schwartz PJ, Towbin JA, Vincent GM, Keating MT. Spectrum of mutations in long-QT syndrome genes. KVLQT1, HERG, SCN5A, KCNE1, and KCNE2. Circulation. 2000;102:1178–85. doi: 10.1161/01.cir.102.10.1178. [DOI] [PubMed] [Google Scholar]
- 6.Tyson J, Tranebjaerg L, McEntagart M, Larsen LA, Christiansen M, Whiteford ML, Bathen J, Aslaksen B, Sorland SJ, Lund O, Pembrey ME, Malcolm S, Bitner-Glindzicz M. Mutational spectrum in the cardioauditory syndrome of Jervell and Lange-Nielsen. Hum Genet. 2000;107:499–503. doi: 10.1007/s004390000402. [DOI] [PubMed] [Google Scholar]
- 7.Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KVLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–3. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- 8.Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KVLQT1 and IsK (minK) proteins associate to form the IKs cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- 9.Roepke TK, Anantharam A, Kirchhoff P, Busque SM, Young JB, Geibel JP, Lerner DJ, Abbott GW. The KCNE2 potassium channel ancillary subunit is essential for gastric acid secretion. J Biol Chem. 2006;281:23740–7. doi: 10.1074/jbc.M604155200. [DOI] [PubMed] [Google Scholar]
- 10.Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–9. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- 11.Lundquist AL, Manderfield LJ, Vanoye CG, Rogers CS, Donahue BS, Chang PA, Drinkwater DC, Murray KT, George AL., Jr Expression of multiple KCNE genes in human heart may enable variable modulation of IKs. J Mol Cell Cardiol. 2005;38:277–87. doi: 10.1016/j.yjmcc.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Bendahhou S, Marionneau C, Haurogne K, Larroque MM, Derand R, Szuts V, Escande D, Demolombe S, Barhanin J. In vitro molecular interactions and distribution of KCNE family with KCNQ1 in the human heart. Cardiovasc Res. 2005;67:529–38. doi: 10.1016/j.cardiores.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Lundquist AL, Turner CL, Ballester LY, George AL., Jr Expression and transcriptional control of human KCNE genes. Genomics. 2006;87:119–28. doi: 10.1016/j.ygeno.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Wu DM, Jiang M, Zhang M, Liu XS, Korolkova YV, Tseng GN. KCNE2 is colocalized with KCNQ1 and KCNE1 in cardiac myocytes and may function as a negative modulator of IKs current amplitude in the heart. Heart Rhythm. 2006;3:1469–80. doi: 10.1016/j.hrthm.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Grunnet M, Jespersen T, Rasmussen HB, Ljungstrom T, Jorgensen NK, Olesen SP, Klaerke DA. KCNE4 is an inhibitory subunit to the KCNQ1 channel. J Physiol. 2002;542:119–30. doi: 10.1113/jphysiol.2002.017301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith C, Phillips M, Miller C. Purification of charybdotoxin, a specific inhibitor of the high-conductance Ca2+-activated K+ channel. J Biol Chem. 1986;261:14607–13. [PubMed] [Google Scholar]
- 17.Goldstein SA, Pheasant DJ, Miller C. The charybdotoxin receptor of a Shaker K+ channel: peptide and channel residues mediating molecular recognition. Neuron. 1994;12:1377–88. doi: 10.1016/0896-6273(94)90452-9. [DOI] [PubMed] [Google Scholar]
- 18.Shimony E, Sun T, Kolmakova-Partensky L, Miller C. Engineering a uniquely reactive thiol into a cysteine-rich peptide. Protein Eng. 1994;7:503–7. doi: 10.1093/protein/7.4.503. [DOI] [PubMed] [Google Scholar]
- 19.Posson DJ, Ge P, Miller C, Bezanilla F, Selvin PR. Small vertical movement of a K+ channel voltage sensor measured with luminescence energy transfer. Nature. 2005;436:848–51. doi: 10.1038/nature03819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takumi T, Moriyoshi K, Aramori I, Ishii T, Oiki S, Okada Y, Ohkubo H, Nakanishi S. Alteration of channel activities and gating by mutations of slow ISKpotassium channel. J Biol Chem. 1991;266:22192–8. [PubMed] [Google Scholar]
- 21.Gage SD, Kobertz WR. KCNE3 Truncation Mutants Reveal a Bipartite Modulation of KCNQ1 K+ Channels. J Gen Physiol. 2004;124:759–771. doi: 10.1085/jgp.200409114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blaustein RO, Cole PA, Williams C, Miller C. Tethered blockers as molecular ‘tape measures’ for a voltage-gated K+ channel. Nat Struct Biol. 2000;7:309–11. doi: 10.1038/74076. [DOI] [PubMed] [Google Scholar]
- 23.Yu L, Sun C, Song D, Shen J, Xu N, Gunasekera A, Hajduk PJ, Olejniczak ET. Nuclear magnetic resonance structural studies of a potassium channel-charybdotoxin complex. Biochemistry. 2005;44:15834–41. doi: 10.1021/bi051656d. [DOI] [PubMed] [Google Scholar]
- 24.Vetter DE, Mann JR, Wangemann P, Liu J, McLaughlin KJ, Lesage F, Marcus DC, Lazdunski M, Heinemann SF, Barhanin J. Inner ear defects induced by null mutation of the isk gene. Neuron. 1996;17:1251–64. doi: 10.1016/s0896-6273(00)80255-x. [DOI] [PubMed] [Google Scholar]
- 25.Chandrasekhar KD, Bas T, Kobertz WR. KCNE1 subunits require co-assembly with K+ channels for efficient trafficking and cell surface expression. J Biol Chem. 2006;281:40015–40023. doi: 10.1074/jbc.M604398200. [DOI] [PubMed] [Google Scholar]
- 26.Rudy B, Iverson LE. Methods Enzymol. 1992:225–345. [Google Scholar]