Abstract
We investigated the protein expression of three glioma-associated antigens (GAAs) in pediatric brain stem glioma (BSG) and non-brain stem glioma (NBSG) cases with a view to their possible use in immunotherapy. Expression of EphA2, IL-13Rα2 and Survivin were studied by immunohistochemistry on paraffin-embedded tissues using a series of 15 BSG cases and 12 NBSG cases. Thirteen of 15 BSGs and all 12 NBSGs expressed at least one of GAAs; and 7 BSGs and 9 NBSGs expressed at least two of these GAAs at higher levels than non-neoplastic brain. There was no association between the tumor grade and levels of GAA expression. Although many cases demonstrated diffuse expression of GAAs throughout specimens, partial or patchy expression was noted in a small number of cases, suggesting a need for targeting multiple GAAs in immunotherapy. These results suggest that EphA2, IL-13Ralpha2 and Survivin are suitable targets for developing vaccine strategies for pediatric glioma.
Keywords: Brain stem, EphA2, Glioma, Glioma-associated antigen, Immunohistochemistry, interleukin-13 receptor alpha 2, Pediatric glioma, Survivin
Introduction
Children with intrinsic brainstem malignant gliomas have 1- and 5-year progression-free survival rates of less than 25% and 10%, respectively [1, 2]. Radiologically, these lesions generally have a characteristic appearance on magnetic resonance imaging that, in most instances, obviates the need for a biopsy to establish the histological diagnosis in children with an appropriate clinical history [3]. Accordingly, in recent years, these lesions have seldom been biopsied. In the limited numbers of cases where tissue has been obtained, the diagnosis was most often high-grade glioma [3–7]. Given the paucity of information about the biology of brain stem gliomas, it is often necessary to make inferences about their molecular characteristics based on studies of pediatric highgrade gliomas at non-brain stem sites. Other than radiation therapy, no other therapy has demonstrated efficacy in prolonging the survival of these patients [8].
Similarly, pediatric patients with incompletely resected anaplastic astrocytoma or glioblastoma multiforme also have a dismal prognosis with contemporary therapy, with 1- and 5-year progression-free survival rates of less than 50% and 20%, respectively. These lesions show a low frequency of objective responses to conventional chemotherapeutic agents, which is less than 20% even for some promising agents, such as temozolomide [2].
In view of these discouraging results, there is a strong need for identifying new therapeutic approaches that target those features of the tumor cell that distinguish it from surrounding normal cells, such as vaccine strategies targeting glioma-associated antigens. In recent years, we have examined the applicability of a series of tumor cellbased immunization approaches for patients with gliomas [9–15]. Although promising results have been achieved [9, 16, 17], a limitation of this strategy is the need for autologous tumor and the time delay involved in generating a patient-specific vaccine. In contrast, vaccines in the form of synthetic peptides encoding T-cell epitopes in GAA would eliminate the need for autologous fresh glioma explants to generate clinical grade vaccines, facilitate timely vaccine production, and potentially reduce the risk of autoimmune encephalitis. To this end, we have focused attention on the identification and characterization of human GAA-derived cytotoxic T-lymphocyte (CTL) epitopes, such as those in the interleukin-13 receptor (IL-13R)α2 [18, 19] and EphA2 [20]. Recent studies by others have shown that Survivin, which contains HLA-A2-restricted CTL epitopes [21, 22], are frequently expressed at high levels in grade II, III and IV astrocytomas in adults [23]. In vivo, glioma cells may undergo “immuno-editing”, in which heterogeneous progressive lesions exhibit loss of specific antigens [24]. Hence, an optimal glioma vaccine design should include multiple GAA-derived T cell epitopes.
In the current study, we performed immunohistochemical evaluation of three GAAs, EphA2, IL-13Rα2 and Survivin in paraffin-embedded tissues derived from pediatric gliomas arising from both brain stem and non-brain stem locations. Our results suggest that EphA2, IL-13Rα2 and Survivin are suitable targets for developing vaccine strategies for pediatric glioma.
Materials and methods
Tissues
Archival formalin-fixed, paraffin-embedded glioma specimens (15 BSG, 12 NBSG), obtained at the time of tumor biopsy or resection or by autopsy, were provided for this study under an Institutional Review Board-approved protocol. The vast majority of the BSG samples were obtained prior to the MR era, and in many cases prior to the availability of high resolution CT, when open biopsy was often performed to establish the diagnosis, whereas the NBSG samples were obtained more recently. Normal brain sections were obtained from Human Brain Tissue Bank, Division of Neuropathology of the University of Pittsburgh.
Antibodies
The following primary antibodies were used at indicated dilutions or concentrations (in parentheses): anti-human EphA2 monoclonal antibody (mAb) (1:100; Ab 208, mouse IgG1, MedImmune), anti-human IL-13Rα2 polyclonal antibody (pAb) (15 μg/ml; AF146, goat polyclonal, R&D), anti-human Survivin pAb (1:100 in 1% BSA, C-19, goat polyclonal, Santa Cruz Biotechnology, Inc.) and antiglial fibrillary acidic protein (GFAP) (1:500, rabbit polyclonal, DakoCytomation). The secondary antibodies used in the current study were peroxidase-conjugated rabbit polyclonal anti-goat IgG heavy and light chains (1:200; ab6741, Abcam), goat polyclonal anti-mouse IgG heavy and light chains (1:200; AP124P, Upstate Cell Signaling) and goat polyclonal anti-rabbit IgG heavy and light chains (1:500; AP132P, Chemicon International).
Immunohistochemistry
Paraffin-embedded tissue sections (6–8 μm) were deparaffinized in xylene and rehydrated in a series of ethanol/phosphate-buffered saline (PBS) washes. Antigenicity was retrieved with modified citrate solution (Dako) in a pressure cooker for 20 min. Endogenous peroxidase was blocked by incubation in 3.0% hydrogen peroxide in PBS, and the nonspecific binding of antibodies was blocked by incubation in serum-free blocking solution (Dako) for 1 h. The slides were then incubated with each of antigen-specific antibodies diluted in 1% bovine serum albumin (BSA) in PBS overnight at 4°C. After washing, slides were then incubated with corresponding peroxidase-conjugated secondary antibodies for 1 h at room temperature. Peroxidase labeling was visualized using Vectastain’s Nova Red kit. The sections were lightly counterstained with Gill’s hematoxylin. The sections were then reviewed. Positive staining (+) was defined by definite, but moderate staining in the tumor. Strong positive staining (2+) was defined by intense immunoreactivity. In both positive (+) and strong positive (2+) cases, it was also con- firmed that positive signals in the tumor tissues were higher than signals from normal tissue elements in the same sections or normal brain control sections.
Results
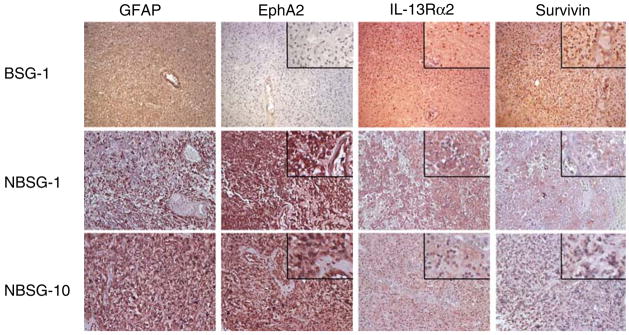
The expression of EphA2, IL-13Rα2 and Survivin protein was evaluated by immunohistochemistry in 15 BSG and 12 NBSG samples. Figure 1 demonstrates representative cases. A brain stem (medullary) low grade glioma (BSG-1) showed diffuse expression of IL-13Rα2 and Survivin, but was negative for EphA2.A temporo-frontal GBM (NBSG-1) and a thalamic GBM (NBSG-10) both exhibited strong expression of EphA2, and moderate IL-13Rα2 and Survivin. Interestingly, EphA2 was also expressed in tumor-associated vascular endothelial cells (NSBG-1), consistent with previous findings by us [25] and others [26].
Fig. 1.
Immunohistochemical analysis of EphA2, IL-13Rα2 and Survivin in pediatric glioma specimens. Tissue specimens were stained with specific antibodies against GFAP, EphA2, IL-13Rα2, or Survivin as described in Materials and Methods. Original magnification ×200. High power insets for EphA2, IL-13Rα2, and Survivin were derived from the corresponding low power fields
Staining results of all evaluated cases are summarized in Table 1. Negative staining is defined by lack of staining or weak background staining that was not higher than the background signal observed in negative control samples. Although many cases demonstrated diffuse expression of GAAs throughout specimens, partial or patchy expression was noted in a small number of cases, exemplified by Survivin expression in NBSG-1 (Fig. 1).
Table 1.
Expression of glioma-associated antigen (GAA) in pediatric brain tumor sections by immunohistochemistry
| Case | Age | Gender | Diagnosisa | Site | GFAP | EphA2 | IL-13Ra2 | Survivin |
|---|---|---|---|---|---|---|---|---|
| BSG | ||||||||
| 1 | 5 | f | Low grade | Medulla | 1+ | – | 1+ | 1+ |
| 2 | 15 | m | High-grade | Pons | 2+ | 2+ | 2+ | – |
| 3 | 7 | f | High-grade | Pons | 2+ | – | 1+ | – |
| 4 | 8 | f | Low-grade | Medulla | 1+ | – | 1+ | 1+ |
| 5 | 17 | m | High-grade | Pons | 1+ | 1+ | 1+ | 2+ |
| 6 | 6 | m | High-grade | Pons | 2+ | – | – | – |
| 7 | 4 | f | High-grade | Pons | 1+ | 1+ | 1+ | 1+ |
| 8 | 7.5 | f | High-grade | Pons | 1+ | – | 1+ | – |
| 9 | 4 | f | Low-grade | Medulla | 2+ | 1+ | 1+ | 2+ |
| 10 | 7 | m | High-grade | Pons | 2+ | 1+ | – | – |
| 11 | N/A | Malignant | Pons | 1+ | – | – | 2+ | |
| 12 | N/A | Malignant | Pons | 1+ | – | – | 1+ | |
| 13 | N/A | Malignant | Pons | 1+ | – | – | 2+ | |
| 14 | 4.9 | m | Low-grade | Medulla | 1+ | 2+ | 2+ | – |
| 15 | 0.7 | m | Low-grade | Pons | 1+ | 2+ | 1+ | – |
| NBSG | ||||||||
| 1 | 1.2 | f | gbm | Temporofrontal | 1+ | 2+ | 1+ | 1+ |
| 2 | 1.2 | f | aa | Thalamus | 1+ | 1+ | – | – |
| 3 | 16.7 | f | aa | Frontoparietal | 1+ | 2+ | 1+ | 1+ |
| 4 | 0.8 | f | Low-grade | Frontal | 1+ | 2+ | 2+ | 2+ |
| 5 | 1.4 | f | aa | Basal ganglia | 1+ | 2+ | N/A | 1+ |
| 6 | 15.6 | m | aa | Frontal | 1+ | 2+ | N/A | 1+ |
| 7 | 18 | f | gbm | Frontal | 1+ | 1+ | N/A | – |
| 8 | 11.5 | m | Low-grade | Parieto-frontal | 1+ | 2+ | 1+ | 1+ |
| 9 | 9.5 | m | Low-grade | Parietal | 1+ | 1+ | 1+ | – |
| 10 | 12.9 | m | gbm | Thalamus | 1+ | 2+ | 1+ | 1+ |
| 11 | 6.5 | m | Low-grade | Frontal | 1+ | 2+ | 1+ | 1+ |
| 12 | 6.3 | f | gbm | Parietofrontal | 1+ | 1+ | – | – |
N/A, information not available
Because of the small sizes of many of the specimens, histological diagnoses of the brainstem gliomas are broadly characterized into low-grade and high-grade lesions—no attempt was made to distinguish presumptive grade III (AA) from grade IV (GBM) tumors
Of our the entire series of 15 BSG and 12 NSBG samples, 7 of 15 BSG and 12 of 12 NBSG cases were positive for EphA2, 10 of 15 BSG and 7 of 9 evaluated NBSG cases exhibited positive staining for IL-13Rα2, and 8 of 15 BSG and 8 of 12 NBSG cases were positive for Survivin. There were three cases that were not evaluated for IL-13Rα2 due to the limited number of available slides. Overall, 13 of 15 BSG cases and all 12 NBSG cases expressed at least one of GAAs; and 7 of 15 BSG and 9 of 12 NBSG expressed at least two of these GAAs. Table 2 summarizes expression of the three GAAs, subdivided by tumor location and histological grade.
Table 2.
Glioma-associated antigen (GAA) expression in brain stem (BS) and non-brain stem (NBS) pediatric gliomas Diagnosis No. of patients EphA2
| Diagnosis | No. of patients | EphA2 −/1+/2+ | IL-13Rα2 −/+/++ | Survivin −/1+/2+ |
|---|---|---|---|---|
| BS LGG | 5 | 2/1/2 | 0/4/1 | 2/2/1 |
| BS HGG | 10 | 6/3/1 | 5/4/1 | 5/2/3 |
| NBS LGA | 4 | 0/1/3 | 0/3/1 | 1/2/1 |
| NBS AA | 4 | 0/1/3 | 1/1/0a | 1/3/0 |
| NBS GBM | 4 | 0/2/2 | 1/2/0a | 2/2/0 |
BS, brain stem; NBS, non-brain stem; LGG, low grade glioma; HGG, high grade glioma; LGA, low grade astrocytoma; AA, anaplastic astrocytoma, GBM; glioblastoma multiforme
There were one GBM and two AA cases that were not evaluated for IL-13Rα2 due to the limited number of slides
Our current series does not demonstrate any clear association between the tumor grade and levels of GAA expression.
Discussion
To our knowledge, this is the first report evaluating expression of three GAAs: EphA2, IL-13Rα2 and Survivin, in pediatric gliomas, including brain stem gliomas. We chose to evaluate these three antigens because they are expressed in adult gliomas and are known to contain CTL epitopes, especially in the context of HLA-A2 [18, 20, 23]. Our results suggest that EphA2, IL-13α2 and Survivin are suitable targets for developing vaccine strategies for pediatric glioma.
EphA2, which was expressed in 7 of 15 BSG and all 12 NBSG cases in the current study, is a tyrosine kinase receptor that plays a role in carcinogenesis [27, 28]. In normal cells, EphA2 localizes to sites of cell-to-cell contact [29, 30], where it may negatively regulate cell growth. EphA2 is frequently overexpressed and often functionally dysregulated in advanced cancers, contributing to their malignant phenotype [31]. We have previously reported that EphA2883–891 can elicit an HLA-A2-restricted CTL response against glioma cell lines [20]. In addition, recent studies from us [20] and others [32] have revealed that a majority of adult malignant gliomas express high levels of EphA2. More recently, EphA2 mRNA overexpression was found to correlate inversely with survival in a panel of 21 adult GBM cases, and ligand-mediated EphA2 receptor activation increased GBM cell proliferation via a mitogen-activated protein kinase-dependent pathway [33]. These findings suggest that targeting of EphA2 by immunotherapy may have efficacy in controlling tumor growth.
In the current study, more than a half (8 of 15) BSGs were negative for EphA2, whereas all 12 NBSG cases were positive. Although this may represent biological differences between these tumor types, it is important to emphasize that the BSG tissues evaluated in the current study were much older than the NBSGs, in some cases from specimens obtained in 1950s and 1960s, or obtained by autopsy. Thus, even though the BSG tissues generally demonstrated strong immunoreactivity for GFAP, it is possible that the antigenicity of these specimens for less robust targets may not have been as well preserved as NBSG tissues.
IL-13Rα2 is a membrane glycoprotein that mediates activation of the TGF-β1 promoter upon stimulation by IL-13 (or IL-4) and tumor necrosis factor (TNF)-α [34]. IL-13Rα2 has attracted significant attention as a target for glioma therapy [35] because this receptor is overexpressed by more than 80% of adult malignant gliomas but is not expressed at detectable levels in normal brain tissues or at high levels in other normal organs except the testis [36]. We recently found that an analogue peptide of natural IL-13Rα2345–353 [19], in which the first and ninth amino-acid residues, tryptophan and isoleucine, were replaced by valine and alanine, respectively, could elicit a greater CTL response against HLA-A2+, IL-13Rα2+ glioma cells compared to the natural peptide (IL-13Rα2345–353:1A9V) [37]. Kawakami et al. have previously observed with a total 58 pediatric brain tumor cases that one hundred percent (11 of 11) high-grade astrocytoma, 79% (26 of 33) low-grade astrocytoma, 67% (4 of 6) medulloblastoma, and 67% (2 of 3) ependymoma samples expressed IL-13Rα2 [38]. Taken together with our current study, in which 10 of 15 BSGs and 7 of 9 NBSGs demonstrated immunoreactivity for IL-13Rα2, it seems promising to target IL-13Rα2 in future vaccine trials for pediatric brain tumor patients.
The apoptosis inhibitor protein Survivin also appeared to be expressed at high levels in a majority of cases evaluated in the current study. Survivin is overexpressed in most human cancers, and inhibition of its function results in increased apoptosis [39]. Induction of cytotoxic immunity against Survivin may, therefore, be an attractive strategy [40]. Survivin has multiple T cell epitopes, including Survivin 96–104 as an HLA-A2-restricted CTL epitope [22, 41]. Moreover, vaccination of a pancreatic cancer patient with a modified Survivin epitope peptide, Survivin96–104:2M in which the second residue threonine was replaced by methionine, induced complete remission of a liver metastasis [42]. Further, vaccinations using dendritic cells loaded with Survivin96 –104 induced positive interferon (IFN)-γ ELISPOT responses in four of five patients with advanced melanoma [21]. A recent immunohistochemical study demonstrated that 100% of adult astrocytoma specimens (n = 29; grades II–IV), but not normal brain tissues, contained Survivin-positive cells [23]. The mean percentage of immunoreactive cells in each specimen was 70.0 in grade II, 81.3 in grade III, and 85.0 in grade IV. Interestingly, high level expression of Survivin was correlated with poor prognosis in patients with grade II or III astrocytomas [23]. Even though we have not been able to demonstrate a correlation between Survivin expression levels and survival of pediatric patients in the current study, these expression data support the inclusion of Survivin in vaccine strategies for pediatric patients with glioma.
In conclusion, this study demonstrates the frequent expression of three therapeutically exploitable GAAs (EphA2, IL-13Rα2 and Survivin) in archival cohorts of BSGs and hemispheric NBSGs of childhood. All but two of 15 BSG cases and all 12 NBSG cases evaluated in the current study expressed at least one of these antigens. In addition, although many cases in the current study demonstrated diffuse expression of GAAs throughout specimens, partial or patchy expression was also noted in a small number of cases, suggesting the need for targeting multiple GAAs in immunotherapy.
Given the availability of CTL epitopes for these antigens, development of novel vaccine trials targeting these GAAs for pediatric gliomas is warranted.
Acknowledgments
This work was supported by P01 NS40923 [H.O and IFP], P01 CA100327 [H.O.], the Doris Duke Charitable Foundation and James S. McDonnell Foundation [H.O.].
References
- 1.Pollack IF. Brain tumors in children. N Engl J Med. 1994;331:1500–1507. doi: 10.1056/NEJM199412013312207. [DOI] [PubMed] [Google Scholar]
- 2.Jennings MT, Freeman ML, Murray MJ. Strategies in the treatment of diffuse pontine gliomas: the therapeutic role of hyperfractionated radiotherapy and chemotherapy. J Neurooncol. 1996;28:207–222. doi: 10.1007/BF00250200. [DOI] [PubMed] [Google Scholar]
- 3.Albright AL, Packer RJ, Zimmerman R, Rorke LB, Boyett J, Hammond GD. Magnetic resonance scans should replace biopsies for the diagnosis of diffuse brain stem gliomas: a report from the Children’s Cancer Group. Neurosurgery. 1993;33:1026–1029. doi: 10.1227/00006123-199312000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Berger MS, Edwards MS, LaMasters D, Davis RL, Wilson CB. Pediatric brain stem tumors: radiographic, pathological, and clinical correlations. Neurosurgery. 1983;12:298–302. doi: 10.1227/00006123-198303000-00008. [DOI] [PubMed] [Google Scholar]
- 5.Mantravadi RV, Phatak R, Bellur S, Liebner EJ, Haas R. Brain stem gliomas: an autopsy study of 25 cases. Cancer. 1982;49:1294– 1296. doi: 10.1002/1097-0142(19820315)49:6<1294::aid-cncr2820490636>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 6.Epstein F, Wisoff JH. Intrinsic brainstem tumors in childhood: surgical indications. J Neurooncol. 1988;6:309–317. doi: 10.1007/BF00177425. [DOI] [PubMed] [Google Scholar]
- 7.Cartmill M, Punt J. Diffuse brain stem glioma. A review of stereotactic biopsies. Childs Nerv Syst. 1999;15:235–237. doi: 10.1007/s003810050379. [DOI] [PubMed] [Google Scholar]
- 8.Packer RJ, Boyett JM, Zimmerman RA, Rorke LB, Kaplan AM, Albright AL, Selch MT, Finlay JL, Hammond GD, Wara WM. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer. 1993;72:1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Okada H, Lieberman FS, Edington HD, Witham TF, Wargo MJ, Cai Q, Elder EH, Whiteside TL, Schold SC, Jr, Pollack IF. Autologous glioma cell vaccine admixed with interleukin-4 gene transfected fibroblasts in the treatment of recurrent glioblastoma: preliminary observations in a patient with a favorable response to therapy. J Neurooncol. 2003;64:13–20. doi: 10.1007/BF02700016. [DOI] [PubMed] [Google Scholar]
- 10.Okada H, Attanucci J, Giezeman-Smits KM, Brissette-Storkus SC, Fellows KW, Pollack IF, Pogue-Geile K, Lotze MT, Bozik ME, Chambers WH. An antigen identified by cytokine tumor vaccine-assisted SEREX (CAS) suppressed growth of a rat 9L glioma in vivo. Cancer Res. 2001;61:2625–2631. [PubMed] [Google Scholar]
- 11.Okada H, Villa LA, Attanucci J, Erff M, Fellows WK, Lotze MT, Pollack IF, Chambers WH. Cytokine gene therapy of gliomas: effective induction of therapeutic immunity to intracranial tumors by peripheral immunization with interleukin-4 transduced glioma cells. Gene Ther. 2001;8:1157–1166. doi: 10.1038/sj.gt.3301496. [DOI] [PubMed] [Google Scholar]
- 12.Okada H, Pollack IF, Lieberman F, Lunsford LD, Kondziolka D, Schiff D, Attanucci J, Edington H, Chambers W, Kalinski P, et al. Gene therapy of malignant gliomas: a pilot study of vaccination with irradiated autologous glioma and dendritic cells admixed with IL-4 transduced fibroblasts to elicit an immune response. Hum Gene Ther. 2001;12:575–595. doi: 10.1089/104303401300042528. [DOI] [PubMed] [Google Scholar]
- 13.Giezeman-Smits KM, Okada H, Brissette-Storkus SC, Villa LA, Attanucci J, Lotze MT, Pollack IF, Bozik ME, Chambers WH. Cytokine gene therapy of gliomas: induction of reactive CD4+ T cells by interleukin-4 transfected 9L gliosarcoma is essential for protective immunity. Cancer Res. 2000;60:2449–2457. [PubMed] [Google Scholar]
- 14.Okada H, Giezeman-Smits KM, Tahara H, Attanucci J, Fellows WK, Lotze MT, Chambers WH, Bozik ME. Effective cytokine gene therapy against an intracranial glioma using a retrovirally transduced IL-4 plus HSV-TK tumor vaccine. Gene Ther. 1999;6:219–226. doi: 10.1038/sj.gt.3300798. [DOI] [PubMed] [Google Scholar]
- 15.Okada H, Tahara H, Shurin MR, Attanucci J, Giezeman-Smits KM, Fellows KW, Lotze MT, Chambers WH, Bozik ME. Bone marrow derived dendritic cells pulsed with a tumor specific peptide elicit effective anti-tumor immunity against intracranial neoplasms. Int J Cancer. 1998;78:196–201. doi: 10.1002/(sici)1097-0215(19981005)78:2<196::aid-ijc13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Yu JS, Liu G, Ying H, Yong WH, Black KL, Wheeler CJ. Vaccination with tumor lysate-pulsed dendritic cells elicits antigen- specific, cytotoxic T-cells in patients with malignant glioma. Cancer Res. 2004;64:4973–4979. doi: 10.1158/0008-5472.CAN-03-3505. [DOI] [PubMed] [Google Scholar]
- 17.Liau LM, Prins RM, Kiertscher SM, Odesa SK, Kremen TJ, Giovannone AJ, Lin JW, Chute DJ, Mischel PS, Cloughesy TF, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11:5515–5525. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 18.Eguchi J, Hatano M, Nishimura F, Zhu X, Dusak JE, Sato H, Pollack IF, Storkus WJ, Okada H. Identification of interleukin- 13 receptor alpha2 peptide analogues capable of inducing improved antiglioma CTL responses. Cancer Res. 2006;66:5883–5891. doi: 10.1158/0008-5472.CAN-06-0363. [DOI] [PubMed] [Google Scholar]
- 19.Okano F, Storkus WJ, Chambers WH, Pollack IF, Okada H. Identification of a novel HLA-A*0201 restricted cytotoxic T lymphocyte epitope in a human glioma associated antigen, interleukin-13 receptor 2 chain. Clin Cancer Res. 2002;8:2851–2855. [PubMed] [Google Scholar]
- 20.Hatano M, Eguchi J, Tatsumi T, Kuwashima N, Dusak JE, Kinch MS, Pollack IF, Hamilton RL, Storkus WJ, Okada H. EphA2 as a glioma-associated antigen: a novel target for glioma vaccines. Neoplasia. 2005;7:717–722. doi: 10.1593/neo.05277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Otto K, Andersen MH, Eggert A, Keikavoussi P, Pedersen LO, Rath JC, Bock M, Brocker EB, Straten PT, Kampgen E, et al. Lack of toxicity of therapy-induced T cell responses against the universal tumour antigen survivin. Vaccine. 2005;23:884–889. doi: 10.1016/j.vaccine.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 22.Andersen MH, Pedersen LO, Becker JC, Straten PT. Identification of a cytotoxic T lymphocyte response to the apoptosis inhibitor protein survivin in cancer patients. Cancer Res. 2001;61:869–872. [PubMed] [Google Scholar]
- 23.Uematsu M, Ohsawa I, Aokage T, Nishimaki K, Matsumoto K, Takahashi H, Asoh S, Teramoto A, Ohta S. Prognostic significance of the immunohistochemical index of survivin in glioma: a comparative study with the MIB-1 index. J Neurooncol. 2005;72:231–238. doi: 10.1007/s11060-004-2353-3. [DOI] [PubMed] [Google Scholar]
- 24.Jager E, Ringhoffer M, Karbach J, Arand M, Oesch F, Knuth A. Inverse relationship of melanocyte differentiation antigen expression in melanoma tissues and CD8+ cytotoxic-T-cell responses: evidence for immunoselection of antigen-loss variants in vivo. Int J Cancer. 1996;66:470–476. doi: 10.1002/(SICI)1097-0215(19960516)66:4<470::AID-IJC10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 25.Hatano M, Kuwashima N, Tatsumi T, Dusak JE, Nishimura F, Reilly KM, Storkus WJ, Okada H. Vaccination with EphA2- derived T cell-epitopes promotes immunity against both EphA2- expressing and EphA2-negative tumors. J Transl Med. 2004;2:40. doi: 10.1186/1479-5876-2-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brantley DM, Cheng N, Thompson EJ, Lin Q, Brekken RA, Thorpe PE, Muraoka RS, Cerretti DP, Pozzi A, Jackson D, et al. Soluble Eph A receptors inhibit tumor angiogenesis and progression in vivo. Oncogene. 2002;21:7011–7026. doi: 10.1038/sj.onc.1205679. [DOI] [PubMed] [Google Scholar]
- 27.Oba SM, Wang YJ, Song JP, Li ZY, Kobayashi K, Tsugane S, Hamada GS, Tanaka M, Sugimura H. Genomic structure and loss of heterozygosity of EPHB2 in colorectal cancer. Cancer Lett. 2001;164:97–104. doi: 10.1016/s0304-3835(00)00716-3. [DOI] [PubMed] [Google Scholar]
- 28.Dodelet VC, Pasquale EB. Eph receptors and ephrin ligands: embryogenesis to tumorigenesis. Oncogene. 2000;19:5614– 5619. doi: 10.1038/sj.onc.1203856. [DOI] [PubMed] [Google Scholar]
- 29.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 30.Miao H, Wei BR, Peehl DM, Li Q, Alexandrou T, Schelling JR, Rhim JS, Sedor JR, Burnett E, Wang B. Activation of EphA receptor tyrosine kinase inhibits the Ras/MAPK pathway. Nat Cell Biol. 2001;3:527–530. doi: 10.1038/35074604. [DOI] [PubMed] [Google Scholar]
- 31.Kinch MS, Moore MB, Harpole DH., Jr Predictive value of the EphA2 receptor tyrosine kinase in lung cancer recurrence and survival. Clin Cancer Res. 2003;9:613–618. [PubMed] [Google Scholar]
- 32.Wykosky J, Gibo DM, Stanton C, Debinski W. EphA2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3:541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Park PJ, Lai W, Maher E, Chakravarti A, Durso L, Jiang X, Yu Y, Brosius A, Thomas M, et al. A genome-wide screen reveals functional gene clusters in the cancer genome and identifies EphA2 as a mitogen in glioblastoma. Cancer Res. 2006;66:10815– 10823. doi: 10.1158/0008-5472.CAN-06-1408. [DOI] [PubMed] [Google Scholar]
- 34.Fichtner-Feigl S, Strober W, Kawakami K, Puri RK, Kitani A. IL-13 signaling through the IL-13alpha2 receptor is involved in induction of TGF-beta1 production and fibrosis. Nat Med. 2006;12:99–106. doi: 10.1038/nm1332. [DOI] [PubMed] [Google Scholar]
- 35.Kahlon KS, Brown C, Cooper LJ, Raubitschek A, Forman SJ, Jensen MC. Specific recognition and killing of glioblastoma multiforme by interleukin 13-zetakine redirected cytolytic T cells. Cancer Res. 2004;64:9160–9166. doi: 10.1158/0008-5472.CAN-04-0454. [DOI] [PubMed] [Google Scholar]
- 36.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–449. [PMC free article] [PubMed] [Google Scholar]
- 37.Eguchi J, Hatano M, Nishimura F, Zhu X, Dusak JE, Sato H, Pollack IF, Storkus WJ, Okada H. Identification of interleukin- 13 receptor 2 peptide analogues capable of inducing improved anti-glioma CTL responses. Cancer Res. 2006;66:5883–5891. doi: 10.1158/0008-5472.CAN-06-0363. [DOI] [PubMed] [Google Scholar]
- 38.Kawakami M, Kawakami K, Takahashi S, Abe M, Puri RK. Analysis of interleukin-13 receptor alpha2 expression in human pediatric brain tumors. Cancer. 2004;101:1036–1042. doi: 10.1002/cncr.20470. [DOI] [PubMed] [Google Scholar]
- 39.Blanc-Brude OP, Yu J, Simosa H, Conte MS, Sessa WC, Altieri DC. Inhibitor of apoptosis protein survivin regulates vascular injury. Nat Med. 2002;8:987–994. doi: 10.1038/nm750. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt SM, Schag K, Muller MR, Weck MM, Appel S, Kanz L, Grunebach F, Brossart P. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 41.Andersen MH, Pedersen LO, Capeller B, Brocker EB, Becker JC, Thor SP. Spontaneous cytotoxic T-cell responses against survivin-derived MHC class I-restricted T-cell epitopes in situ as well as ex vivo in cancer patients. Cancer Res. 2001;61:5964–5968. [PubMed] [Google Scholar]
- 42.Wobser M, Keikavoussi P, Kunzmann V, Weininger M, Andersen MH, Becker JC. Complete remission of liver metastasis of pancreatic cancer under vaccination with a HLA-A2 restricted peptide derived from the universal tumor antigen survivin. Cancer Immunol Immunother. 2005;55:1294–1298. doi: 10.1007/s00262-005-0102-x. [DOI] [PMC free article] [PubMed] [Google Scholar]