Abstract
The MAL proteolipid is a nonglycosylated integral membrane protein found in glycolipid-enriched membrane microdomains. In polarized epithelial Madin-Darby canine kidney cells, MAL is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin. MAL is thus part of the integral machinery for glycolipid-enriched membrane–mediated apical transport. At steady state, MAL is predominantly located in perinuclear vesicles that probably arise from the trans-Golgi network (TGN). To act on membrane traffic and to prevent their accumulation in the target compartment, integral membrane elements of the protein-sorting machinery should be itinerant proteins that cycle between the donor and target compartments. To establish whether MAL is an itinerant protein, we engineered the last extracellular loop of MAL by insertion of sequences containing the FLAG epitope or with sequences containing residues that became O-glycosylated within the cells or that displayed biotinylatable groups. The ectopic expression of these modified MAL proteins allowed us to investigate the surface expression of MAL and its movement through different compartments after internalization with the use of a combination of assays, including surface biotinylation, surface binding of anti-FLAG antibodies, neuraminidase sensitivity, and drug treatments. Immunofluorescence and flow cytometric analyses indicated that, in addition to its Golgi localization, MAL was also expressed on the cell surface, from which it was rapidly internalized. This retrieval implies transport through the endosomal pathway and requires endosomal acidification, because it can be inhibited by drugs such as chloroquine, monensin, and NH4Cl. Resialylation experiments of surface MAL treated with neuraminidase indicated that ∼30% of the internalized MAL molecules were delivered to the TGN, probably to start a new cycle of cargo transport. Together, these observations suggest that, as predicted for integral membrane members of the late protein transport machinery, MAL is an itinerant protein cycling between the TGN and the plasma membrane.
INTRODUCTION
Asymmetric organization of the plasma membrane is fundamental for most cells and is characterized by the cell’s ability to create and maintain functionally, morphologically, and biochemically distinct plasma membrane subdomains (Drubin and Nelson, 1996). Polarized epithelial cells displaying apical and basolateral surfaces represent a paradigmatic system in which to investigate the mechanism of generation of different plasma membrane subdomains (Matter and Mellman, 1994). In epithelial Madin-Darby canine kidney (MDCK) cells (Rodriguez-Boulan and Powell, 1992), segregation of newly synthesized proteins destined for the apical or basolateral surfaces takes place in the trans-Golgi network (TGN) (Traub and Kornfeld, 1997). Whereas basolateral sorting takes place by the recognition of peptide motifs that may or may not be related to tyrosine- or dileucine-based sorting determinants (Matter and Mellman, 1994), apical transport appears to occur by a completely different mechanism. The apical membrane of MDCK cells is highly enriched in glycosylphosphatidylinositol (GPI)-anchored proteins (Lisanti et al., 1988). In these cells, the influenza virus hemagglutinin (HA) is also specifically targeted to the apical surface (Roth et al., 1979; Rodriguez-Boulan and Pendergast, 1980). The GPI anchor in the case of GPI-anchored proteins (Brown et al., 1989; Lisanti et al., 1989), and specific residues in the transmembrane segment in the case of HA (Scheiffele et al., 1997; Lin et al., 1998), are required for targeting to the apical membrane. For these two types of protein, apical transport appears to be mediated by a novel pathway mediated by cholesterol- and glycolipid-enriched membrane (GEM) microdomains or rafts (Simons and Wandinger-Ness, 1990). In accordance with this model, the inclusion of cargo proteins into GEMs at the TGN determines their delivery to the apical surface. Although partitioning into GEMs is necessary for apical sorting, protein-sorting machinery may help in the recruitment of cargo proteins and should also be required to guarantee the processes of vesiculation, targeting, and fusion to the apical membrane (Simons and Wandinger-Ness, 1990).
The MAL gene was first identified during a search for genes that are differentially expressed during T-cell ontogeny (Alonso and Weissman, 1987). More recently, MAL gene expression was detected in polarized epithelial cells (Zacchetti et al., 1995; Millán et al., 1997a; Martín-Belmonte et al., 1998) and myelin-forming cells (Kim et al., 1995; Schaeren-Wiemers et al., 1995). The generation of mAbs to human, rat, and canine MAL proteins allowed us to identify MAL as an exclusive resident of GEMs in human T-lymphocytes (Millán and Alonso, 1998), polarized epithelial Fischer rat thyroid (FRT) cell (Martín-Belmonte et al., 1998), and canine MDCK cells (Puertollano et al., 1999). Specific amino acids at the carboxyl terminus are required for incorporation of MAL in GEMs (Puertollano and Alonso, 1998). MAL appears mainly distributed in TGN-derived vesicular structures and to a lesser extent in early endosomes and the plasma membrane (Millán et al., 1997b). The observation that MAL expression leads to massive de novo formation of vesicles in heterologous insect cells led to MAL being proposed as an element of the apical sorting machinery for GEM vesiculation (Puertollano et al., 1997). We have recently demonstrated that the in vivo depletion of endogenous MAL impairs apical transport and produces basolateral missorting of HA in MDCK cells, thus providing evidence in support of this hypothesis. Moreover, these effects are corrected by ectopic expression of MAL in cells in which the endogenous protein was depleted (Puertollano et al., 1999). This finding indicates that MAL is necessary for normal apical transport and accurate sorting of HA in MDCK cells. The accomplishment of this stringent requirement highlights the importance of MAL as a component of the apical sorting machinery.
Protein traffic between intracellular compartments involves the accurate selection of cargo proteins at specific sites and their inclusion in the appropriate vesicle. Part of the sorting machinery includes soluble proteins that are normally dispersed throughout the cytosol. When required, these proteins are recruited into specific sites and, once they have performed their specific task in the transport process, are then released into the cytosol for recycling (Farquhar and Hauri, 1997). One of the most remarkable characteristics of integral membrane protein components of the transport machinery is that they have to cycle continuously between different compartments to function adequately without accumulating in the target compartment. This is the case for the mannose-6-phosphate receptors, which recognize residues of mannose-6-phosphate in soluble proteins that are destined for lysosomes (Kornfeld and Mellman, 1989; Riederer et al., 1994). In the early secretory pathway, the KDEL receptor, which is responsible for the continuous retrieval of endoplasmic reticulum–soluble proteins (Townsley et al., 1993), and the putative cargo receptors of the p24 family proteins (Fiedler et al., 1996; Blum et al., 1999) and p53 (ERGIC-53) (Kappeler et al., 1997) move continuously between the endoplasmic reticulum and the Golgi apparatus. TGN38, a putative element of the apparatus involved in the late stages of the secretory pathway, is an example of an itinerant protein that cycles between the TGN and the plasma membrane (Bosshart et al., 1994; Ghosh et al., 1998).
The involvement of MAL in apical transport (Puertollano et al., 1999) has prompted us to investigate whether MAL does indeed behave as an itinerant protein. To follow its movement to and from the plasma membrane, we have engineered the last extracellular loop of MAL with a FLAG epitope (MAL-FLAG) or with sequences that become O-glycosylated within the cells (MAL-T). These modifications do not affect the predominant steady-state Golgi localization of MAL. Internalization of MAL was monitored by endocytosis of anti-FLAG mAb bound to MAL/FLAG or traced by neuraminidase treatment of surface MAL-T. These assays revealed that 1) MAL expression is detected on the cell surface, 2) surface MAL is rapidly endocytosed, 3) some of the internalized MAL molecules are recycled to the TGN, and 4) TGN delivery of surface MAL requires endosomal acidification. These findings suggest that MAL is an itinerant component of the sorting machinery that continuously cycles between the plasma membrane and the TGN.
MATERIALS AND METHODS
Materials
The mouse hybridoma that produces mAb to the 9E10 c-Myc epitope (EQKLISEED) (Evan et al., 1985) was purchased from the American Type Culture Collection (Rockville, MD). Rabbit polyclonal antibodies to transferrin were obtained from Dako A/S (Glostrup, Denmark). Antiserum 4, which recognizes the endogenous TGN38 protein of COS-7 cells (Wilde et al., 1992), was a generous gift from Dr. G. Banting (University of Bristol, Bristol, United Kingdom). Peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG) antibodies, peroxidase-coupled streptavidin, and sulfo-N-hydroxyl-succinimido-biotin (sulfo-NHS-biotin) were from Pierce (Rockford, IL). Fluorescein- and Texas Red–conjugated secondary antibodies were from Southern Biotech (Birmingham, AL). Anti-FLAG M2 antibodies, Triton X-100, octyl-glucoside, neuraminidase, nocodazole, brefeldin A (BFA), chloroquine, monensin, NH4Cl, and iron-saturated transferrin were obtained from Sigma (St. Louis, MO).
Cell Culture Conditions, Transfections, and DNA Constructs
Epithelial MDCK cells and COS-7 cells were grown on Petri dishes or glass coverslips in DMEM supplemented with 10% FBS (Life Technologies-BRL, Gaithersburg, MD), penicillin (50 U/ml), and streptomycin (50 μg/ml) at 37°C in an atmosphere of 5% CO2/95% air. Transfections were done by electroporation with the ECM 600 electroporation instrument (BTX, San Diego, CA). Selection of stable MDCK cell transfectants was carried out by treatment with 0.5 mg/ml G418 sulfate (Life Technologies-BRL) for 3 wk after transfection. Drug-resistant clones were picked up with cloning rings, and individual clones were screened for expression of MAL-FLAG by immunofluorescence analysis.
The MAL-FLAG construct, encoding MAL modified at its last extracellular loop by insertion of the sequence DYKDDDDK, which contains the FLAG epitope (DYKD), was generated by PCR with the use of the overlap extension technique (Ho et al., 1989) and appropriate oligonucleotide primers. We have previously described the generation of an N-glycosylated MAL protein, named MAL-Z, by insertion at the last extracellular loop of MAL of the 27-amino acid sequence (DYKDDDDKGNLSANITPYPYDVPDYAS) containing two tandem consensus N-glycosylation sites (NLS and NIT) flanked by the FLAG and HA (YPYDVPYAS) epitopes and additional amino acids used as spacers (Puertollano and Alonso, 1999). MAL-T was generated by substituting the asparagine residue within the two N-glycosylation consensus sites that are present in MAL-Z with threonine with the use of the overlap extension technique (Ho et al., 1989) and appropriate oligonucleotide primers. After cloning of the modified MAL cDNAs into the pCR3 eukaryotic expression vector (Invitrogen, Carlsbad, CA), the sequence of the inserted DNA was verified to check for possible amplification errors.
Detergent Extraction Procedures
GEMs were isolated by standard procedures (Brown and Rose, 1992). Cells grown to confluence in 100-mm dishes were rinsed with PBS and lysed for 20 min in 1 ml of 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100 at 4°C. The lysate was scraped from the dishes with a rubber policeman, the dishes were rinsed with 1 ml of the same buffer at 4°C, and the lysate was homogenized by passing the sample through a 22-gauge needle. The extract was finally brought to 40% sucrose in a final volume of 4 ml and placed at the bottom of an 8-ml 5–30% linear sucrose gradient. Gradients were centrifuged for 18 h at 39,000 rpm at 4°C in a Beckman (Fullerton, CA) SW41 rotor. Fractions of 1 ml were harvested from the bottom of the tube, and aliquots were subjected to immunoblot analysis.
Surface Labeling
After repeated washings with ice-cold PBS containing 0.1 mM CaCl2 and 1 mM MgCl2, cells were incubated with 0.5 mg/ml sulfo-NHS-biotin. After 30 min at 4°C, the solution was removed and the remaining unreacted biotin was quenched by incubation with ice-cold serum-free culture medium. Cell monolayers were washed with PBS and extracted with 0.5 ml of 25 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 60 mM octyl-glucoside for 30 min on ice. Extracts were then immunoprecipitated with mAb 9E10, and the immunoprecipitates were, or were not, digested with neuraminidase (0.05 U/ml) for 1 h at 4°C. Samples were finally fractionated by SDS-PAGE and analyzed with peroxidase-coupled streptavidin to trace the biotin groups incorporated during the surface-labeling procedure. Quantitative analyses were carried out with a computing densitometer.
Immunoblot and Immunoprecipitation Analyses
For immunoblot analysis, samples were subjected to SDS-PAGE in 15% acrylamide gels under reducing conditions and transferred to Immobilon-P membranes (Millipore, Bedford, MA). After blocking with 5% (wt/vol) nonfat dry milk, 0.05% (vol/vol) Tween-20 in PBS, blots were incubated with the indicated primary antibody. After several washings, blots were incubated for 1 h with goat anti-mouse IgG antibodies coupled to HRP, washed extensively, and developed using an ECL Western blotting kit (Amersham, Arlington Heights, IL).
For use in immunoprecipitation studies, antibodies were prebound overnight at 4°C to protein G–Sepharose in 10 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 1% Triton X-100. Postnuclear supernatants prepared with 1% Triton X-100 plus 60 mM octyl-glucoside were incubated for 4 h at 4°C with a control anti-CD4 mAb bound to protein G–Sepharose. After centrifugation, the supernatant was immunoprecipitated by incubation for 4 h at 4°C with mAb 9E10 bound to protein G–Sepharose. The immunoprecipitates were collected, washed six times with 1 ml of 10 mM Tris-HCl, pH 8.0, 0.15 M NaCl, 1% Triton X-100, and analyzed by SDS-PAGE under reducing conditions.
Endocytosis Assays by Immunofluorescence Analysis or Neuraminidase Treatment
To detect the presence of MAL-FLAG on the cell surface or to monitor its internalization by immunofluorescence analysis, intact cells were washed several times with ice-cold PBS and incubated for 1 h at 4°C with anti-FLAG antibodies. After extensive washing with PBS to remove excess antibody, cells were incubated in normal medium at either 4 or 37°C to prevent or allow endocytosis, respectively. The cells were then fixed and permeabilized (surface plus internalized MAL-FLAG) or not (surface MAL-FLAG) with 0.2% Triton X-100 before the addition of the secondary fluorescent antibody. Samples were processed as described under Immunofluorescence Microscopy.
To monitor MAL-T internalization with the use of neuraminidase digestion, the surfaces of transfected cells were first subjected to biotinylation and then the cells were incubated at either 4 or 37°C for the indicated times to prevent or allow internalization, respectively. After washing extensively with PBS at 4°C, intact cells were rinsed twice with 25 mM 2-(N-morpholino)ethanesulfonic acid, pH 5.5, 2 mM CaCl2, 140 mM NaCl and incubated with 0.05 U/ml neuraminidase at 4°C for 1 h. After lysis, the extracts were subjected to immunoprecipitation with mAb 9E10, and the immunoprecipitates were subjected to immunoblot analysis with streptavidin conjugated to peroxidase and developed using the ECL Western blotting kit.
Flow Cytometric Internalization Assay
MDCK cells stably expressing MAL-FLAG were incubated for 60 min in the presence of anti-FLAG M2 mAb at 4°C, washed, and incubated for 15 min at either 4 or 37°C. Cells were then fixed with 3% paraformaldehyde and incubated with a fluorescein-conjugated secondary antibody to label the FLAG antibodies bound to MAL-FLAG that remained at the surface. Finally, samples were subjected to flow cytometric analysis using the Becton-Dickinson (Mountain View, CA) FACScan cytofluorimeter. To calculate the extent of internalization, the mean fluorescence intensity of each sample was calculated. The value obtained with background staining (anti-CD4 OKT4 mAb) was subtracted from that of each sample. The value corresponding to that of the cells incubated for 15 min at 37°C was divided by that of the cells incubated at 4°C to obtain the percentage of molecules remaining at the cell surface.
Drug Treatments
Treatments with 20 μM nocodazole or 5 μg/ml BFA were carried out in COS-7 cells incubated at 37°C for 30 min to allow internalization of anti-FLAG antibodies previously bound to surface MAL-FLAG for 1 h at 4°C. In the case of treatments with inhibitors of endosomal transport, 100 μM chloroquine, 10 μM monensin, or 50 mM NH4Cl was added to the culture medium either 30 min before or immediately before incubation at 37°C. In both cases, the drugs were maintained in the medium during the 37°C incubation period.
Immunofluorescence Microscopy
To analyze the total steady-state distribution of MAL, transfected cells grown on coverslips were washed twice with PBS, fixed in 3% paraformaldehyde for 15 min, rinsed, and treated with 10 mM glycine for 10 min to quench the aldehyde groups. The cells were then permeabilized with 0.2% Triton X-100, rinsed, and incubated with 3% BSA in PBS for 20 min. Coverslips were then incubated for 1 h with the primary antibody, rinsed several times, and incubated for 1 h with the appropriate fluorescent secondary antibody. For double-label immunofluorescence analysis, the procedure was repeated with the second primary and secondary antibodies. After extensive washing, the coverslips were mounted on slides. The cells were photographed with a Zeiss (Thornwood, NY) Axioskop photomicroscope using Kodak (Rochester, NY) T-Max 400 film. Primary antibodies included mouse 9E10 (IgG1) and anti-FLAG M2 (IgG1) mAbs and rabbit polyclonal antibodies to transferrin or to TGN38. Secondary antibodies included Texas Red–conjugated goat anti-mouse Igγ1 chain and fluorescein-conjugated anti-rabbit IgG antibodies absorbed against mouse IgG. Controls to assess the specificity and the lack of cross-labeling included incubations with control primary antibodies and omission of either of the primary antibodies.
RESULTS
Characterization of a MAL Protein Engineered by Insertion of the FLAG Epitope at Its Last Extracellular Loop
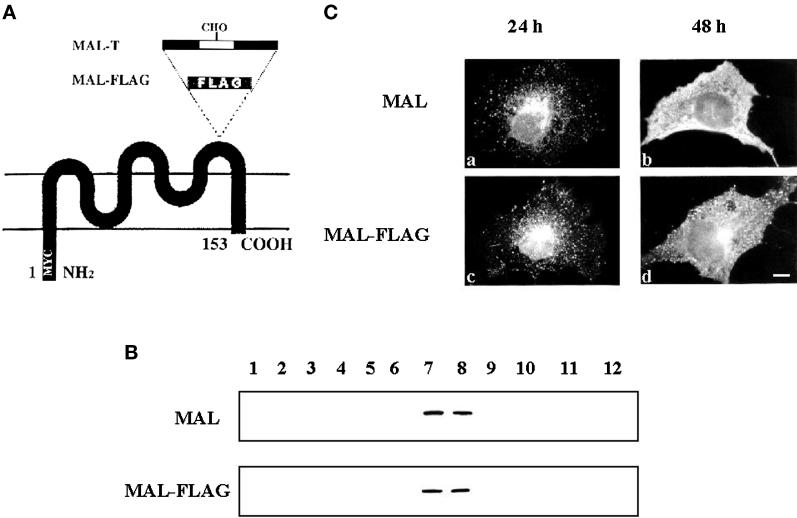
We have previously generated a construct in which the c-Myc 9E10 epitope was added to the amino terminus of MAL and shown that this tag does not interfere with MAL incorporation into GEMs (Millán et al., 1997b). However, this tagged molecule, referred to as MAL, is not of use in internalization studies because the tag is cytosolically oriented and, thus, is only accessible to mAb 9E10 after permeabilization. To detect MAL on the cell surface, we made a new construct encoding a MAL protein (MAL-FLAG) that, in addition to the amino-terminal 9E10 tag, bore a FLAG epitope in the last extracellular loop (Figure 1A). We know that the loop is extracellular because we have previously obtained N-glycosylated MAL after inserting two N-glycosylation consensus sites in the same position (Puertollano and Alonso, 1999). The presence of the FLAG epitope does not alter the incorporation of MAL into GEMs, as revealed by immunoblot analysis of the fractions obtained by centrifugation to equilibrium in sucrose density gradients of COS-7 cells transiently expressing MAL-FLAG (Figure 1B). To determine whether the presence of the FLAG tag influences MAL localization, we compared the distributions of MAL and MAL-FLAG by immunofluorescence analysis of COS-7 cells at either 24 or 48 h after transfection using mAb 9E10. Figure 1C shows that at 24 h after transfection, MAL (a) and MAL-FLAG (c) occur in perinuclear vesicular structures in accordance with their presence in TGN-derived transport vesicles. This pattern is also consistent with that obtained in A498 cells that stably expressed MAL (Millán et al., 1997b). At 48 h after transfection, the enormous overexpression typically observed in COS-7 cells produced strong staining on the plasma membrane for both MAL (b) and MAL-FLAG (d).
Figure 1.
Comparative analysis of MAL and MAL-FLAG expression in COS-7 cells. (A) Schematic diagram of the engineered MAL proteins used in this study. MAL-FLAG was constructed by adding sequences containing the FLAG epitope to the last extracellular loop of MAL. MAL-T was derived from the previously reported MAL-N construct (Puertollano and Alonso, 1999), which contains two consensus N-glycosylation sites in an extended loop, by substituting the asparagine residues within those sites with threonine. (B) To compare the incorporation of MAL and MAL-FLAG into COS-7, cells transiently expressing either MAL or MAL-FLAG were extracted with 1% Triton X-100 at 4°C and subjected to centrifugation to equilibrium in sucrose density gradients at 48 h after transfection. Aliquots from each fraction were subjected to immunoblot analysis with mAb 9E10. Fractions 1–4 are the 40% sucrose layer and contain the bulk of cellular membranes and cytosolic proteins, whereas fractions 5–12 are the 5–30% sucrose layer and contain GEMs. In parallel experiments using MDCK cells, the distribution of calnexin, an endoplasmic reticulum protein that is excluded from GEMs, and caveolin, a GEM resident protein, was used as a control of the fractionation procedure (not shown). (C) The intracellular distribution of MAL (a and b) and MAL-FLAG (c and d) was compared by immunofluorescence analysis with the 9E10 antibody in permeabilized COS-7 cells at either 24 h (a and c) or 48 h (b and d) after transfection. Bar, 10 μm.
Internalization of MAL in Transiently Transfected COS-7 Cells
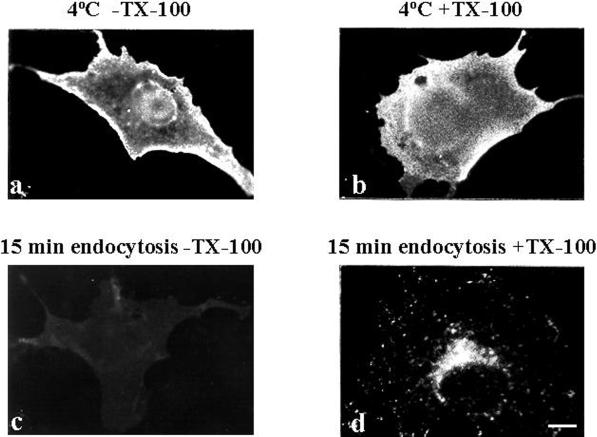
We took advantage of the strong plasma membrane expression of MAL-FLAG obtained at 48 h after transfection to investigate MAL endocytosis using the COS-7 cell system and anti-FLAG antibodies. To detect only surface MAL-FLAG, COS-7 cells were transfected with the appropriate construct and analyzed at 48 h after transfection by incubating intact cells with anti-FLAG antibodies at 4°C for 1 h before fixation, followed by incubation with a fluorescent secondary antibody (Figure 2a). In this way, surface fluorescence was not swamped by fluorescence from intracellular staining and only surface MAL-FLAG was stained. To explore the uptake of surface MAL-FLAG into the cell, intact cells were incubated with anti-FLAG antibody at 4°C for 1 h, washed to remove excess antibody, and incubated for 15 min at either 4 (b) or 37°C (c and d). Cells were then fixed, permeabilized (b and d) or not (c), and incubated with the secondary antibody to detect the antibody-bound MAL-FLAG complexes that were originally present on the cell surface. Figure 2d shows that surface MAL-FLAG was rapidly internalized, adopting a perinuclear vesicular distribution. This intracellular accumulation correlated with a loss in MAL-FLAG surface staining (Figure 2, compare a and c). This loss was prevented when the 15-min incubation at 37°C was replaced by incubation at 4°C (Figure 2, compare a and b), indicating that the effect observed in Figure 2c was due to MAL-FLAG endocytosis.
Figure 2.
Endocytosis of MAL-FLAG in COS-7 cells. COS-7 cells were transiently transfected with MAL-FLAG and, after 48 h, incubated with anti-FLAG antibody for 1 h at 4°C, and then for 15 min at either 4°C (a and b) or 37°C (c and d). Finally, the cells were fixed, permeabilized (b and d) or not (a and c) with 0.2% Triton X-100, and subjected to immunofluorescence analysis with fluorescent secondary antibodies. Bar, 10 μm.
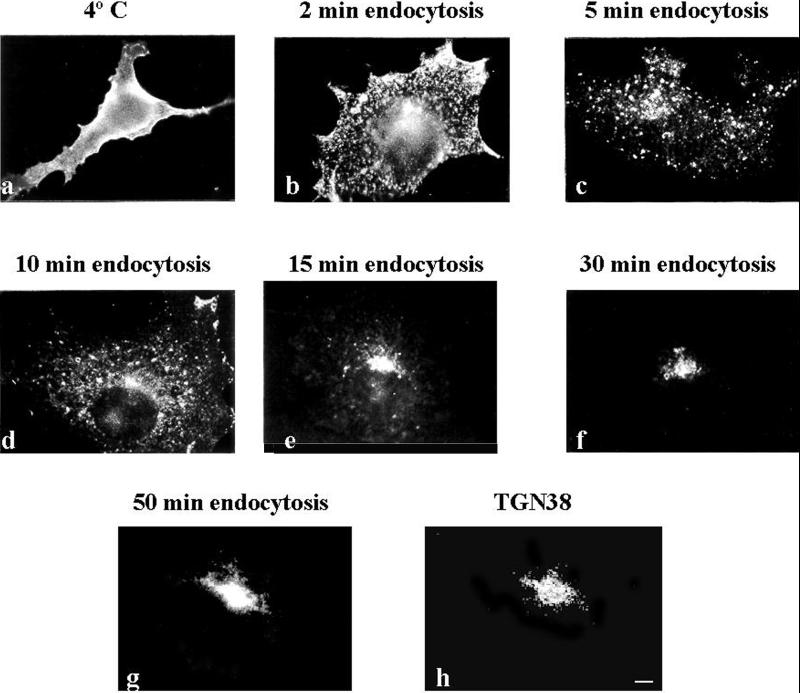
To analyze the kinetics of MAL internalization in greater detail, COS-7 cells transfected with MAL-FLAG for 48 h were incubated with anti-FLAG antibody for 1 h at 4°C and then incubated at 37°C for different times. Figure 3 shows that MAL-FLAG was clustered on the cell surface after 2 min (b), it began to be internalized 3 min later (c), and it progressively accumulated in the perinuclear region (d–g). Most of the internalized MAL-FLAG colocalized with the TGN38 marker in the Golgi region after 50 min of MAL-FLAG endocytosis, as revealed by double-label immunofluorescence analysis (g and h). The quantification of MAL endocytosis by flow cytometric analysis indicated that 10% of the protein originally on the surface had become intracellular after 5 min of incubation at 37°C. After 15 min, this value was 70%, and it exceeded 90% after 50 min (not shown).
Figure 3.
Kinetics of MAL-FLAG internalization. COS-7 cells transfected with MAL-FLAG were incubated for 1 h at 4°C with anti-FLAG mAb and then fixed (a) or incubated at 37°C for the indicated times to allow MAL-FLAG internalization (b–g). Cells were permeabilized (b–g) or not (a) with 0.2% Triton X-100 and incubated with fluorescent secondary antibodies. (g and h) Double-label immunofluorescence analysis of MAL-FLAG internalized for 50 min (g) and steady-state TGN38 (h). Bar, 10 μm.
MAL Endocytosis at Steady State
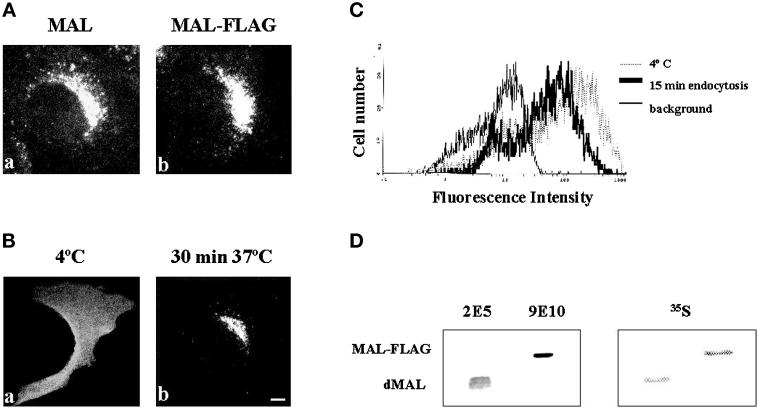
Overexpression in transiently transfected COS-7 cells greatly facilitates the analysis of MAL endocytosis. To avoid massive overexpression and to establish whether MAL endocytosis also takes place at steady state in cells with endogenous MAL expression, we generated MDCK cells that stably expresses MAL-FLAG. The insertion of the FLAG tag did not affect the predominant perinuclear vesicular distribution characteristic of MAL at steady state, as revealed by immunofluorescence analysis with mAb 9E10 (Figure 4A). To detect steady-state surface expression and internalization of MAL-FLAG, cells were incubated with anti-FLAG antibodies at 4°C for 1 h, and then for 30 min at either 4 or 37°C, to prevent or allow MAL internalization, respectively. Cells were then fixed and stained with fluorescent secondary anti-mouse IgG antibodies. Figure 4B shows that most of the MAL-FLAG originally present on the cell surface (a) was internalized at 37°C and accumulated in the perinuclear region (b). In a similar experiment, the anti-FLAG antibodies bound to MAL-FLAG remaining at the cell surface after 15 min of endocytosis at 37°C were compared by flow cytometry analysis with those existing when the incubation was done at 4°C. Quantitative analysis of the profiles shown in Figure 4C indicated that ∼40% of the MAL molecules present on the cell surface were endocytosed after 15 min of incubation at 37°C. As there are no anti-MAL antibodies available that recognize simultaneously both the canine and the human species, to estimate the level of MAL-FLAG overexpression we labeled the cells with [35S]methionine/cysteine and, after extraction of the proteolipids present in GEMs, we quantified the intensity of the signals corresponding to MAL-FLAG and endogenous MAL. This analysis indicated that MAL-FLAG expression was approximately twofold higher than that of endogenous MAL in the MDCK cell transfectant (Figure 4D). This finding excludes the possibility that the observed endocytosis of MAL-FLAG was caused by massive overexpression of this molecule.
Figure 4.
MAL internalization in stably transfected MDCK cells. (A) MDCK cells stably expressing either MAL (a) or MAL-FLAG (b) were fixed, permeabilized with 0.2% Triton X-100, and subjected to immunofluorescence analysis with mAb 9E10. (B) Intact MDCK cells stably expressing MAL-FLAG were incubated with anti-FLAG antibody for 60 min at 4°C to label surface MAL, washed, and incubated for 30 min at either 4°C, to prevent endocytosis (a), or 37°C, to allow MAL internalization (b). After fixing, cells were permeabilized with 0.2% Triton X-100 and bound antibodies were visualized with fluorescent anti-mouse IgG antibodies. Bar, 10 μm. (C) The experiment in B was repeated omitting the fixation and permeabilization steps. The anti-FLAG antibodies bound to MAL-FLAG remaining at the cell surface after incubation at 37°C for 15 min were analyzed by flow cytometry using a fluorescent secondary antibody. Irrelevant control antibody at 4°C for 75 min (thick line) and anti-FLAG antibodies at 4°C for 60 min followed by 15 min of incubation at either 4°C (thin line) or 37°C (dotted line) are shown. (D) Comparative analysis of the levels of expression of MAL-FLAG and endogenous MAL in transfected MDCK cells. MDCK cells expressing MAL-FLAG were labeled with [35S]methionine/cysteine for 16 h and then extracted with 1% Triton X-100 at 4°C. The GEM fraction was collected and solubilized with 60 mM octyl-glucoside to solubilize GEMs. The solubilized material was then extracted with n-butanol, and the organic phase containing proteolipids was dried and analyzed by SDS-PAGE. Two major proteolipid bands were observed by autoradiography with short exposures. Additional minor bands were detected with longer exposures (not shown). The major bands were identified as endogenous MAL (dMAL) and exogenous MAL-FLAG by immunoblot analysis with antibodies 2E5 and 9E10, respectively. Quantification of the radiolabeled bands indicated that MAL-FLAG expression was approximately twofold higher than that of endogenous MAL.
Treatments with Endosomal Transport Inhibitors
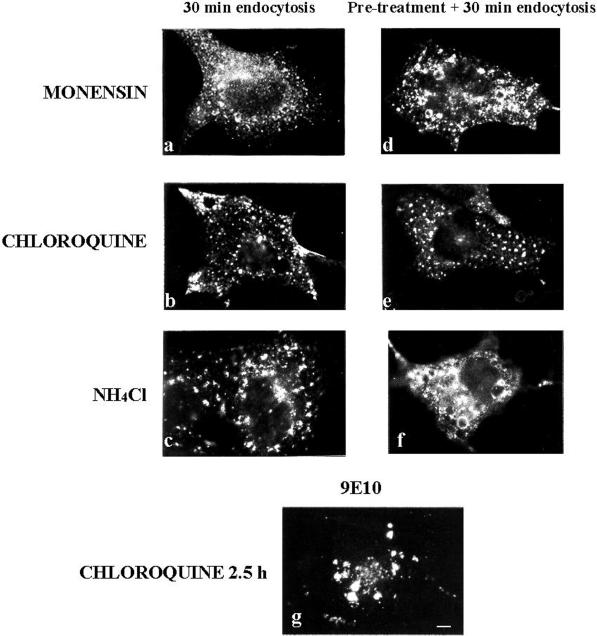
Agents that increase the lumenal pH of acidic organelles, such as chloroquine, monensin, and NH4Cl, are known to block transport through the endosomal system (Chapman and Munro, 1994). To examine whether these drugs were able to affect MAL endocytosis, COS-7 cells transiently expressing MAL-FLAG were incubated with anti-FLAG antibody for 1 h at 4°C and the unbound antibody was removed by extensive washing. MAL-FLAG internalization was then allowed to proceed at 37°C for 30 min in the presence of 10 μM monensin, 100 μM chloroquine, or 50 mM NH4Cl. Figure 5, a–c, shows that MAL-FLAG can still be endocytosed in the presence of these agents, but further progress along the endosomal pathway was blocked and the protein appeared within swollen vesicular structures scattered through the cytoplasm. When cells were treated with the drugs for 30 min before antibody binding and maintained throughout the procedure, the effect on MAL-FLAG internalization was more evident (Figure 5, d–f) than in nonpretreated cells (Figure 5, a–c). Similar observations were reported for TGN38 and furin, two TGN proteins (Chapman and Munro, 1994; Reaves and Banting, 1994, Reaves et al., 1998). These results indicate that MAL passes through endosomes before accumulation in the perinuclear region. Finally, when cells were treated with chloroquine for long periods (2.5 h) and then analyzed by immunofluorescence with 9E10 mAb, all of the protein appeared in large intracellular accumulations (Figure 5g). This might be interpreted as indicating that after this time all of the MAL-FLAG molecules had arrived at the plasma membrane and had been endocytosed. The vesicular structures observed in Figure 5g are larger than those in Figure 5e, probably because of the longer incubation period with the drug.
Figure 5.
Effect of agents that dissipate endosomal pH gradients on MAL-FLAG internalization. (a–c) COS-7 cells were transiently transfected with MAL-FLAG. After 48 h, cells were incubated with anti-FLAG mAb for 1 h at 4°C and then for 30 min at 37°C in the presence of 10 μM monensin (a), 100 μM chloroquine (b), or 50 mM NH4Cl (c). (d–f) Cells were pretreated with monensin (d), chloroquine (e), or NH4Cl (f) for 30 min before binding of the anti-FLAG antibody, and the drugs were maintained during both the incubation with the primary antibody and the 30-min incubation at 37°C. Finally, cells were fixed, permeabilized, and incubated with the secondary antibody. (g) Cells were treated with 100 μM chloroquine for 2.5 h at 37°C, fixed, permeabilized, and subjected to immunofluorescence analysis with mAb 9E10. Note that panel g shows the distribution of the total MAL-FLAG protein, whereas panels a–f show only the distribution of the MAL-FLAG originally present on the cell surface. Bar, 10 μm.
Characterization of the Perinuclear Compartment Where MAL Accumulates after Endocytosis
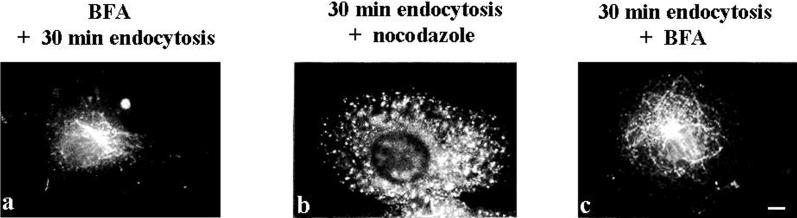
Treatment with the fungal metabolite BFA had no effect on the delivery of MAL-FLAG from the plasma membrane to the perinuclear region (Figure 6a). This is consistent with the behavior previously described for TGN38 (Reaves et al., 1998) in BFA-treated cells and indicates that BFA affects the morphology more dramatically than the function of endosomes. To characterize further the perinuclear compartment in which MAL accumulates, cells were incubated for 1 h at 4°C with the anti-FLAG antibody, followed by 30 min of incubation at 37°C to allow endocytosis, and then 30 min in the presence of nocodazole or BFA. Figure 6b shows that the MAL-FLAG previously on the surface dispersed throughout the cytoplasm in the presence of nocodazole, indicating that the perinuclear compartment where MAL accumulated was sensitive to microtubule depolymerization. This compartment tubulated in the presence of BFA (Figure 6c), suggesting that it may correspond to the TGN and/or early endosomes (Wood et al., 1989; Lippincott-Schwartz et al., 1991).
Figure 6.
Effect of nocodazole and BFA on MAL-FLAG internalization. (a) COS-7 cells transiently expressing MAL-FLAG were treated with 5 μg/ml BFA for 30 min, followed by sequential incubations in the presence of BFA with anti-FLAG antibodies for 1 h at 4°C and for 30 min at 37°C after removal of the unbound antibodies. (b and c) Transfected cells were incubated with anti-FLAG antibody for 1 h at 4°C. After incubation for 30 min at 37°C to allow the anti-FLAG mAb/MAL-FLAG complex to accumulate in the perinuclear region, cells were incubated for 30 min in the presence of 20 μM nocodazole (b) or 5 μg/ml BFA (c), fixed, permeabilized, and subjected to immunofluorescence analysis with an anti-mouse IgG secondary antibody. Bar, 10 μm.
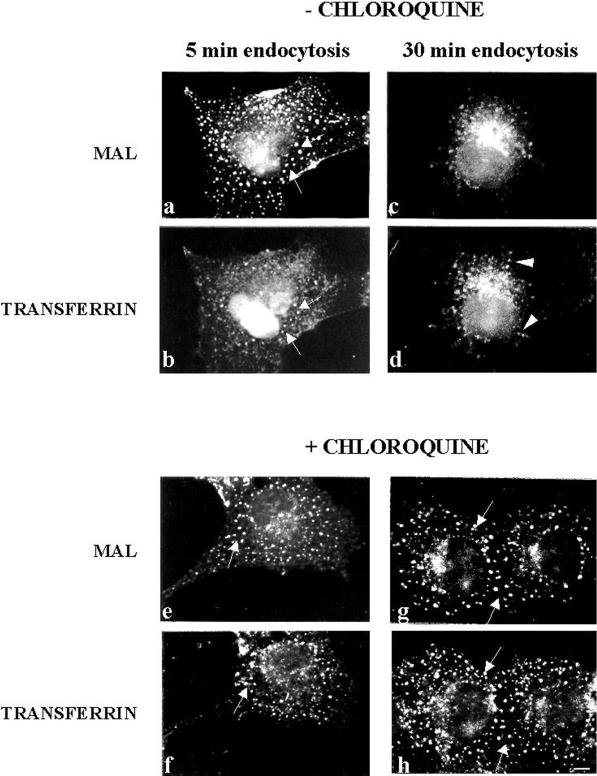
We next investigated whether surface MAL travels to early endosomes after internalization. Cells were incubated with 0.5 mg/ml transferrin and anti-FLAG antibodies for 1 h at 4°C, washed, incubated for either 5 or 30 min at 37°C, and analyzed by double-label immunofluorescence. Figure 7 shows that after 5 min at 37°C MAL-FLAG (a) largely colocalized with transferrin (b) in the same vesicular structures. After 30 min of endocytosis, although there was still some colocalization, a fraction of MAL-FLAG (c) was excluded from the structures containing transferrin (d). Moreover, when the experiment was done in chloroquine-pretreated cells, MAL-FLAG (e and g) colocalized with transferrin (f and h) after 5 min (e and f) and 30 min (g and h) of incubation at 37°C. Endosomal acidification has been suggested as being necessary for the formation of carrier vesicles from early endosomes (Aniento et al., 1996). The results in Figure 7 indicate that a sorting event takes place between MAL-FLAG and transferrin in early endosomes after internalization. The predominant colocalization of internalized MAL-FLAG with TGN38 (Figure 3, g and h) but not with transferrin (Figure 7, c and d) after incubation at 37°C for periods longer than 30 min suggests that MAL-FLAG might accumulate in the TGN after endocytosis.
Figure 7.
Comparative analysis of MAL-FLAG and transferrin internalization. COS-7 cells transiently transfected with MAL-FLAG were incubated with 0.5 mg/ml transferrin for 1 h at 37°C in the absence (a–d) or presence (e–h) of 100 μM chloroquine. Cells were then incubated with anti-FLAG antibody for 1 h at 4°C and for 5 min (a, b, e, and f) or 30 min (c, d, g, and h) at 37°C. Finally, cells were subjected to double-label immunofluorescence analysis with anti-FLAG mAb (a, c, e, and g) and polyclonal anti-transferrin antibodies (b, d, f, and h). Arrows indicate vesicles in which MAL-FLAG and transferrin colocalize. Arrowheads in d indicate vesicles with only transferrin. Bar, 10 μm.
MAL Recycling from the Plasma Membrane to the TGN
We have previously described the glycosylation of an engineered MAL molecule bearing two engineered consensus N-glycosylation sites in its last extracellular loop (Puertollano and Alonso, 1999), indicating accessibility of the inserted sequence to the N-glycosylation machinery. In an attempt to confer O-glycosylation on MAL, we substituted the asparagine residues within the consensus N-glycosylation sites with threonine (Figure 1A, MAL-T construct). This modification impedes N-glycosylation and simultaneously provides additional hydroxyl residues to the O-glycosylation machinery. In addition, the extended loop contains residues with biotinylatable free amino groups for the detection of surface expression of the protein. Figure 8A shows that expression of MAL-T in COS-7 cells resulted in sialylation of the protein, as implied by its sensitivity to neuraminidase treatment, indicating passage of MAL through the TGN en route to the plasma membrane. Furthermore, centrifugation to equilibrium in a sucrose density gradient confirmed that the presence of the O-glycans did not interfere with the incorporation of MAL into detergent-insoluble membranes (Figure 8B), although MAL-T peaked in fractions with higher density than that of MAL (compare Figures 1B and 8B). The presence of glycosylation intermediates in the soluble but not in the insoluble fractions is consistent with previous reports showing that the incorporation of glycoproteins into GEMs takes place in the Golgi apparatus concomitantly with processing of attached carbohydrates (Brown and Rose, 1992).
Figure 8.
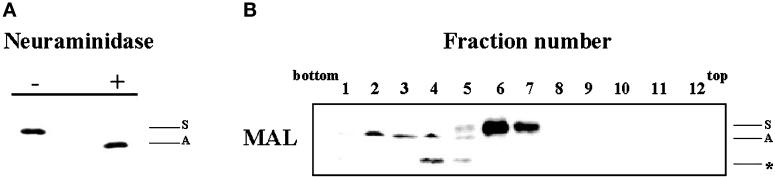
O-Glycosylation of MAL does not interfere with its ability to gain access to GEMs. (A) COS-7 cells transiently expressing MAL-T cells were surface biotinylated 48 h after transfection and lysed with 1% Triton X-100 plus octyl-glucoside at 4°C. The extract was then subjected to immunoprecipitation with mAb 9E10, and the immunoprecipitate was incubated in the absence or presence of neuraminidase. After SDS-PAGE analysis, surface MAL-T was visualized with streptavidin-peroxidase. The positions of the sialylated (S) and asialo (A) MAL-T species are indicated. (B) COS-7 cells transiently expressing MAL-T cells were extracted with 1% Triton X-100 at 4°C at 48 h after transfection and subjected to centrifugation to equilibrium in sucrose density gradients. Aliquots from each fraction were subjected to immunoblot analysis with mAb 9E10. The positions of the sialylated (S) and asialo (A) MAL-T species are indicated. The position of a third band (*), detected by immunoblot analysis but not by surface labeling, likely corresponds to nonglycosylated MAL-T species.
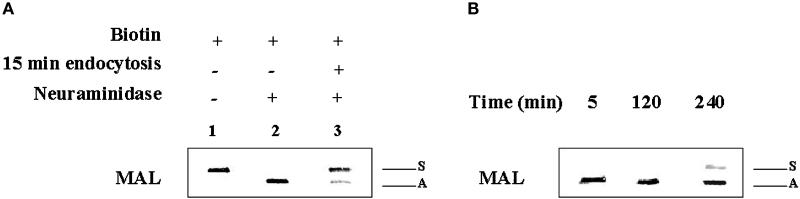
Antibody binding to surface proteins might trigger their internalization. To determine whether or not MAL-FLAG endocytosis is independent of anti-FLAG mAb binding, we analyzed the acquisition of resistance to neuraminidase in surface-labeled MAL-FLAG after incubation at 37°C in the absence of anti-FLAG antibodies. Figure 9A shows that MAL-T was able to reach the plasma membrane, as detected by surface biotinylation of transfected COS-7 cells (lane 1). Moreover, surface MAL-T was sensitive to the treatment of intact cells with neuraminidase, indicating that it had been sialylated (lane 2). It is of note that when MAL-T was allowed to internalize by incubation at 37°C for 15 min before the treatment with neuraminidase, most of the sialic acid became resistant to the enzyme (lane 3). Quantitative analysis indicated that ∼70% of the MAL-T that was originally present on the cell surface was not accessible to neuraminidase after incubation at 37°C. This result is consistent with the data obtained using fluorescence cytometric analysis (Figure 4) showing that ∼70% of surface MAL-FLAG was endocytosed after 15 min at 37°C. Thus, the results in Figure 9A indicate that MAL endocytosis is a process that occurs without antibody triggering.
Figure 9.
Recycling of MAL from the plasma membrane to the TGN. (A) COS-7 cells were transfected with MAL-T and, 48 h later, surface labeled with sulfo-NHS-biotin for 30 min at 4°C and incubated for 30 min at 4°C in the absence (lane 1) or presence (lane 2) of neuraminidase, or for 15 min at 37°C to allow MAL internalization and then for 1 h with neuraminidase at 4°C (lane 3). Finally, cells were lysed in 25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 5 mM EDTA, 1% Triton X-100, 60 mM octyl-glucoside for 30 min at 4°C. The extracts were subjected to immunoprecipitation with mAb 9E10 and analyzed by immunoblot with streptavidin-peroxidase to detect the biotinylated protein originally on the surface. (B) COS-7 cells expressing MAL-T were labeled with sulfo-NHS-biotin for 30 min at 4°C, incubated with neuraminidase for 1 h at 4°C, and placed in normal medium at 37°C for 5 min (lane 1), 120 min (lane 2), or 240 min (lane 3). Cells were then lysed and subjected to immunoprecipitation analysis as described in A. The positions of the sialylated (S) and asialo (A) MAL-T species are indicated. The intensity of the bands shown in A and B were quantified by densitometric analysis (see text). SEs were ∼10% of the mean values.
Acquisition of sialic acid on O-linked carbohydrates takes place at the TGN by the action of resident sialyltransferases (Roth, 1997). We exploited the ability of neuraminidase to remove sialic acid from surface MAL-T (Figure 9A) to establish whether internalized MAL-T is delivered to the TGN after endocytosis. To this end, MAL-T was surface labeled with biotin at 4°C, treated with neuraminidase at the same temperature to convert all the surface MAL-T to the asialo species (as in Figure 9A), and incubated for 5, 120, or 240 min to allow endocytosis and processing of MAL-T in internal compartments. The transit of asialo–MAL-T through the TGN was then monitored by resialylation of the biotinylated MAL-T, as measured by the acquisition of sialic acid resistant to the treatment of intact cells with external neuraminidase. As observed in Figure 9B, only a single band corresponding to the asialo–MAL-T species was evident after 5 min of incubation at 37°C. Some neuraminidase-resistant resialylated protein was detected after 240 min at 37°C. Quantitative analysis indicated that, after this time, ∼30% of the MAL-T originally present on the surface was recycled to the TGN for resialylation. The discrepancy between the value obtained for MAL-T resialylation and the extent of the colocalization of internalized MAL-FLAG with TGN38 observed after 50 min of incubation at 37°C (Figure 3, g and h) might be due to the different size of the engineered extracellular loop present in MAL-FLAG and MAL-T, which possibly affects their kinetics of transport to the TGN after internalization.
DISCUSSION
Integral membrane components of the machinery for membrane trafficking must possess a retrieval mechanism to prevent their accumulation in the target compartment and to ensure their continuous recycling. In the case of epithelial polarized cells, the apical machinery leaving the TGN with cargo molecules should travel to the plasma membrane for cargo delivery and then be endocytosed and subsequently either degraded or recycled for new cycles of transport. We previously demonstrated MAL to be an integral membrane element necessary for apical transport of the influenza virus HA in polarized MDCK cells (Puertollano et al., 1999). In the current study, we have investigated the surface expression of MAL, its internalization, and its recycling to the TGN to examine the putative itinerant character of MAL.
The expression of engineered MAL proteins in transiently and stably transfected cells allowed us to detect surface expression of MAL. We also observed a rapid internalization of MAL in both systems. This process was independent of antibody binding and resulted in the endocytosis of ∼70% of surface MAL protein after 15 min of incubation at 37°C, which increased to 90% after 30 min at this temperature. Internalized MAL accumulated in the perinuclear region when the cells were incubated for longer than 30 min at 37°C. This accumulation was prevented by inhibitors of endosomal transport, such as chloroquine, monensin, and NH4Cl. The observed accumulation of MAL does not appear to be a nonspecific effect of these drugs, because although chloroquine and NH4Cl are both weak bases capable of dissipating pH gradients across organelle membranes, they are structurally unrelated, and the ionophore monensin has a completely different structure and mechanism of action. A similar effect to that observed for MAL has been described for furin (Chapman and Munro, 1994), a TGN endoprotease responsible for proteolytic processing of a number of substrates, and for TGN38, a TGN protein of unknown function (Chapman and Munro, 1994; Reaves and Banting, 1994). In the presence of chloroquine, these proteins accumulate in the neutralized endosomes. The same treatment had no effect on the distribution of Golgi or TGN resident enzymes, such as mannosidase II or sialyltransferase, respectively (Chapman and Munro, 1994). This finding rules out the possibility that the observed effect was due to drug-induced fragmentation of the TGN. It is of particular note that α-2,6-sialyltransferase is a permanent resident of the TGN and that its localization is determined by a retention process involving its transmembrane domain (Munro, 1995), whereas TGN38, furin, and MAL appear to be itinerant proteins requiring continuous retrieval from the cell surface to maintain their predominant steady-state localization in the TGN.
Most of the internalized MAL molecules colocalize with the early endosome tracer transferrin in chloroquine-treated cells, consistent with the passage of MAL through early endosomes after internalization. Moreover, the trapping of MAL in early endosomes in chloroquine-treated cells indicates that endosome acidification is required for MAL to leave the endosomes. This is consistent with the observation that endosome acidification is necessary for the formation of at least some types of endosome-derived carrier vesicles (Aniento et al., 1996). Once inside the sorting endosomes, some receptor molecules are returned directly to the plasma membrane (direct recycling). However, the bulk of the receptor population is transferred to a recycling endosomal compartment consisting of a juxtanuclear pericentriolar collection of membranous tubular elements before being recycled to the plasma membrane. Previous observations have shown that internalized transferrin molecules concentrate in this pericentriolar recycling compartment (Ren et al., 1998). The preponderant colocalization of internalized MAL with transferrin in chloroquine-treated cells, and the partial colocalization of these proteins after 30 min of incubation a 37°C, might be interpreted as indicating that MAL is transported from early endosomes to the recycling compartment after endocytosis. Our results from surface MAL-T resialylation after internalization indicate that ∼30% of the protein in recycling endosomes would be directed to the TGN for recycling and 70% would be directed to the plasma membrane for new rounds of endocytosis. Kinetic analyses of trafficking of antibodies bound to Tac TGN38 previously indicated that 80% of internalized anti-Tac returned to the cell surface, whereas the remaining 20% moved from the recycling compartment to the TGN (Ghosh et al., 1998). Similarly, furin is also internalized from the plasma membrane and is then either retained in a local cycling loop between early endosomes and the cell surface or directed to the TGN (Molloy et al., 1998). Thus, it appears that MAL might follow an intracellular route from the cell surface to the TGN via the endocytic pathway similar to that used by TGN38 or furin.
In summary, the results of this and previous studies suggest a model for MAL trafficking in which at steady state a small fraction of MAL is present at the plasma membrane, and this surface pool undergoes dynamic exchange with intracellular MAL, which is predominantly present in the Golgi apparatus. The rate of MAL exit from the Golgi would be relatively slow compared with the other steps in MAL trafficking and would thus largely determine the steady-state distribution of the protein. The prolonged presence of MAL in the Golgi could facilitate the recruitment and stabilization of cargo proteins in GEMs, as we have previously observed in the influenza virus HA (Puertollano et al., 1999). According to our model, the presence of some MAL molecules in the plasma membrane is a consequence of the role of MAL in cargo delivery to the surface. In addition to preventing surface accumulation of MAL, the observed internalization of MAL would be necessary for participation in new rounds of cargo transport in the TGN and, possibly, for assuming novel roles in sorting in the endosomal system.
ACKNOWLEDGMENTS
R.P. is the recipient of a predoctoral fellowship from the Comunidad de Madrid. This work was supported by grants (PM96-0004) from the Dirección General de Enseñanza Superior and the Comunidad de Madrid (08.3/0020/1998). An institutional grant from the Fundación Ramón Areces to the Centro de Biología Molecular “Severo Ochoa” is acknowledged.
Abbreviations used:
- BFA
brefeldin A
- GEM
glycolipid-enriched membrane
- GPI
glycosylphosphatidylinositol
- HA
hemagglutinin
- MDCK
Madin-Darby canine kidney
- sulfo-NHS-biotin
sulfo-N-hydroxyl-succinimido-biotin
- TGN
trans-Golgi network
REFERENCES
- Alonso MA, Weissman SM. cDNA cloning and sequence of MAL, a hydrophobic protein associated with human T cell differentiation. Proc Natl Acad Sci USA. 1987;84:1997–2001. doi: 10.1073/pnas.84.7.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal βCOP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum R, Pfeiffer F, Feick P, Nastainczyk W, Kohler B, Schafer K-H, Schultz I. Intracellular localization and in vivo trafficking of p24A and p23. J Cell Sci. 1999;112:537–548. doi: 10.1242/jcs.112.4.537. [DOI] [PubMed] [Google Scholar]
- Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MG, Hauri H-P. Protein sorting and vesicular traffic in the Golgi apparatus. In: Berger EG, Roth J, editors. The Golgi Apparatus. Basel, Switzerland: Birkhäuser Verlag; 1997. pp. 63–130. [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of the coatomer with the p24 family of putative cargo receptors. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Ghosh RN, Mallet WG, Soe TT, McGraw TE, Maxfield FR. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J Cell Biol. 1998;142:923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SN, Hunt HD, Horton RM, Pullen JK, Pease LR. Site-directed mutagenesis by overlap extension using the PCR. Gene. 1989;77:51–59. doi: 10.1016/0378-1119(89)90358-2. [DOI] [PubMed] [Google Scholar]
- Kappeler F, Klopfenstein DRC, Foguet M, Paccaud JP, Hauri H-P. The recycling of ERGIC-53 in the early secretory pathway. J Biol Chem. 1997;272:31801–31808. doi: 10.1074/jbc.272.50.31801. [DOI] [PubMed] [Google Scholar]
- Kim T, Fiedler K, Madison DL, Krueger WH, Pfeiffer SE. Cloning and characterization of MVP17: a developmentally regulated myelin protein in oligodendrocytes. J Neurosci Res. 1995;42:413–422. doi: 10.1002/jnr.490420316. [DOI] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Lin S, Naim HY, Rodriguez AC, Roth MG. Mutations in the middle of the transmembrane domain reverse the polarity of transport of the influenza virus hemagglutinin in MDCK epithelial cells. J Cell Biol. 1998;142:51–57. doi: 10.1083/jcb.142.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A’s effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Caras IW, Davitz MA, Rodriguez-Boulan R. A glycophospholipid membrane anchor acts as an apical targeting signal in polarized epithelial cells. J Cell Biol. 1989;109:2145–2156. doi: 10.1083/jcb.109.5.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti MP, Sargiacomo M, Graeve L, Saltiel AR, Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci USA. 1988;85:9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Belmonte F, Kremer L, Albar JP, Marazuela M, Alonso MA. Expression of the MAL gene in the thyroid: the MAL proteolipid, a component of glycolipid-enriched membranes, is apically distributed in the thyroid follicles. Endocrinology. 1998;139:2077–2084. doi: 10.1210/endo.139.4.5875. [DOI] [PubMed] [Google Scholar]
- Matter K, Mellman I. Mechanisms of cell polarity: sorting and transport in epithelial cells. Curr Opin Cell Biol. 1994;6:545–554. doi: 10.1016/0955-0674(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Millán J, Alonso MA. MAL, a novel integral membrane protein of human T lymphocytes, associates with glycosylphosphatidylinositol-anchored proteins and Src-like tyrosine kinases. Eur J Immunol. 1998;28:3675–3684. doi: 10.1002/(SICI)1521-4141(199811)28:11<3675::AID-IMMU3675>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Millán J, Puertollano R, Fan L, Alonso MA. Caveolin and MAL, two protein components of internal detergent-insoluble membranes, are in distinct lipid microenvironments in MDCK cells. Biochem Biophys Res Commun. 1997a;233:707–712. doi: 10.1006/bbrc.1997.6530. [DOI] [PubMed] [Google Scholar]
- Millán J, Puertollano R, Fan L, Rancaño C, Alonso MA. The MAL proteolipid is a component of the detergent-insoluble membrane subdomains of human T-lymphocytes. Biochem J. 1997b;321:247–252. doi: 10.1042/bj3210247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy SS, Thomas L, Kamibayashi C, Mumby MC, Thomas G. Regulation of endosome sorting by a specific PP2A isoform. J Cell Biol. 1998;142:1399–1411. doi: 10.1083/jcb.142.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S. An investigation of the role of transmembrane domains in Golgi retention. EMBO J. 1995;14:4695–4704. doi: 10.1002/j.1460-2075.1995.tb00151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puertollano R, Alonso MA. A short peptide motif at the carboxyl terminus is required for incorporation of the integral membrane MAL protein to glycolipid-enriched membranes. J Biol Chem. 1998;273:12740–12745. doi: 10.1074/jbc.273.21.12740. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Alonso MA. Targeting of MAL to glycolipid-enriched membranes requires a preGolgi sorting event. Biochem Biophys Res Commun. 1999;254:689–692. doi: 10.1006/bbrc.1998.0122. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Li S, Lisanti MP, Alonso M A. Recombinant expression of the MAL proteolipid, a component of glycolipid-enriched membrane microdomains, induces the formation of vesicular structures in insect cells. J Biol Chem. 1997;272:18311–18315. doi: 10.1074/jbc.272.29.18311. [DOI] [PubMed] [Google Scholar]
- Puertollano R, Martín-Belmonte F, Millán J, de Marco MC, Albar JP, Kremer L, Alonso MA. The MAL proteolipid is necessary for normal apical transport and accurate sorting of the influenza virus hemagglutinin in Madin-Darby canine kidney cells. J Cell Biol. 1999;145:141–151. doi: 10.1083/jcb.145.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaves B, Banting G. Vacuolar ATPase inactivation blocks recycling to the trans-Golgi network from the plasma membrane. FEBS Lett. 1994;345:61–66. doi: 10.1016/0014-5793(94)00437-4. [DOI] [PubMed] [Google Scholar]
- Reaves BJ, Banting G, Luzio JP. Lumenal and transmembrane domains play a role in sorting type I membrane proteins on endocytic pathways. Mol Biol Cell. 1998;9:1107–1122. doi: 10.1091/mbc.9.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Xu G, Zeng J, Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab 11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riederer MA, Soldati T, Shapiro AD, Lin J, Pfeffer SR. Lysosome biogenesis requires Rab9 function and receptor recycling from endosomes to the trans-Golgi network. J Cell Biol. 1994;125:573–582. doi: 10.1083/jcb.125.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Pendergast M. Polarized distribution of viral envelope glycoproteins in the plasma membrane of infected epithelial cells. Cell. 1980;20:45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Roth J. Topology of glycosylation in the Golgi apparatus. In: Berger EG, Roth J, editors. The Golgi Apparatus. Basel, Switzerland: Birkhäuser Verlag; 1997. pp. 131–162. [Google Scholar]
- Roth MG, Fitzpatrick JP, Compans RW. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci USA. 1979;76:6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeren-Wiemers N, Valenzuela DM, Frank M, Schawb ME. Characterization of the rat gene, rMAL, encoding a protein with four hydrophobic domains in central and peripheral myelin. J Neurosci. 1995;15:5753–5764. doi: 10.1523/JNEUROSCI.15-08-05753.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Roth MG, Simons K. Interaction of influenza virus hemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Wandinger-Ness A. Polarized sorting in epithelia. Cell. 1990;62:207–210. doi: 10.1016/0092-8674(90)90357-k. [DOI] [PubMed] [Google Scholar]
- Townsley FM, Wilson DW, Pelham HRB. Mutational analysis of the human KDEL receptor: distinct structural requirements for Golgi retention, ligand binding and retrograde transport. EMBO J. 1993;12:2821–2829. doi: 10.1002/j.1460-2075.1993.tb05943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Kornfeld S. The trans-Golgi-network: a late secretory sorting station. Curr Opin Cell Biol. 1997;9:527–533. doi: 10.1016/s0955-0674(97)80029-4. [DOI] [PubMed] [Google Scholar]
- Wilde A, Reaves B, Banting G. Epitope mapping of two isoforms of a trans Golgi network specific integral membrane protein TGN38/42. FEBS Lett. 1992;313:235–238. doi: 10.1016/0014-5793(92)81199-v. [DOI] [PubMed] [Google Scholar]
- Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1989;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- Zacchetti D, Peranën J, Murata M, Fiedler K, Simons K. VIP17/MAL, a proteolipid in apical transport vesicles. FEBS Lett. 1995;377:465–469. doi: 10.1016/0014-5793(95)01396-2. [DOI] [PubMed] [Google Scholar]