Abstract
The intent of this article is to review the numerous factors that affect the mechanical properties of particle or fiber filler containing, indirect dental resin composite materials. The focus will be on degradation due to aging in different media, mainly water and water and ethanol, cyclic loading, and mixed mode loading on the flexure strength and fracture toughness. Next several selected papers will be examined in detail with respect to mixed and cyclic loading and then an examination of 3D tomography using multiaxial compression specimens. The main cause of failure, for most dental resin composites, is the breakdown of the resin matrix and or the interface between the filler and the resin matrix. In clinical studies, it appears that failure in the first 5 years is a restoration issue (technique or material selection) and after that time period from secondary decay.
Keywords: dental composites, cyclic loading, aging, multiaxial compression, 3D tomography
Background
The intent of this review paper is to focus on restorative resin based composite materials, specifically those reinforced with an individual, separated filler, either particles and/or short fibers. Development of modern dental composite restorative materials started in the late 1950s and early 1960s, when Bowen (O’Brien, 2002) began experiments to reinforce epoxy resins with filler particles. Deficiencies in the epoxy resin system, such as a slow curing rate and a tendency to discolor, stimulated his work on combining the advantages of epoxies and acrylates. Dental composites consist of a polymerizable resin matrix, reinforcing glass particle fillers, and silane coupling agents (Ferracane, 1995). These glass particle/resin matrix composites have good aesthetic properties and strength, making them the most widely used materials for restorations of anterior teeth (Nicholson, 2000). The polymerizable resin matrix typically contains one or more monomers such as bis-phenol-A-diglycidyl dimethacrylate (Bis-GMA), urethane dimethacrylate (UDMA) and triethylene glycol dimethacrylate (TEGDMA). Polymerization of the resin matrix may be chemically initiated in “self cure” composites, light activated, or a combination of both. Various inorganic materials such as glass fillers are utilized as fine or micro-fine particles and serve as reinforcing components. These fillers make up the bulk of the composites and they vary in size and composition among different composites, Figure 1. In addition to silica (SiO2), these composites also incorporate barium (Ba) and strontium (Sr) glasses, which add x-ray opacity to facilitate radiological monitoring of the composite in vivo.
Figure 1.
SEM of typical filer particles showing a colloidal filler, OX 50 and two micro fillers Z100 and a Sr-SiO2 glass showing the differences in size and shape.
The issue with restorative composites is to increase the flexure strength and fracture toughness, and thereby lengthen their service life in the oral cavity, but still maintain their esthetic value. However, longevity and survival studies in posterior teeth continue to show that amalgam has a better track record than composite, further reinforcing the need to understand the failure mechanisms of dental composites to enhance their survival (Bernardo et al., 2007, Soncine, et al., 2007). Damage in dental composites may result in matrix and/or filler deterioration due to mechanical and/or environmental loads, interfacial debonding, microcracking, and/or filler particle fracture. A continuous application of mechanical and environmental loads eventually leads to progressive degradation and crack initiation and growth, resulting in catastrophic failure of dental restorations. This process is further assisted by preexisting voids introduced during material processing, imperfect interfaces, and residual stresses, making resistance to crack initiation and growth an important consideration for a reliable assessment of dental restorations. Most of the published work is concerned with mode I straight-line crack growth, and toughness characterization of various composites, which have been exposed to air, water, ethanol, and other environments.
Fracture mechanics is an important tool in understanding and predicting the life of materials. In its most basic form fracture mechanics can be applied to relate the maximum permissible applied loads acting upon a structural component to the size and location of a crack (either real or hypothetical) in the component (Kanninen and Popelar, 1985). Fracture mechanics can also be used to predict the rate at which a crack can approach a critical size in fatigue or by environmental influences, and can be used to determine the conditions under which a rapidly propagating crack can be arrested. Fracture occurs when the stress concentration inside the material reaches the critical level known as the “plane strain fracture toughness.” The “plane strain fracture toughness”, KIC is a measure for the crack resistance of a material. Characterization of this material property can thus help prevent catastrophic failures. Procedures for plane strain fracture toughness testing are standardized by the American Society for Testing and Materials (ASTM, 1990, 1997). A test method that has been used extensively in the study of fracture properties of brittle materials is the diametral compression test, also referred to as the Brazilian disk test or the indirect tension test (Awaji and Sato, 1978; ASM Handbook, 1996; Huang et al., 1996). The Brazilian disk test involves loading a disk specimen in compression (edgewise) along a diameter. The loading generates a biaxial stress state in the specimen with a compressive principal stress in the direction of loading and a transverse tensile stress. For a valid plane strain fracture toughness measurement, in addition to the linearity of the load displacement curves and the plane strain conditions, the crack tip should be “atomically” sharp. The crack tip condition is difficult to satisfy in brittle materials due to problems associated with growing a sharp crack normal to the applied load. Researchers have implemented various techniques to introduce sharp notches in brittle specimens (Sanchez, 1979; Shetty et al., 1986). Atkinson et al. (1982) have analyzed the case of combined mode fracture via the Brazilian disk test and they have provided expressions for the stress intensity factor.
Aging Environments of Dental Composites
Söderholm and Roberts (1990) found that tensile strength specimens stored dry were significantly stronger then those stored wet for six months or those stored wet and then dried. The specimens were dried in a desiccator for two weeks at 60°C. The aging in water appeared to increase filler particle pull out on the fractured surface, possibly due to break down of the silane bond between the resin and the filler particle. Ferracane and Marker (1992) used fracture toughness specimens utilizing a razor blade insert to form the starter crack. They found no difference in wet versus dry testing. Aging in water for fourteen months had no statistically significant effect on KIC for the filled composites or the unfilled resin. A significant reduction in KIC was observed in ethanol after fourteen months aging, but the value at the end of fourteen months was the same as after two months aging. Ferracane and Condon (1992) used single edge notched specimens heat-treated at 120°C and found an increase in KIC (16–47%) and modulus of elasticity (E) (12–60%). The heat treatment of the small particle hybrid enhanced crack propagation through the matrix resulting in less filler-matrix debonding. For the microfill, the heat treatment resulted in less filler-matrix delamination and enhanced fracture of the prepolymerized resin filler. The increase in KIC and E was attributed to an increase in the degree of conversion. A more recent study by Ferracane et al. (1995) showed that long term aging (up to 2 years) in water slightly, but significantly reduced fracture toughness for all the composites investigated, but had little effect on flexural modulus and flexural strength. Lloyd (1982) found that microfine fillers lowered fracture toughness more than small or large filler particles with fracture occurring through the resin matrix. Ferracane et al. (1987) used two sets of single edge notched (SEN) specimens, pre-cracked and not pre-cracked, with no polished surface stored in water 24 hours before testing. There was no correlation between degree of cure and fracture toughness. The fracture toughness was greater for the filled composites than the unfilled resin. This increase in fracture toughness was attributed to increased fracture energy due to crack pinning and bowing between particles rather than increased fracture surface energy as compared to unfilled resin. Pilliar et al. (1986) found a decrease in fracture toughness with aging over 1 month using a short rod fracture specimen, but no change less than 1 month. Microfills had a lower fracture toughness than small particle composites. Aging in ethanol caused a significant decrease in the fracture toughness (Wright and Burton, 1976). Goldman (1985) used a double torsion specimen with a groove along the length of the specimen with no surface polish. He attributed fracture toughness to a crack-pinning mechanism of hard particles in a soft matrix. The fracture toughness reached a maximum, then fell off due to overlapping of particle strain energy fields with the particles acting as stress concentrators and crack precursors. Kovarik and Engle (1992) evaluated long-term aging in water and air of dental composites. The fracture toughness for dry specimens at 7 days was 1.69±0.13 MPa-m1/2 and at 4 years 1.68±0.19 MPa-m1/2 and for wet specimens at 7 days 1.94±0.13 MPa-m1/2 and at 4 years 1.55±0.18 MPa-m1/2. Jones et al. (1992) stored composite specimens in water up to 29 months and found no effect on the elastic modulus.
Aging in water, on the other hand may have a beneficial effect on dental composites as the water is absorbed into the resin matrix, making the composite more flexible resulting in an apparent increase in the mechanical properties. However over time the leaching of the components and swelling and degradation of the cross-linked matrix in the dental composite and hydrolysis of the filler-matrix interfaces eventually leads to a decrease in the mechanical properties (Ferracane et al., 1995; Takeshige et al., 2007). Other theories as to the cause of the degradation to the dental resin include the formation of microcracks through repeated sorption/desorption cycles leading to hydrolytic degradation of the polymer (Musto et al., 2002: Yiu et al., 2004). With respect to fracture toughness, water seems to lower the yield stress, release internal stress accumulated during polymerization shrinkage, and increase the plastic zone ahead of the crack, which causes the increase in observed fracture toughness (Indrani et al., 1995; Takeshige et al., 2007). A five-week study on water absorption of dental composites suggested that the decrease in the mechanical properties was a result of residual stress between the wet and dry regions of the composite (Oshida et al., 1995). Whether this might be a carry over to possible breakdown of the resin matrix or separation of the filler particles from the resin matrix for complete sorption of water following a longer aging time was not discussed.
Aging in an ethanol and water mixture results in probable absorption of ethanol and water, resulting in penetration of the cross linked-matrix, weakening the resin and a decrease in the mechanical properties (Ferracane and Berge, 1995). Hydroxyapatite as micrometric or nanometric particles was used as a filler in a Bis-GMA/TEGDMA resin. The flexure strength was increased over the unfilled resin, but was still lower than traditional glass filler particle composites. The nano particles also tended to agglomerate making a uniform dispersion in the resin difficult and the agglomeration lead to reduced mechanical properties (Domingo et al., 2001).
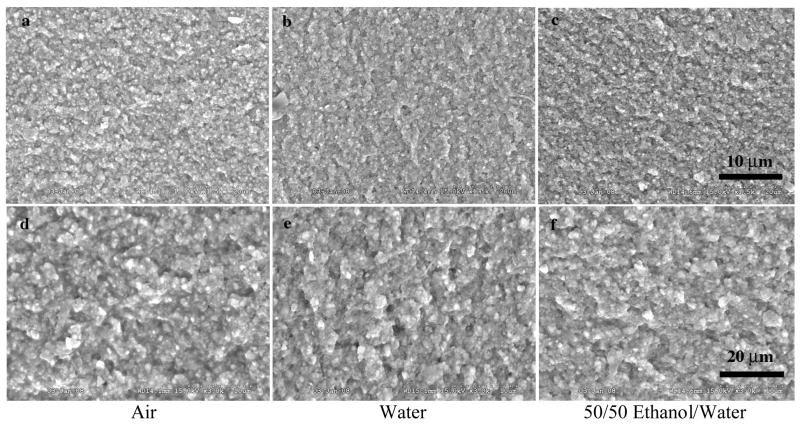
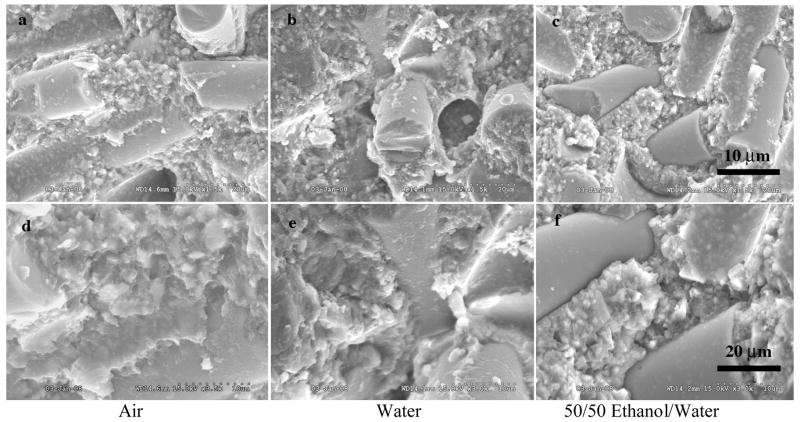
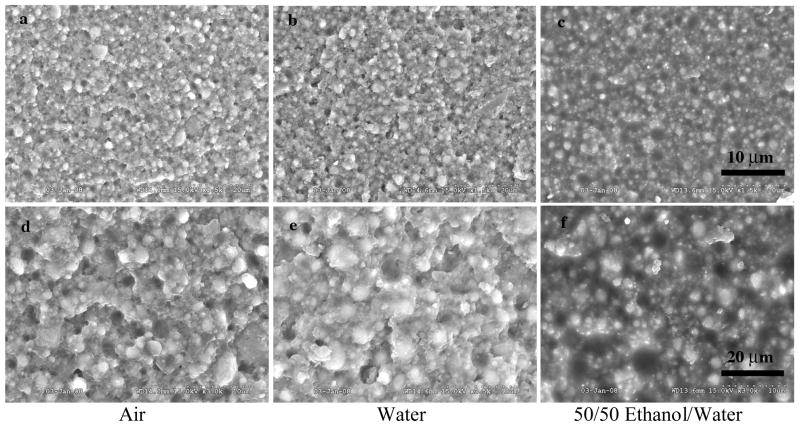
The variation in fracture of dental composites with different fillers can be demonstrated in Figures 2–4. These figures represent a micro hybrid (Figure 2), Renew (Bisco Inc., Schaumburg, IL, USA); a nanofiller (Figure 3), Filtek Supreme (3M ESPE, St. Paul, MN USA); and a fiber filler (Figure 4), Restolux (Lee Pharmaceutical, South El Monte, CA USA). Renew is by weight 28% resin and 72% glass filler particles with an average particle size distribution of 5% 0.004 μm, 62% 0.7 μm and 5% 3–7 μm particles. Filtek is 78.5% by weight 25–75 nm filler particles and 21.5% resin. Restolux is 85% filler and 15% resin by weight with the filler composed of 3–4 μm particles (~ 27%) and 80–120 μm fibers (~52%). The figures represent aging for 6 months in three media; air, distilled water, and a 50/50 by volume mixture of ethanol and distilled water. The trend appears to be the same for all materials, in that, the fracture tends to occur in the resin matrix between the silanated filler for the specimens aged in air and distilled water. For those specimens aged in the 50/50 mixture of distilled water and ethanol, the resin is severely weakened, such that the failure is in the resin for Renew and Filtek, and for Restolux, the fiber filler is separated from the resin matrix completely, Figure 4c and 4f. This separation of the fiber from the resin matrix may be unique for the Restolux composite in that the fiber filler is much larger than any current fiber filler being used and may be an issue of polymerization shrinkage stress separating the fiber from the resin matrix in addition to the aging in the 50/50 mixture. Another feature of the nanofiller composite is that due to the small size of the nano particles, the actual filler is in the form of clusters, around 5 μm in size (Figure 3), and serve as the limiting factor with respect to the mechanical properties.
Figure 2.
SEM of Renew, a micro hybrid by weight 28% resin and 72% glass filler particles with an average particle size distribution of 5% 0.004 mm, 62% 0.7 mm and 5% 3–7 mm particles The figures represent the fracture of specimens aged for 6 months in three media air, distilled water, and a 50/50 by volume mixture of ethanol and distilled water.
Figure 4.
SEM of Restolux, a fiber filler by weight 85% filler and 15% resin with the filler composed of 3–4 mm particles (~ 27%) and 80–120 mm fibers (~52%). The SEMs indicate the relative large size of the fiber filler compared to the surrounding particle filler and the separation of the fiber filler from the resin matrix, c and f, compared to the other aging media. The separation is moist likely a combination of aging in the 50/50 mixture and polymerization shrinkage stress release. The figures represent the fracture of specimens aged for 6 months in three media air, distilled water, and a 50/50 by volume mixture of ethanol and distilled water.
Figure 3.
SEM of Filtek, a nano filler by weight 25–75 nm filler particles and 21.5% resin. The figures show how the nano particles are formed as 5 mm clusters and are pulled out of the resin matrix during fracture. The specimens aged in the 50/50 mixture demonstrate the degradation (weakening of mechanical properties) by a lack of sharpness in the fracture surface. The figures represent the fracture of specimens aged for 6 months in three media air, distilled water, and a 50/50 by volume mixture of ethanol and distilled water.
Another approach is to use nano-silica-fused whiskers (the silicon nitride whiskers have a mean diameter of 0.4 mm and a mean length of 5 mm and the fused silica particles have a mean of 80 nm) to improve the mechanical properties of resin composites, Figures 5 and 6. The best results obtained were 74% by weight of the fused-silica whiskers, which resulted in a doubling of the fracture toughness to 3 MPa-m1/2 and better wear resistance compared to the controls (Xu, 1999, 2000, Xu et al., 2000, Xu et al., 2002a, 2003, 2004). The silica-fused whiskers demonstrated toughening and crack deflection. Aging of these whisker composites for 730 days in water showed an increased resistance to flexure strength loss compared to a control; silica-fused SiN4 whiskers 185(33) MPa, SiC whiskers 146(44) MPa and the control 67(23) MPa (Artglass™, Heraeus Kulzer GmbH, Wehrhein, Germany). The decrease in strength of the non-silica-fused SiN4 whiskers was attributed to the breakdown of the SiN4 whiskers allowing for their fracture and no crack deflection reinforcement. When SiC whiskers thermally fused with silica particles were used to reinforced dental resin composites, no decrease in the flexure strength was observed after 105 thermal cycles between 5°C and 60ºC (Xu et al., 2002b). An experimental and theoretical study of a fiber-matrix interface using polypropylene with 40% in mass of short glass fibers for tensile and creep tests indicated zones of behavior that were linear, non-linear without damage, and non-linear with damage for either treated or untreated fibers (Ségard et al., 2003). This would seem to indicate that the fracture is more complicated than just crack deflection (Manhart et al., (2000).
Figure 5.
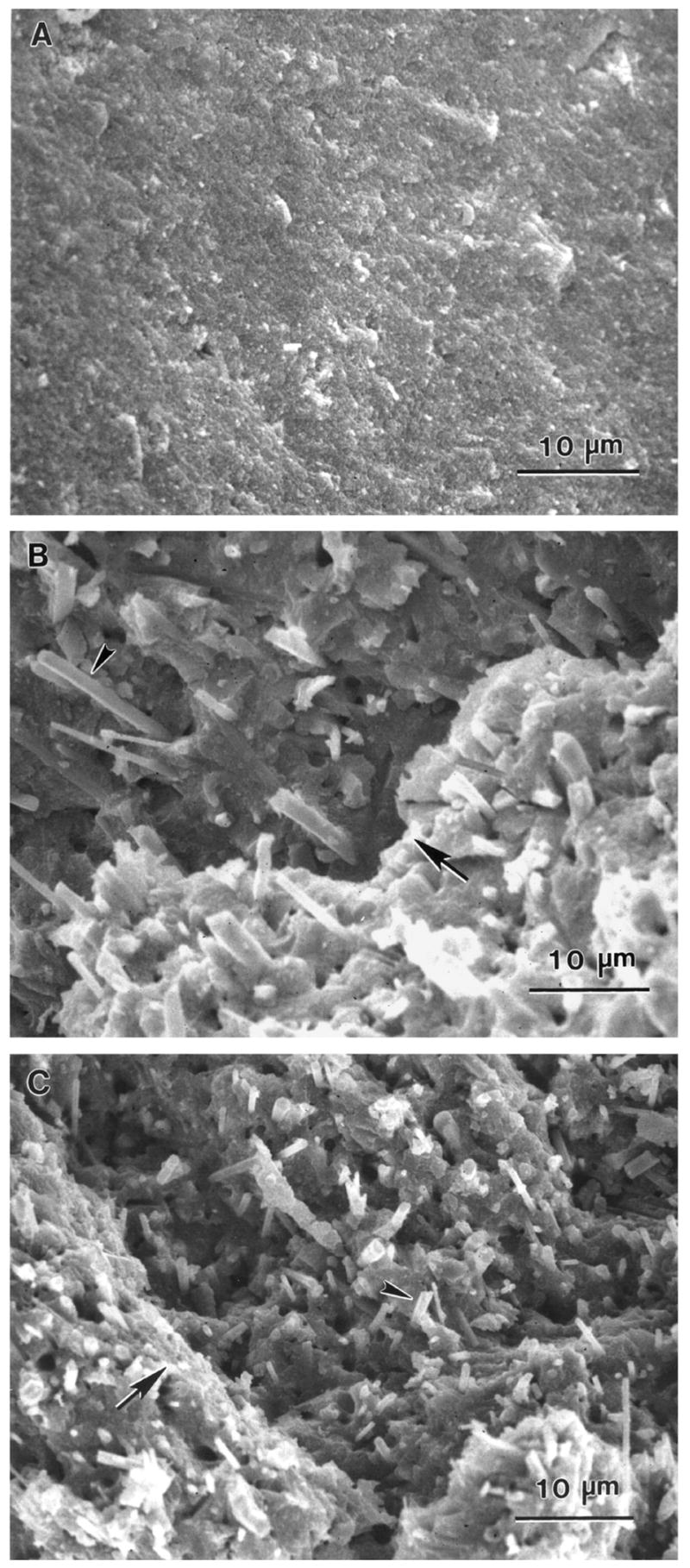
SEM of fracture surfaces of specimens: (A) control A, (B) SiC whisker composite, and (C) Si3N4 whisker composite, all after 1-day immersion. The fracture surfaces of the controls were relatively flat. In contrast, the whisker composites had much rougher surfaces with fracture steps (large arrows) and whisker pullout (small arrows) from the paper Xu HHK (2003). Long-term water aging of whisker-reinforced polymer-matrix composites, J Dent Res, 82:48–52.
Figure 6.
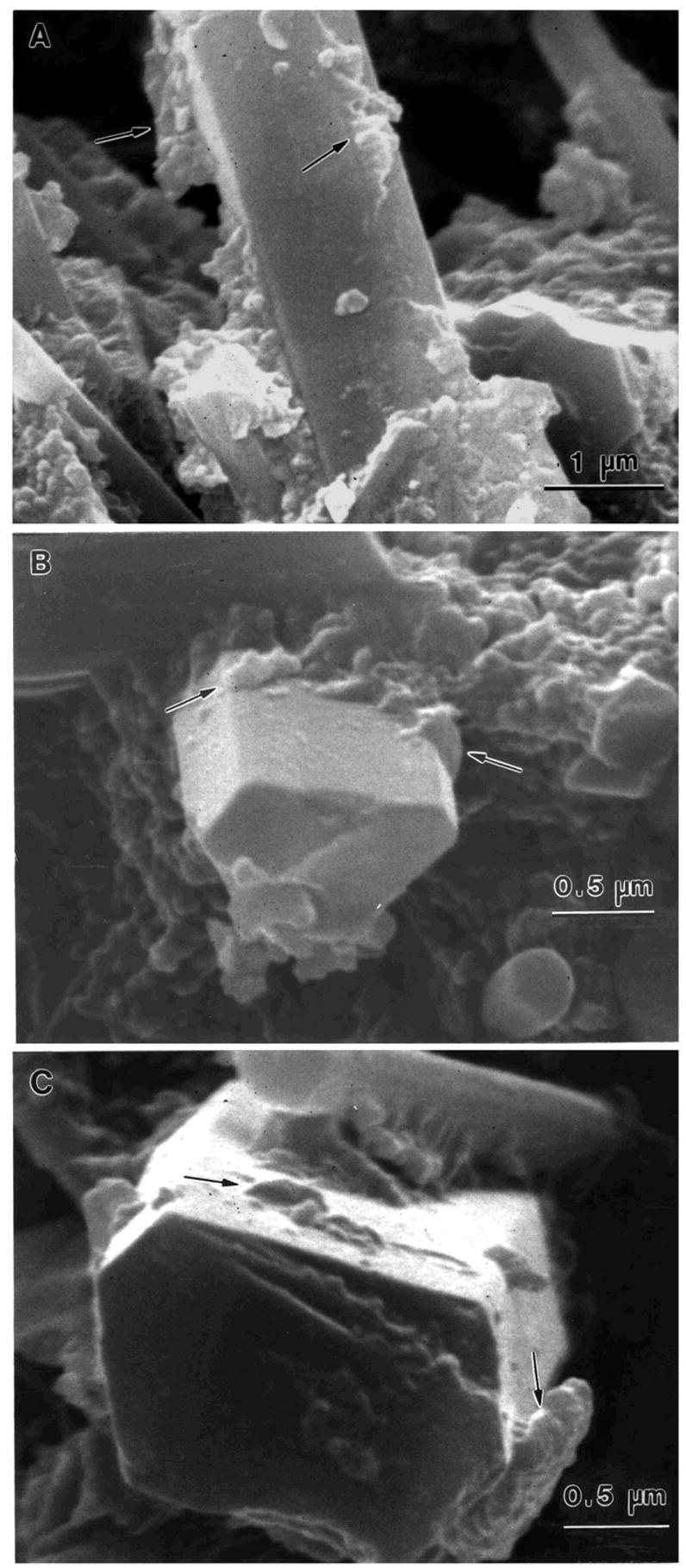
SEM of whisker pullout on fracture surfaces of Si3N4 composite: (A) 1 d, (B) 400 d, and (C) 730 d of water aging, with shorter whisker pullout at 400 d and 730 d. Polymer remnants were observed on the pulled-out whiskers (arrows), indicating good whisker-polymer matrix bonding even after 730 d of water aging from the paper Xu HHK (2003). Long-term water aging of whisker-reinforced polymer-matrix composites. J Dent Res, 82:48–52.
Garoushi et al. (2007) employed short fibers (3 mm in length and ~15–25 mm in diameter) in a restorative resin composition and found a doubling of the flexure strength, a slight increase in the flexure modulus, and a 3 time increase in the flexure toughness compared to a commercial particle filled composite (Z250, 3M-ESPE, St. Paul, MN, USA). The increase in material properties is attributed to the ability of the fibers to withstand a higher stress and to stop and/or deflect the crack propagation. Nano fibrillar silicate has also been used as a reinforcement to conventional Bis-GMA/TEGDMA composite resins (Tian et al., 2007). The nano fiber filler was added in small amounts, 1-2-5% by weight, which resulted in an increase in the mechanical properties of flexure strength, elastic modulus, and work of fracture, but over 7.5% by weight showed no improvement and an actual decrease from the starting composite material. This reinforcing at the low weight concentrations is attributed to the uniform distribution of the nano fiber and the decrease in clumping of the fibers. The highly dispersed fibers may deflect the micro cracks, whereas, the agglomerates seem to function as stress concentrators, weakening the composite.
Silanes such as methacryloxypropyltrimethoxysilane (MPS) and other silanes are used to strengthen dental composites by forming a covalent bond between the glass filler particles and the various methylmethacrylate based compounds comprising the resin (Liu et al., 2001). Control of silanation will affect the wear properties of dental composite (Condon and Ferracane, 1997), presumably by modulating the silane bonding at the organic-inorganic interface of the filler-resin composite. For example, control of moisture in silanation of silica is also known to control the extent of silane polymerization, which competes with the initial surface binding (Ulman, 1996). One end of MPS will form Si-O-Si bonds with hydroxy groups on silica and other oxides of the filler particles. Hydrolysis of the Si-O-Si bond by water is a well-known phenomenon, which is expected to weaken the polymer-filler filler interface during aging (Xiao et al., 1998; Lateef et al., 2002). The other end of the silane also strengthens the composite by forming covalent bonds with the resin matrix (Liu et al., 2001). Hydrolysis of the ester linkage that serves as the silane-resin bond is also feasible and therefore another potential, but largely unexplored, degradation mechanism of dental composites.
The effect of silanization is to move the fracture of the dental composite from between the filler particles to the resin composite adjacent to the filler particles (Jandt, 1999; Lin et al., 2000, Debnath et al., 2004). The silanization also results in an increase in the mechanical properties of the composite. The bonding of glass to resin through silane agents (formation of oxane bonds) other than simple chemical reactivity is best explained by interdiffusion and interpolymer network formation in the interphase region (Plueddemann, 1988). In dental composites, studies on the effects of interphase on the overall properties have been limited.
Cracks and Flaws and Cyclic Loading
During exposure to various environments, dental composites are subjected to material property changes due to degradation and aging. These changes are due to: (a) chemical break down by hydrolysis (b) chemical break down by stress induced effects associated with swelling and applied stress, (c) chemical composition changes by leaching, (d) precipitation and swelling phenomena to produce voids and cracks, leaching the interface, and (e) loss of strength due to corrosion (McKinney and Wu, 1982; Draughn, 1979; Lloyd, 1984; Roulet, 1987; Drummond, 1989). All of these processes may lead to nucleation and growth of microcracks. These cracks, however, are not always normal to the applied load.
In many instances, fracture and failure of dental composites occur from a surface or subsurface crack or a flaw, which is oriented at an angle with respect to that applied load (mode I and mode II loading) (Ferracane et al., 1992). Fracture initiation for bar specimens results from cracks, voids, inclusions or other defects most likely resulting from the processing of the dental composite or the fabrication (polishing, grinding) of the specimens (Rodrigues et al., 2007). Thus upon loading, normal (mode I) and shear (mode II) loads drive the crack originating from such defects. Inclined flaws or cracks are also observed in wear loading in many composite materials. Baran et al. (1994, 1998) using an indenter to study wear, observed, that although the majority of surface cracks ran originally orthogonal to the surface, they changed direction to run 20 to 30° to the horizon in the direction of the indenter movement. In fact, it is believed that fracture characteristics of any composite material could be realistically investigated under combined fracture modes because the highly heterogeneous materials' microstructures give rise to curved crack paths. In addition, it offers a more realistic approach to fatigue and fracture of dental restorations since it is more likely that a flaw is at an angle with the force of mastication.
The traditional approach to testing brittle materials has been monotonic loading (Guiberteau et al., 1993). These materials are polycrystalline and have enhanced toughening from crack bridging from various monophase and multi-phase components. Many materials, especially those in the oral cavity, are subject to concentrated contact stresses at the microstructural level, rather than macroscopically distributed stresses as represented in conventional crack propagation and strength tests (Lawn and Wilshaw, 1975). Lawn and his group have proposed a new procedure for studying fatigue properties of brittle ceramics using an spherical indenter. The initial load of stress fields is purely elastic and beyond a critical load the material undergoes permanent deformation and/or fracture (Hertz, 1896; Lawn and Wilshaw, 1975). For a well-behaved, highly brittle material (glasses and ultra-fine polycrystalline ceramics), a well define, cone shaped crack (Hertzian fracture), occurs around the contact circle and spreads downward and outward into the material. In less brittle material, the material deforms plastically beyond the elastic limit and the cracks form radial or lateral geometries.
Cyclic loading of materials has gained increased importance as it has been realized that a static evaluation of a material may not be as important as cyclic fatigue values for materials utilized in the oral cavity. Numerous dental restorative materials have shown susceptibility to cyclic loading: ceramics, glass ionomers, fiber reinforced resins, and composites (Drummond, 1989; Drummond et al., 1995, 1998; Braem et al., 1994, 1995; Bapna et al., 2002). A recent study on flexural fatigue behavior concluded that static strengths do not correlate with fatigue values and also that contact fatigue is different than flexural fatigue (McCabe et al, 2000).
Flexural cyclic loading results in lower observed flexure strengths (30–50%) than static testing and is considered more sensitive for evaluating the performance of clinical materials (Yoshida et al., 2003; Lohbauer et al., 2006). Papadogiannis et al. (2006) using a dynamic mechanical technique to relate to the viscoelastic properties of dental composites concluded that fatigue strength is not only related to the type of filler, but also the silanization of the fillers and the resin matrix play a role.
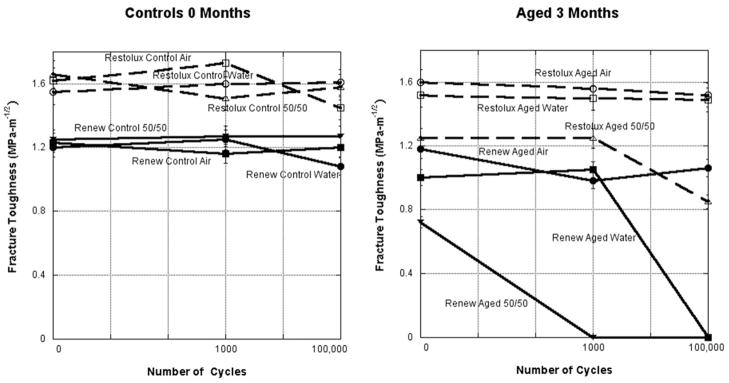
Mixed-mode loading conditions were used to investigate the effects of cyclic loading and environmental aging on three dental resin composites with different filler compositions: a fiber filler, a hybrid filler, and a microfill (Ravindranath, et al., 2007). Diametral (Brazilian) disk specimens 25 mm in diameter and 2 mm in thickness were used in this study. The specimens were aged for 4 months in air, water, artificial saliva, and a 50/50 (by volume) mixture of ethanol and water at room temperature in sealed polyethylene containers. Both unaged and aged specimens were subjected to cyclic loading at a frequency of 5 Hz with sinusoidal loads cycling for 1, 1000, and 100,000 cycles at a load level 60% of the fracture load for non-cycled specimens. Test results showed that aging in a 50/50 alcohol–water mixture lowered the fracture toughness of dental resin composite, which was further reduced by cyclic loading, Figure 7. The loads at failure were used as input into a finite element model. After obtaining the stress field in the specimens by the finite element method, the mixed-mode stress intensity factors were calculated using an interaction energy integral method. Good agreement was obtained between the fracture envelope predicted by the maximum tensile stress criterion and the experimental fracture toughness data. Hence, it can be concluded that it is only necessary to characterize the mode I fracture toughness to fully characterize the mixed-mode behavior of dental resin composites. Scherrer et al. (2000) found no difference in the KIC of composites using diametral specimens following aging for 12 months, however the specimens were not subjected to cyclic loading. Tikare and Choi (1993) found a rage of (0.7–2.0) for KIC with different fracture behavior for different fracture initiating flaws. They stated that indentation methods of crack formation are not able to obtain pure mode II fracture data from flexure loading conditions and the fracture toughness is dependent on the microstructure.
Figure 7.
Fracture toughness versus number of cycles completed for Renew and Restolux using a Diametral (Brazilian) disc specimen of controls and aged specimens for 3 months. Cyclic loading had little effect on the fracture toughness for the control specimens, but in conjunction with aging had a major effect for the aged specimens. Aging in the 50/50 mixture of ethanol and distilled water caused the greatest decrease in the fracture toughness.
Two different modes of fatigue loading, contact and flexure, were investigated to determine the effect on the flexure strength of a fiber filled dental composite (Al-Turki et al., 2007). The composite was Restolux (a fiber filled composite) tested under cyclic loading ranges of 30–50 N, 60–80 N, and 90–110 N for contact loading and 20–40 N and 40–60 N for the flexure loading. Statistical analysis indicated a significantly lower flexure strength for the specimens flexure loaded versus contact loaded. For the flexure-loaded specimens, the number of cycles had no significant effect, but the aging, load, and the media were all significant. For the contact loaded specimens, a significant effect was observed for the media, aging, and cycles completed, but no effect for the different cycling loads. The decrease in flexure strength from flexure loading was mainly affected by the aging media, whereas, the decrease from contact loading was attributed mainly to the number of cycles.
Multiaxial Compression and 3D Tomography
Since surface fracture analysis only shows the region where the final failure occurred, a goal has always been to determine the degree of cracking in three dimensions in dental composites under various loading and environmental conditions. An initial attempt used 3-point bend bars (Drummond et al., 2005) and then hourglass specimens (Drummond et al., 2006). However, these method possessed several shortcomings; the image contrasts varied between samples due to reconstruction artifacts; there was difficulty in controlling the applied load; the load fluctuated while the sample was being examined; and occasionally the specimen splintered during testing.
To overcome these issues of using uniaxial compression, a method of multiaxial compression loading was employed. Dental materials are commonly tested for strength using a bar specimen in a three or four-point bend configuration. However, dental composites when placed within natural teeth are subject to radial as well as axial stresses, thereby introducing a three-dimensional (3D) compressive stress state. Therefore it is of interest to experimentally examine these materials subjected to multiaxial compression loads, rather than uniaxial compression. Using a method described by Ma and Ravi-Chandar (2000) to constitutively characterize materials under confined compression, it is possible to determine the principle components of stress and strain. The loading configuration that composite specimens experience in this method of multiaxial testing better replicates the loading that restorations experience in the oral cavity, and may yield greater insight into the failure mechanisms of dental composites.
This method also allows for better control of compressive load conditions: with the dental composite fabricated as cylindrical specimens 3.7 mm in height and 2.4 mm in diameter in aluminum ring molds with a strain gage placed on the outer wall of the aluminum (Al) confining ring to record hoop strain during compression. Assuming plane stress conditions for a thick-walled cylinder subject to internal pressure, the confining stress can be calculated at the interface between the inner wall of the ring and the sample during elastic deformation of the ring (Kotche et al., 2008).
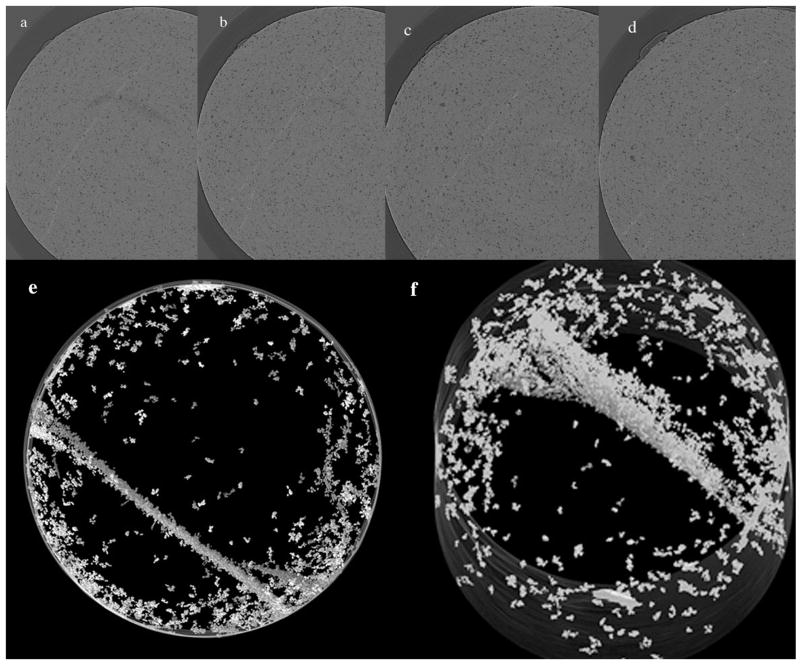
Following mechanical loading, tomographic data is generated for each specimen using the microtomography system at beamline 2-BM of the Advanced Photon Source (APS) at Argonne National Laboratory. Preliminary results are extremely encouraging, yielding clean images with few artifacts, Figure 8a–d. Multiaxial compression also eliminates any concern for splintering of the specimen during mechanical loading since the sample is confined. Furthermore, multiaxially loaded specimens are more consistent between specimens, permitting reasonable baseline thresholds to be established for an entire sample population, thus allowing a more reliable, automated method of processing the tomographic images and allowing more accurate reconstructions of the 3D cracking pattern, Figure 8e–f. Data from this multiaxial approach is given in Table 1. The preliminary data investigated, Renew, a hybrid microfilled composite. The objective of this study was to quantify the crack edge area/total volume of multiaxial confined compression specimens of dental composite. The specimens were controls and specimens subjected to 6 and 12% strain, two different loading ring configurations, and cyclic loading at 400N for 100,000 cycles. The multiaxial compression specimens were contained within Al rings, similar to a class I occlusal restoration and loaded via stainless steel plungers on the composite only. The axial loaded is known and the constrained load is measured via a strain gage on the exterior of the Al ring. The Al rings varied with respect to the inner diameter versus the outer diameter, a variable known as λ, 2.0 and 2.7. The higher the λ value, the higher the load on the confined composite. The cracks developed during loading were quantified using image analysis of the data sets obtained at the APS. This preliminary 3D image analysis indicates as the loading conditions are intensified, from controls to confined compression at 6 and 12% and an increase in λ from 2.0 to 2.7, the amount of cracking observed in the composite also increases. No effect was observed for the cyclic loading under these conditions. This data would indicate that class I occlusal restorations are subject to increased cracking depending on the thickness of the surrounding tooth structure. Continuation of this technique will hopefully allow comparison of the work of strain (the area under the stress strain curve during loading of the multiaxial confined compression specimen) with the amount of cracking measured in the 3D tomography analysis.
Figure 8.
Reconstructions (a–d) of the images taken at the Advanced Photon Source of a Renew specimen subjected to multiaxial compression at a strain level of 12% demonstrating the crack pattern after loading. The 3D reconstruction of the same Renew specimen in (a–d) indicating the complexity and distribution of the cracking within the specimen with (e) looking down the axial axis and (f) off axis.
Table 1.
Comparison of Different Loading Conditions on the Crack Edge Area (CA) to Total Volume (V) of Multiaxial Confined Compression Specimens of Renew Dental Composite
| Testing Conditions | N | Mean(SD) %CA/V |
|---|---|---|
| Control | 2 | 2.5(2.2) |
| 6% strain, λ =2.0 | 11 | 4.2(2.9) |
| 12% strain, λ =2.0 | 8 | 5.8(4.1) |
| 12% strain, λ =2.0, load cycled 400N | 7 | 4.9(7.3) |
| 12% stain, λ =2.7 | 3 | 36.7(10.5) |
Ultimately, the key evaluation of posterior resin composite restoratives is clinical survival. Clinical data has suggested that microfills might be more susceptible to bulk fracture, but the sample size was extremely small (Collins et al., 1998). Studies have indicated a range of failures attributed to bulk fracture of the composite from very low (Mjör and Moorhed, 1998), to 7% (Raskin et al., 1999), and 32% (Wu et al., 1984). Many studies exist that indicate that composites on average have a failure rate which includes all sources of failure i.e., secondary decay, fracture of the composite or cusp, esthetics, wear etc. from, 0–9% after 1 year (Hickel and Manhart, 2001), 8% for packable composites after 1.5 years (Brackett et al., 2007), 7% after 2 years (Scheibenbogen-Fuchsbrunner et al., 1999), 7–14% after 2 years (Krämer et al., 2005), for fiber filled composites 2–5% after 2 years and 13–25% after 6 years (van Dijken and Sunnegårdh-Grönberg, 2006), after 3.5 years 19% for a packable composite and 8% for a hybrid composite(Poon et al., 2005), 14% after 8 years (Collins et al., 1998), and 35% after 17 years (da Rosa Rodolpho et al., 2006), Cusp fracture as a source of failure is essentially the same for either amalgam or composite restorations (Wahl et al., 2004). The incidence of cusp fracture increases with the patient’s age and the number of cusp involved in the restoration. However the main reason for replacement of dental restorations is secondary decay (Burke et al., 2001). The study by Brunthaler et al. (2003) of posterior composite restorations summarized data published between 1996 and 2002 and concluded that failure of composite restorations between 0–5 years was a restoration issue (technique or material selection) followed by secondary decay and from 6–17 years secondary caries was the reason to replace the restoration. As expected the longer a restoration is in use, the higher the failure rate, but the failure of resin composite restorative materials is far more complicated than just the material properties. As with all dental restorative materials, the proper technique, the appropriate materials, and proper patient selection usually ensure a successful clinical restoration.
Acknowledgments
Assistance and thanks for this paper goes to: Miiri Kotche, Kang Sun, Lulwa Al-Turki, Lihong Lin, Francesco DeCarlo, Manshui Zhou, Luke Hanley, Donglei Zhao, and John Botsis. The research in part was supported by NIDCR grant HHS DE07979 and the dental composites provided by 3M-ESPE, Bisco Dental Products, and Lee Pharmaceuticals. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DE-AC02-06CH11357.
References
- Al-Turki LI, Drummond JL, Agojci M, Gosz M, Tyrus JM, Lin L. Contact versus flexure fatigue of a fiber-filled composite. Dental Materials. 2007;23(5):648–653. doi: 10.1016/j.dental.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Annual Book of ASTM Standards, Part 10. 1990. pp. C-1161–90. [Google Scholar]
- ASM Handbook. Fatigue and Fracture. Vol. 19. ASM International, The Materials Information Society; 1996. [Google Scholar]
- Standard Test Methods for the Determination of Fracture Toughness of Advanced Ceramics at Ambient Temperature ASTM PS070–97. 1997 [Google Scholar]
- Atkinson C, Smelser RE, Sanchez J. Combined Mode Fracture via the Cracked Brazilian Disk Test. Inter J Frac. 1982;18(4):279–291. [Google Scholar]
- Awaji H, Sato S. Combined Mode Fracture Toughness Measurement by the Disk Test. J Eng Mater Tech. 1978;100:175–182. [Google Scholar]
- Bapna MS, Gadia CM, Drummond JL. Effect of aging and cyclic loading on the mechanical properties of glass ionomer cements. Eur J Oral Sci. 2002;110:330–334. doi: 10.1034/j.1600-0722.2002.21225.x. [DOI] [PubMed] [Google Scholar]
- Baran G, Sadeghipour K, Jayaraman S, Silage D, Paul D, Boberick K. Crack propagation directions in unfilled resins. J Dent Res. 1998;77(11):1864–1873. doi: 10.1177/00220345980770110201. [DOI] [PubMed] [Google Scholar]
- Baran G, Shin W, Abbas A, Wunder S. Indentation cracking of composite matrix materials. J Dent Res. 1994;73(8):1450–1456. doi: 10.1177/00220345940730080901. [DOI] [PubMed] [Google Scholar]
- Bernardo M, Luis H, Martin MD, Leroux BG, Rue T, Leitão J, DeRouen TA. Survival and reasons for failure of amalgam versus composite posterior restorations placed in a randomized clinical trail. JADA. 2007;138:775–783. doi: 10.14219/jada.archive.2007.0265. [DOI] [PubMed] [Google Scholar]
- Braem MJA, Davidson CL, Lambrechts P, Vanherle G. In vitro fatigue behavior of restorative composites and glass ionomers. J Biomed Mater Res. 1994;28:1397–1402. [Google Scholar]
- Braem MJA, Lambrechts P, Gladys S, Vanherle G. In vitro flexural fatigue limits of dental composites. Dent Mater Res. 1995;11:137–141. doi: 10.1002/jbm.820281203. [DOI] [PubMed] [Google Scholar]
- Brackett WW, Browing WD, Brackett MG, Callan RS, Blalock JS. Effect of restoration size on the clinical performance of posterior “packable” resin composites over 18 months. Oper Dent. 2007;32(3):212–216. doi: 10.2341/06-87. [DOI] [PubMed] [Google Scholar]
- Brunthaler A, König F, Lucas T, Sperr W, Schedle A. Longevity of direct composite restorations in posterior teeth. Clin Oral Invest. 2003;7:63–70. doi: 10.1007/s00784-003-0206-7. [DOI] [PubMed] [Google Scholar]
- Burke FLT, Wilson NHF, Cheung SW, Mjör IA. Influence of patient factors on age of restorations at failure and reasons for their placement and replacement. J Dent. 2001;29:317–324. doi: 10.1016/s0300-5712(01)00022-7. [DOI] [PubMed] [Google Scholar]
- Collins CJ, Bryant RW, Hodge K-LV. A clinical evaluation of posterior composite restorations: 8-year findings. J Dent. 1998;26(4):311–317. doi: 10.1016/s0300-5712(97)00019-5. [DOI] [PubMed] [Google Scholar]
- Condon JR, Ferracane JL. In vitro wear of composite with varied cure, filler level and filler treatment. J Dent Res. 1997;76:1405–1411. doi: 10.1177/00220345970760071101. [DOI] [PubMed] [Google Scholar]
- da Rosa Rodolpho PA, Cenci MS, Donassollo TA, Loguércio AD, Demarco FF. A clinical evaluation of posterior composite restorations: 17-year findings. J Dent. 2006;34:427–435. doi: 10.1016/j.jdent.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Debnath S, Ranade R, Wunder SL, McCool J, Boberick K, Baran G. Interface effects on mechanical properties of particle-reinforced composites. Dent Mater. 2004;20:677–686. doi: 10.1016/j.dental.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Domingo C, Arcís RW, López-Macipe A, Osorio R, Rodríguez-Clemente R, Murtra J, Fanovich MA, Toledano M. Dental composites reinforced with hydroxyapatite: Mechanical behavior and absorption/elution characteristics. J Biomed Mater Res. 2001;56:297–305. doi: 10.1002/1097-4636(200108)56:2<297::aid-jbm1098>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Draughn RA. Compressive fatigue limits of composite restorative materials. J Dent Res. 1979;58(3):1093–1096. doi: 10.1177/00220345790580031101. [DOI] [PubMed] [Google Scholar]
- Drummond JL. Cyclic fatigue of composite restorative materials. J Oral Rehab. 1989;(5):509–520. doi: 10.1111/j.1365-2842.1989.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Drummond JL, Botsis J, Zhao D, Samyn J. Fracture properties of aged and postprocessed dental composites. Eur J Oral Sci. 1998;106:661–666. doi: 10.1046/j.0909-8836.1998.eos106208.x. [DOI] [PubMed] [Google Scholar]
- Drummond JL, Botsis J, Zhao D. Encyclopedia of Biomaterials and Bioengineering. Vol. 56. Marcel Dekker, Inc; 1995. Fracture mechanics of dental composites; pp. 2813–2844. chapter. [Google Scholar]
- Drummond JL, De Carlo F, Super BJ. Three-dimensional tomography of composite fracture surfaces. J Biomed Mater Res B:Appl Biomater. 2005;74B(2):669–675. doi: 10.1002/jbm.b.30298. [DOI] [PubMed] [Google Scholar]
- Drummond JL, Sun K, Bedran-Russo A, Koin P, Kotche M, Super BJ. Tomography of dental composites. SPIE. 2006;6318:63182B-1–B-8. [Google Scholar]
- Ferracane JL. Current trends in dental composites. Crit Rev Oral Biol Med. 1995;6:302–318. doi: 10.1177/10454411950060040301. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Antonio RC, Matsumoto H. Variables affecting the fracture toughness of dental composites. J Dent Res. 1987;66(6):1140–1145. doi: 10.1177/00220345870660060901. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Berge HX. Fracture toughness of experimental dental composites aged in ethanol. J Dent Res. 1995;74(7):1418–1423. doi: 10.1177/00220345950740071501. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Berge HX, Condon JR. Aging of composites with varied DC, Vf and filler coupling. J Dent Res. 1995;74(Special Issue 630):90. [Google Scholar]
- Ferracane JL, Condon JR. Post-cure heat treatments of composites: properties and fractography. Dent Mater. 1992;8:290–295. doi: 10.1016/0109-5641(92)90102-i. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Condon JR, Mitchem JC. Evaluation of subsurface defects created during the finishing of composites. J Dent Res. 1992;71(9):1628–1632. doi: 10.1177/00220345920710091601. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Hopkin JK, Condon JR. Properties of heat-treated composites after aging in water. Dent Mater. 1995;11:354–358. doi: 10.1016/0109-5641(95)80034-4. [DOI] [PubMed] [Google Scholar]
- Ferracane JL, Marker VA. Solvent degradation and reduced fracture toughness in aged composites. J Dent Res. 1992;71(1):13–19. doi: 10.1177/00220345920710010101. [DOI] [PubMed] [Google Scholar]
- Garoushi S, Vallittu PK, Lassila LVJ. Short glass fiber reinforced restorative composite resin with semi-inter penetrating polymer network matrix. Dent Mater. 2007;23:1356–1362. doi: 10.1016/j.dental.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Goldman M. Fracture properties of composite and glass ionomer dental restorative materials. J Biomed Mater Res. 1985;19:771–783. doi: 10.1002/jbm.820190705. [DOI] [PubMed] [Google Scholar]
- Guiberteau F, Padture NP, Cai H, Lawn BR. Indentation Fatigue: A simple cyclic Hertzian test for measuring damage accumulation in polycrystalline ceramics. Phil Mag A. 1993;68(5):1003–1016. [Google Scholar]
- Hickel R, Manhart J. Longevity of restorations in posterior teeth and reasons for failure. J Adhes Dent. 2001;3(1):45–64. [PubMed] [Google Scholar]
- Huang Y, Liu C, Stout MG. A Brazilian Disk for Measuring Fracture Toughness of Orthotropic Materials. Acta Mater. 1996;44(3):1223–1232. [Google Scholar]
- Hertz HH. Hertz’s Miscellaneous Papers. London: Macmillan; 1896. chapters 5 and 6 [Google Scholar]
- Indrani DJ, Cook WD, Televantos F, Tyas MJ, Harcourt JK. Fracture toughness of water-aged resin composite restorative materials. Dent Mater. 1995;11:201–207. doi: 10.1016/0109-5641(95)80019-0. [DOI] [PubMed] [Google Scholar]
- Jandt KD. Structure of microfilled dental composite fractured surfaces. Probe Microscopy. 1999;1:323–331. [Google Scholar]
- Jones DW, Rizkalla AS, Sutow EJ, Hall GC. Elastic moduli of wet and dry experimental composite materials. J Dent Res. 1992;71(Abstract 666):599. [Google Scholar]
- Kanninen MF, Popelar CH. Advanced Fracture Mechanics. 3–92. Oxford University Press; New York: 1985. pp. 138–191. [Google Scholar]
- Kovarik RE, Ergle JW. Effects of long term aging on fracture toughness of composites. J Dent Res. 1992;71(Abstract 1662):313. [Google Scholar]
- Kotche M, Drummond JL, Sun K, Vural M, De Carlo F. Multiaxial Analysis of Dental Composite Materials. J Biomed Mater Res B:Appl Biomater. 2008 doi: 10.1002/jbm.b.31111. in press. [DOI] [PubMed] [Google Scholar]
- Kramer N, Garcia-Godoy F, Frankenberger R. Evaluation of resin composite materials. Part II: in vivo investigations. Am J Dent. 2005;18(2):75–81. [PubMed] [Google Scholar]
- Lateef SS, Boateng S, Hartman TJ, Crot CA, Russell B, Hanley L. GRGDSP peptide bound silicone membranes withstand mechanical flexing in vitro and display enhanced fibroblast adhesion. Biomater. 2002;23:3159–3168. doi: 10.1016/s0142-9612(02)00062-5. [DOI] [PubMed] [Google Scholar]
- Lawn BR, Wilshaw TR. Indentation Fracture: principles and applications. J Mater Sci. 1975;10:1049–1081. [Google Scholar]
- Lin C-T, Lee S-Y, Keh E-S, Dong D-R, Huang H-M, Shih Y-H. Influence of silanization and filler fraction on aged dental composites. J Oral Rehab. 2000;27:919–926. doi: 10.1046/j.1365-2842.2000.00573.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Ding J, Chambers DE, Debnath S, Wunder SL, Baran GR. Filler-coupling agent-matrix interactions in silica/polymethylmethacrylate composites. J Biomed Mater Res. 2001:384–393. doi: 10.1002/1097-4636(20011205)57:3<384::aid-jbm1181>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Lloyd CH. The fracture toughness of dental composites, II. J Oral Rehab. 1982;9:133–138. doi: 10.1111/j.1365-2842.1982.tb00543.x. [DOI] [PubMed] [Google Scholar]
- Lloyd CH. The fracture toughness of dental composites. J Oral Rehab. 1984;11:393–398. doi: 10.1111/j.1365-2842.1984.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Lohbauer U, Frankenberger R, Krämer N, Petschelt A. Strength and fatigue performance versus filler fraction of different types of direct dental restoratives. J Biomed Mater Res Part B: Appl Biomater. 2006;76B:114–120. doi: 10.1002/jbm.b.30338. [DOI] [PubMed] [Google Scholar]
- Ma Z, Ravi-Chandar K. Confined compression: A stable homogeneous constitutive characterization. Exper Mech. 2000;40(1):38–45. [Google Scholar]
- Manhart J, Kunzelmann K-H, Chen HY, Hickel R. Mechanical properties of new composite restorative materials. J Biomed Mater Res(Appl Biomater) 2000;53:353–361. doi: 10.1002/1097-4636(2000)53:4<353::aid-jbm9>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- McCabe JF, Wang Y, Braem MJA. Surface contact fatigue and flexural fatigue of dental restorative materials. J Biomed Mater Res. 2000;50:375–380. doi: 10.1002/(sici)1097-4636(20000605)50:3<375::aid-jbm11>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- McKinney JE, Wu W. Relationship between subsurface damage and wear of dental restorative composites. J Dent Res. 1982;61(9):1083–1088. doi: 10.1177/00220345820610091101. [DOI] [PubMed] [Google Scholar]
- Mjör IA, Moorhed JE. Selection of restorative materials, reasons for replacement, and longevity of restorations in Florida. J Am Coll Dent. 1998;65(3):27–33. [PubMed] [Google Scholar]
- Musto P, Ragosta G, Scarinza G, Mascia L. Probing the molecular interactions in the diffusion of water through epoxy and epoxy-bismaleimide networks. J Polym Sci Part B: Polym Phys. 2002;40:922–938. [Google Scholar]
- Nicholson JW. Adhesive dental materials and their durability. Inter J Adhes Adhes. 2000;201:11–16. [Google Scholar]
- O’Brien WJ. Dental Materials and Their Selection. 3. Quintessence Publishing Company, Inc; 2002. Polymeric Restorative Materials; pp. 113–131. [Google Scholar]
- Oshida Y, Hashem A, Elsalawy R. Some mechanistic observation on water-deteriorated dental composite resins. Bio-Med Mater Eng. 1995;5(2):93–115. [PubMed] [Google Scholar]
- Papadogiannis Y, Lakes RS, Palaghias G, Helvatjoglu-Antoniades M, Papadogiannis D. Fatigue of packable dental composites. Dent Mater. 2007;23(2):235–242. doi: 10.1016/j.dental.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Pilliar RM, Smith DC, Maric B. Fracture toughness of dental composite determined using the short-rod fracture toughness test. J Dent Res. 1986;65(11):1308–1314. doi: 10.1177/00220345860650110501. [DOI] [PubMed] [Google Scholar]
- Plueddemann EP. Present Status and Research Needs in Silane Coupling. In: Ishida H, editor. Interfaces in Polymer, Ceramic, and Metal Matrix Composites. Elsevier; New York: 1988. pp. 17–33. [Google Scholar]
- Poon CM, Smales RJ, Yip H-k. Clinical evaluation of packable and conventional hybrid posterior resin-based composites. JADA. 2005;136:1533–1540. doi: 10.14219/jada.archive.2005.0083. [DOI] [PubMed] [Google Scholar]
- Ravindranath V, Gosz M, DeSantiago E, Drummond JL, Mostovoy S. Effect of cyclic loading and environmental aging on the fracture toughness of dental composites. J Biomed Mater Res, Part B, Appl Biomater. 2007;80B:226–235. doi: 10.1002/jbm.b.30588. [DOI] [PubMed] [Google Scholar]
- Raskin A, Michote-Theall B, Verven J, Wilson NHF. Clinical evaluation of a posterior composite 10-year report. J Dent. 1999;27:13–19. doi: 10.1016/s0300-5712(98)00026-8. [DOI] [PubMed] [Google Scholar]
- Rodrigues SA, Ferracane JL, Della Bons A. Flexural strength and Weibull analysis of a microhydrid and a nanofill composite evaluated by 3- and 4-point bending tests. Dent Mater. 2007 doi: 10.1016/j.dental.2007.05.013. [DOI] [PubMed] [Google Scholar]
- Roulet JF. Degradation of Dental Polymers. Karger, Basel; Switzerland: 1987. pp. 1–228. [Google Scholar]
- Sakaguchi RL, Cross M, Douglas WH. A simple model of crack propagation in dental restorations. Dent Mater. 1992;8:131–136. doi: 10.1016/0109-5641(92)90068-n. [DOI] [PubMed] [Google Scholar]
- Sanchez J. Master’s Thesis. University of Pittsburgh; 1979. Application of the Disk Test to Mode I-II Fracture Analysis. [Google Scholar]
- Scheibenbogen-Fuchsbrunner A, Manhart J, Kremers L, Kunzelmann K-H, Hickel R. Two-year clinical evaluation of direct and indirect composite restorations in posterior teeth. J Prosthet Dent. 1999;82:391–397. doi: 10.1016/s0022-3913(99)70025-9. [DOI] [PubMed] [Google Scholar]
- Scherrer SS, Botsis J, Studer M, Pini M, Wiskott HWA, Besler UC. Fracture toughness of aged dental composites in combined mode I and mode II loading. J Biomed Mater Res (Appl Biomater) 2000;53:362–370. doi: 10.1002/1097-4636(2000)53:4<362::aid-jbm10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Ségard E, Benmedakhene S, Laksimi A, Laï D. Damage analysis and fibre-matrix effect in polypropylene reinforced by short glass fibres above glass transition temperature. Comp Struct. 2003;60:67–72. [Google Scholar]
- Shetty DK, Rosenfield AR, Duckworth WH. Mixed-Mode Fracture of Ceramics in Diametral Compression. J Am Cer Soc. 1986;69(6):437–443. [Google Scholar]
- Söderholm K-JM, Roberts MJ. Influence of water exposure on the tensile strength of composites. J Dent Res. 1990;69(12):1812–1816. doi: 10.1177/00220345900690120501. [DOI] [PubMed] [Google Scholar]
- Soncini JA, Maserejian NM, Trachtenberg GH, Tavares M, Hayes C. The longevity of amalgam versus compomer/composite restorations in posterior primary and permanent teeth. JADA. 2007;138:763–772. doi: 10.14219/jada.archive.2007.0264. [DOI] [PubMed] [Google Scholar]
- Takeshige F, Kawakami Y, Hayashi M, Ebisu S. Fatigue behavior of resin composites in aqueous environments. Dent Mater. 2007;23(7):893–899. doi: 10.1016/j.dental.2006.06.031. [DOI] [PubMed] [Google Scholar]
- Tian M, Gao Y, Liu Y, Liao Y, Hedin NE, Fong H. Fabrication and evaluation of Bis-GMA/TEGDMA dental resins/composites containing nano fibrillar silicate. Dent Mater. 2007 doi: 10.1016/j.dental.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tikare V, Choi SR. Combined mode I and mode II fracture of monolithic ceramics. J Am Cer Soc. 1993;76(9):2265–2272. [Google Scholar]
- Ulman A. Formation and structure of self-assembled monolayers. Chem Rev. 1996;96:1533–1554. doi: 10.1021/cr9502357. [DOI] [PubMed] [Google Scholar]
- van Dijken JWV, Sunnegårdh-Grönberg K. Fiber-reinforced packable resin composites in class II cavities. J Dent. 2006;34:763–769. doi: 10.1016/j.jdent.2006.02.003. [DOI] [PubMed] [Google Scholar]
- Wahl MJ, Schmitt MM, Overton DA, Gordon MK. Prevalence of cusp fractures in teeth with amalgam and with resin-based composite. JADA. 2004;135:1127–1132. doi: 10.14219/jada.archive.2004.0371. [DOI] [PubMed] [Google Scholar]
- Wright KHR, Burton AW. Wear of dental tissues and restorative materials. In: Dowson D, Godet M, Taylor M, editors. The Wear of Non-Metallic Materials. Mechanical Engineering Publication; London: 1976. pp. 116–126. [Google Scholar]
- Wu W, Toth EE, Moffa JF, Ellison JA. Subsurface damage layer of in vivo worn dental composite restorations. J Dent Res. 1984;63(5):675–680. doi: 10.1177/00220345840630051401. [DOI] [PubMed] [Google Scholar]
- Xiao S-J, Textor M, Spencer ND, Sigrist H. Covalent attachment of cell-adhesive (Arg-Gly-Asp)-containing peptides to titanium surfaces. Lang. 1998;14:5507–5516. doi: 10.1023/a:1018501804943. [DOI] [PubMed] [Google Scholar]
- Xu HHK. Dental composite resins containing silica-fused ceramic single-crystalline whiskers with various filler levels. J Dent Res. 1999;78(7):1304–1311. doi: 10.1177/00220345990780070401. [DOI] [PubMed] [Google Scholar]
- Xu HHK. Whisker-reinforced heat-cured dental resin composites: effects of filler level and hear-cure temperature and time. J Dent Res. 2000;79(6):1392–1397. doi: 10.1177/00220345000790060701. [DOI] [PubMed] [Google Scholar]
- Xu HHK. Long-term water-water-aging of whisker-reinforced polymer-matrix composites. J Dent Res. 2003;82(1):48–52. doi: 10.1177/154405910308200111. [DOI] [PubMed] [Google Scholar]
- Xu HHK, Eichmiller FC, Smith DT, Quinn JB, Schumacher GE, Giuseppetti AA, Antonucci JM. Effect of thermal cycling on whisker-reinforced dental resin composites. J Mater Sci. 2002a;13:875–883. doi: 10.1023/a:1016504530133. [DOI] [PubMed] [Google Scholar]
- Xu HHK, Quinn JB, Giuseppetti AA. Wear and mechanical properties of nano-silica-fused whisker composites. J Dent Res. 2004;83(12):930–935. doi: 10.1177/154405910408301208. [DOI] [PubMed] [Google Scholar]
- Xu HHK, Quinn JB, Smith DT, Antonucci JM, Schumacher GE, Eichmiller FC. Dental resin composites containing silica-fused whiskers-effects of whisker-to-silica ratio on fracture toughness and indentation properties. Biomater. 2002b;23:735–742. doi: 10.1016/s0142-9612(01)00178-8. [DOI] [PubMed] [Google Scholar]
- Xu HHK, Quinn JB, Smith DT, Giuseppetti AA, Eichmiller FC. Effects of different whiskers on the reinforcement of dental resin composites. Dent Mater. 2003;19:359–367. doi: 10.1016/s0109-5641(02)00078-7. [DOI] [PubMed] [Google Scholar]
- Xu HHK, Smith DT, Eichmiller FC, Schumacher GE, Antonucci JM. Indentation modulus and hardness of whisker-reinforced heat-cured dental resin composites. Dent Mater. 2000;16:248–254. doi: 10.1016/s0109-5641(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Yiu CKY, King NM, Pashley DH, Suh BI, Carvalho RM, Carrihlo MRO, Tay FR. Effect of resin hydrophilicity and water storage on resin strength. Biomater. 2004;25:5789–5796. doi: 10.1016/j.biomaterials.2004.01.026. [DOI] [PubMed] [Google Scholar]
- Yoshida K, Condon JR, Atsuta M, Ferracane JL. Flexural fatigue strength of CAD/CAM composite material and dual-cured resin luting cements. Am J Dent. 2003;16(3):177–180. [PubMed] [Google Scholar]