Abstract
Stress-mediated elevations in circulating glucocorticoid levels lead to corresponding rapid declines in testosterone production by Leydig cells in the testis. In previous studies we have established that glucocorticoids act on Leydig cells directly, through the classic glucocorticoid receptor (GR), and that access to the GR is controlled prior to the GR by a metabolizing pathway mediated by the type 1 isoform of 11β-hydroxysteroid dehydrogenase (11βHSD1). This enzyme is bidirectional (with both oxidase and reductase activities) and in the rat testis is exclusively localized in Leydig cells where it is abundantly expressed and may catalyze the oxidative inactivation of glucocorticoids. The predominant reductase direction of 11βHSD1 activity in liver cells is determined by an enzyme, hexose-6-phosphate dehydrogenase (H6PDH), on the luminal side of the smooth endoplasmic reticulum (SER). Generation of the pyridine nucleotide cofactor NADPH by H6PDH stimulates the reductase direction of 11βHSD1 resulting in increased levels of active glucocorticoids in liver cells. Unlike liver cells, steroidogenic enzymes including 17β-hydroxysteroid dehydrogenase 3 (17βHSD3) forms the coupling with 11βHSD1. Thus the physiological concentrations of androstenedione serve as a substrate for 17βHSD3 utilizing NADPH to generate NADP+, which drives 11βHSD1 in Leydig cells primarily as an oxidase; thus eliminating the adverse effects of glucocorticoids on testosterone production. At the same time 11βHSD1 generates NADPH which promotes testosterone biosynthesis by stimulating 17βHSD3 in a cooperative cycle. This enzymatic coupling constitutes a rapid mechanism for modulating glucocorticoid control of testosterone biosynthesis. Under stress conditions, glucocorticoids also have rapid actions to suppress cAMP formation thus to lower testosterone production.
1. Introduction
In mammalian species, glucocorticoids induce a variety of responses in cells, including proliferation, differentiation and apoptosis with both rapid and delayed responses. The delayed responses of glucocorticoids are mediated mostly via the nuclear glucocorticoid receptor (GR) [1]. Effects of glucocorticoids are exerted through their GRs which when bound to ligand; associate with specific DNA sequences, termed glucocorticoid response elements (GREs). The GREs are present in the promoter regions of target genes, and mediate either increased or repressed transcription of these genes [2]. In addition, GRs can interfere with the transcriptional activities of other factors such as the AP-1 complex by direct protein-to-protein interactions [3]. Accumulation of evidence also points out that the rapid responses of glucocorticoids act via mechanisms not associated with the classic GRs and possibly via the plasma membrane receptor or prereceptor-mediated action by the glucocorticoid metabolizing enzyme 11β-hydroxysteroid dehydrogenase I (11βHSD1). Rapid actions of glucocorticoids may be involved in the regulation of testosterone production in the Leydig cell, the cell that resides in the interstitial area of the testis. The present review will focus on the rapid mechanisms of glucocorticoid regulation of testosterone production in the Leydig cell.
2. Stress and Leydig Cell Function
Stress is a widespread condition and the "wear and tear" experience as animals adjust to the continually changing environment [4]. Although there are some positive influences, stressors result in many harmful actions to the stressed bodies such as the disrupted reproductive system. The stress response is comprised of the physiological adjustments made to compensate for the stressor and preserve balance of the internal milieu of the body [5]. An over abundance of glucocorticoid activity is the hallmark of stress [4]. Increased glucocorticoid levels disrupt and suppress endocrine signaling in the male reproductive axis. Elevations in circulating corticosteroid after stresses result in a significant drop in testosterone secretion [6, 7]. A similar pattern is observed in rats subjected to restrained stress [8–11]. In humans, the severe psychological stress brought on by the death of a relative or spouse consistently lowers the sperm count [12]. This potential reduction in fertility is most likely caused by the stress-induced decline in testosterone, although direct effects of stress on the seminiferous epithelium have also been reported [13]. Some of the earliest studies of gonadal and sexual dysfunction associated with elevated circulating cortisol were made on men with Cushing’s syndrome [14]. These patients already had low plasma testosterone concentrations, which were further suppressed during the administration of dexamethasone given for diagnostic reasons. Other manifestations of stress, including anesthesia, surgery, anticipation of battle, or training stress show the same reciprocal relationship of glucocorticoid rise and testosterone decrease [15].
Stress increases serum glucocorticoids, and a number of in vitro studies have shown that glucocorticoid directly inhibits testosterone production by Leydig cells [10, 16]. Sharp increases in glucocorticoid levels accompany exposure to stressors such as restrained [8–11] and psychosocial interaction with a dominant male [17, 18] are associated with the decline of testosterone levels in these animals. Apparently, the classic GRs are involved in these glucocorticoid actions. Leydig cells express classic GRs [19–21]. The Leydig cell responds to glucocorticoids. Glucocorticoids have been found to directly inhibit the transcription of genes encoding testosterone biosynthetic enzymes such as cytochrome P450-dependent cholesterol side chain cleavage enzyme, and cytochrome P450-dependent 17α-hydroxylase/C17–C20lyase [16, 22]. Cholesterol transporting protein steroidogenic acute regulatory protein (StAR) gene is also repressed by glucocorticoids [23]. Our studies have shown that excessive glucocorticoid exposures not only inhibit androgen synthesis but may also decrease serum testosterone levels by inducing Leydig cell apoptosis, reducing the numbers of Leydig cells per testis [24, 25]. It is important to note that the harmful effects of stress on Leydig cells do not necessarily result only from classic GR-mediated delayed mechanism. A rapid regulation through non-genomic membrane signaling or pre-receptor regulation of classic GRs by glucocorticoid metabolizing enzyme, 11βHSD1, may also be involved.
3. Rapid glucocorticoid actions on Leydig cell
3.1. Rapid glucocorticoid actions via membrane receptors
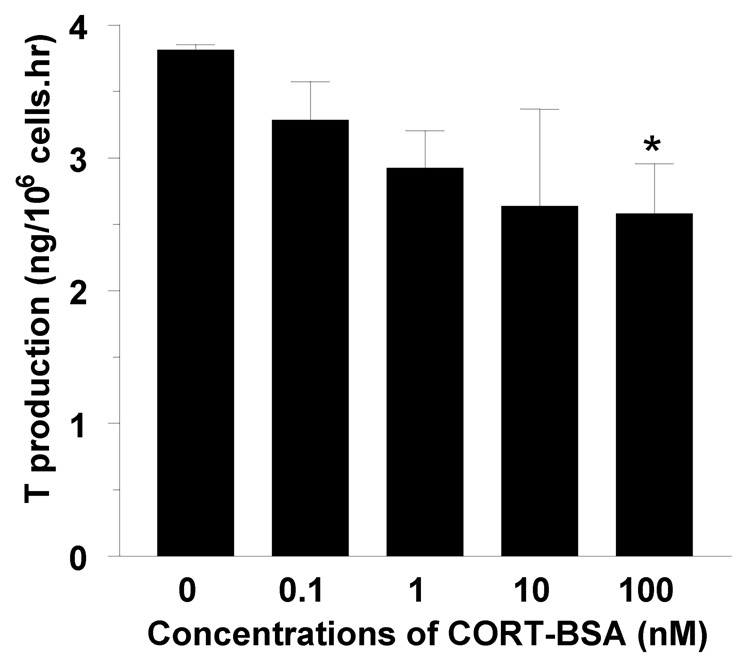
There is growing evidence that glucocorticoids exert various rapid effects on different cells and tissues. The rapid glucocorticoid effects have been confirmed in many cells or tissues including Leydig cells, muscle cells, brain, pancreas, heart, adipose tissue and the immune system. Sometimes, the rapid effects of glucocorticoids appear to be the opposite of classic GR effects. For example, glucocorticoids increase insulin levels via delayed classic GR action, whereas they rapidly inhibit release of insulin from pancreatic β-cells [26]. Sometimes, both rapid and delayed actions of glucocorticoids showed the similar results. For examples, glucocorticoids stimulate preadipocyte differentiation through a non-transcriptional epigenetic mechanism mediated through the ligand-binding domain of the GR [27]. A rapid action of glucocorticoids on Leydig cell function was also demonstrated. A rapid (30-min) decline of testosterone production was seen in mouse Leydig cells when these cells were exposed to 1.44 µM corticosterone (CORT), indicating that a non-genomic mechanism of glucocorticoids in Leydig cells [28], since the genomic mechanism of glucocorticoids requires hours to register at the protein level [29]. CORT can inhibit cAMP formation in mouse Leydig cells within 15 min [28]. The rapid action of glucocorticoids has been hypothesized to occur via the putative membrane corticosteroid receptor. For example, stress-induced elevation of glucocorticoid levels in newt suppressing male reproductive behavior was suggested via rapid actions at a putative membrane corticosteroid receptor [30, 31]. It remains to be investigated whether the nongenomic actions of CORT on testosterone production in Leydig cells are via a membrane receptor or other mechanisms. The membrane receptor hypothesis for CORT rapid action may be explainable since our preliminary data has shown that 100 nM CORT-BSA conjugate can inhibit testosterone production during 15 min (Figure 1). However, there is also a possibility of rapid nongenomic effects of glucocorticoids via the classical intracellular GRs with the membrane components. This case has been confirmed in other nuclear receptor such as estrogen receptor α (ERα). In ERα-expressing pituitary tumor cells (GH3 cells), ERα is located in the plasma membrane and mediates the estrogen-induced rapid release of prolactin, because it is blocked or stimulated directly by antibodies directed to different amino acid sequences of ERα [32].
Figure 1.
Rapid inhibition of testosterone production in rat Leydig cells with corticosterone- BSA conjugate. When 0.5 × 106 Leydig cells cultured with control (n = 6) or different concentrations from 0.1 nM to 100 nM (n = 3, respectively) for 30 minutes. Media were collected for testosterone measurement. *designates the significant difference compared to control at P<0.05.
3.2. A rapid action of glucocorticoids via 11βHSD1
Access of glucocorticoids to the classic GRs is controlled by 11βHSD that catalyzes interconversion of active glucocorticoids (cortisol in human or CORT in rodents) to inert steroids (cortisone in human or 11-dehydrocorticosterone, 11DHC, in rodents). It is now known that there are two isoforms. The type 1 isoform (11βHSD1) first purified from rat liver [33] and then cloned [34] requires pyridine nucleotide cofactor NADP(H) as its cofactor, NADP+ for oxidase which catalyzes the conversion of CORT into 11DHC, or NADPH for reductase to catalyze 11DHC to CORT. It catalyzes both an oxidative and reductive reaction and is referred to as an oxidoreductase. Type 2 isoform (11βHSD2), was identified later [35, 36], and cloned after purification from the kidney [37, 38]. 11βHSD2, having no detectable reductase activity, is known to be an exclusively oxidative enzyme activated by a distinct cofactor, NAD+ [39].
The presence of testicular 11βHSD activity was first noted in the 1960’s by enzyme histochemical labeling of Leydig cells in cryostat sections [40]. Enzyme histochemistry detects the oxidative component of oxidoreductases, with electron transfer to the enzyme’s pyridine cofactor forming NADPH and further electron donation to diaphorase (a ubiquitously-expressed enzyme), and finally to the tetrazolium salt dye, resulting in deposition of blue formazan crystals [40]. When an antibody to 11βHSD1 was produced [41], immunocytochemical staining revealed abundant expression of type 1 protein in rat Leydig cells postnatally [42]. The protein is not present in the fetal Leydig cells that develop in the testis prior to birth, and expressed only weakly in the postnatal generation of Leydig cells until mid-puberty (day 31 postpartum) [42, 43]. Based on an apparent association between the postnatal increases in testicular 11βHSD1 expression and pubertal maturation of testosterone secretion, oxidative inactivation of glucocorticoids by 11βHSD1 within the testis alleviating GR-mediated suppression of androgen biosynthesis in adult rat Leydig cells has been proposed [44]. It has been clearly shown that 11βHSD1 in Leydig cells behaves predominantly as a dehydrogenase, while 11βHSD1 in liver cells behaves predominantly as a reductase [45].
Basal levels of CORT, at approximately 80 nM in the rat [46] are far higher than the dissociation constant of CORT with GR, 5 nM [47] and a mechanism is needed to lower CORT concentrations within Leydig cells, liberating GR from the ligand bound state. Glucocorticoids act directly on Leydig cells to inhibit testosterone biosynthesis [16, 48, 49]. Elevations in circulating glucocorticoids during stress including restraint stresses [8–11] and psychosocial stresses of subordinate rats [17, 18] can, therefore, cause suppression in androgen secretion at the level of the testis. Following this view, 11βHSD in Leydig cells normally acts as a protective gatekeeper, maintaining testosterone biosynthesis in the presence of basal glucocorticoid concentrations: testosterone secretion is suppressed only if the oxidative capacity of 11βHSD is exceeded or the dehydrogenase component of the enzyme is inhibited.
Although the hypothesis for a gatekeeper role of testicular 11βHSD described above appeared reasonable, it fell into conflict with accumulating knowledge of the enzymatic behavior of the two isoforms. When 11βHSD oxidative and reductive activities are measured in freshly isolated Leydig cells, the oxidative activity exceeds the reductive activity by a 2:1 ratio that is the inverse of the ratio measured in freshly isolated hepatocytes assayed in parallel under identical conditions [45]. This primary oxidative activity in rat Leydig cells may be not contributed by 11βHSD2 since it has only one thousandth of 11βHSD1 in this cell type [50].
Evidence for the hypothesis that cell type is a determining influence on whether 11βHSD1 is a net oxidase or net reductase was investigated using two established cell lines, COS1 and CHOP (polyoma virus large T antigen-transfected Chinese Hamster ovary cells, neither of which expresses 11βHSD1 in the unmodified state. The cell lines were transfected with rat 11βHSD1 cDNA, and enzyme activities were assessed 24 hours after exposure to the cDNA. The prevailing direction of the 11βHSD1 reaction was followed by calculating the ratio of oxidative to reductive activity (with estimates equal to one denoting equivalence of the two rates). Transfected COS1 cells behaved similarly to Leydig cells in that 11βHSD1 was initially a net oxidase. The transition in enzyme activity, from a net oxidase to a net reductase, took longer in COS1 cells (2 hours) compared to Leydig cells (40 minutes). In CHOP cells, 11βHSD1 never became a net reductase in vitro: increases in reductase activity over time were offset by increases in oxidase activity [51]. These results indicate that the cellular metabolic environment / milieu of 11βHSD1 is a key determinant of the behavior of the enzyme in vitro.
The oxidative and reductive activities of 11βHSD1 rely on concentrations of pyridine nucleotide cofactor NADP(H). The redox balance of the cell establishes the respective levels of NADP and NADPH, and increased supply of the energy substrate glucose that tips the balance toward NADPH. Depletion of glucose from the culture medium causes a profound suppression of 11βHSD1 reductase activity in Leydig, COS1 and CHOP cells [51]. It has been proposed that 11βHSD1–oxidase activity predominates only when Leydig cells are deprived of glucose [52]. However, the net oxidase observed in Leydig, COS1 and CHOP cells under initial conditions occurred in the presence of a glucose concentration, 5.5 mM, which is characteristic of standard formulations of culture medium. It is therefore unlikely that energy metabolism and redox balance are instrumental in determining the predominance of 11βHSD1 oxidase activity under initial conditions and other NADPH utilizing enzymes which are more reflective of the enzyme’s behavior in vivo. Discrepancies in the literature on the relative predominance oxidation and reduction may have arisen from the use of incubation periods longer than ten minutes, given that linearity of the reaction velocities in assays of intact Leydig cells, necessary for accurate estimation of enzyme kinetic parameters, is lost after ten minutes. Culture of Leydig cells for several days prior to measurement of enzyme activity results in sharp declines in 11βHSD1 oxidative activity and predominance of the reductase [53]. This is not surprising because the two activities are inversely regulated: in rat Leydig cells, protein kinase C increases 11βHSD1 oxidation and inhibits reduction [54].
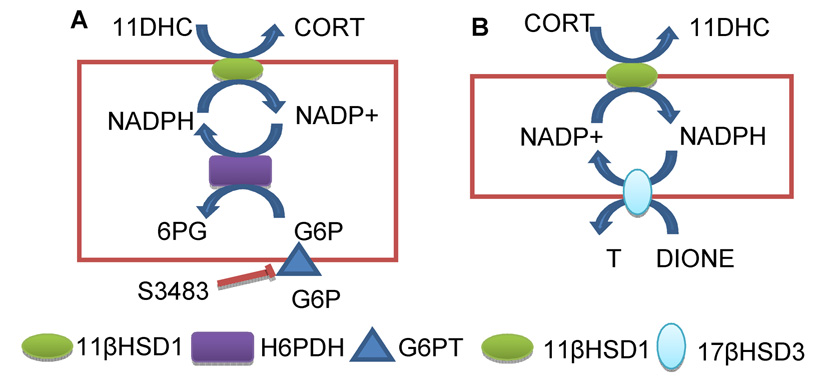
In liver cells 11βHSD1 and hexose-6-phosphate dehydrogenase (H6PDH) are co-localized with their catalytic sites inside the lumen of smooth endoplasmic reticulum (SER) [55, 56]. H6PDH uses NADP+ as a co-factor and glucose-6-phosphate (G6P) as a substrate. It is well established that pyridine nucleotides are impermeable to SER membrane and that G6P can be transported into SER lumen by G6P transporting protein [55–58]. This generates NADPH from NADP+ increasing NADPH/NADP+ ratio which drives 11βHSD1 to function enzymatically as a reductase. Although cytosolic glucose-6-phosphase dehydrogenase (G6PDH) accounts for 95% of NADPH generated by the whole cell, this enzyme does not seem to affect the direction of 11βHSD1. This is supported by the fact that knockdown of this enzyme by RNAi does not change the direction of 11βHSD1 [59]. In contrast, H6PDH, which accounts for only 5% of NADPH production, determines the direction of 11βHSD1 reductase in liver cells, because the enzymes and the co-factors are compartmentalized in the lumen of SER. Therefore, when H6PDH is knocked-out in mice, 11βHSD1 switches from the reductase to oxidase activity in the liver [60]. Similarly, the direction of 11βHSD1 is also switched in the presence of S3483, the inhibitor of G6P transporting protein, which deprives the substrate for H6PDH [55] (Figure 2).
Figure 2.
Models for different couplings between 11βHSD1 and other enzymes in liver and Leydig cells. In liver cells (Panel A), in SER luminal side, H6PDH forms coupling with 11βHSD1: G6P is exported into SER luminal side by G6P exporting protein which can be inhibited by the drug S3483. G6P and NADP+ are used by H6PDH to generate NADPH which renders 11βHSD1 primary as a reductase to activate 11DHC to CORT. In Leydig cells (Panel B), both 11βHSD1 and 17βHSD3 are located in SER membrane and form the coupling. The androgen intermediate androstenedione (DIONE) and NADPH are used by 17βHSD3 to generate NADP+ which renders 11βHSD1 to function primarily as an oxidase to inactivate CORT to 11DHC.
The presence of H6PDH in Leydig cells, however, is controversial since an early histochemical study indicated weak staining of H6PDH in the rat interstitial cells [61], while a recent study has claimed undetectable levels of this enzyme in Leydig cells [62]. In rat Leydig cells, 11βHSD1 reductase activity does not appear to depend on H6PDH, because addition of G6P to Leydig cell microsomes does not increase 11βHSD1 reductase activity, while it robustly stimulates 11βHSD1 reduction in liver microsomes [63].
Unlike the liver, Leydig cell 11βHSD1 directionality may be regulated with different mechanisms. We propose that there is a novel coupling between 11βHSD1 and the enzymes responsible for testosterone biosynthesis in SER, particularly 17β-hydroxysteroid dehydrogenase (17βHSD3). In cells, NADP+ and NADPH are present in catalytic or finite amounts. NADPH generated by 11βHSD1-oxidase reaction is utilized by the steroidogenic enzymes involved in testosterone production, becoming oxidized to NADP+. This is then regenerated to NADPH by 11βHSD1–oxidase. This cyclic reduction/oxidation of this co-factor continues by coupling of these enzymes as long as the substrates for steroidogenic enzymes and 11βHSD1 are available. This is supported by our findings that addition of 17βHSD3 substrate androstenedione in both intact Leydig cells and its microsomes promotes 11βHSD1 oxidase while decreasing the reductase activities. Further, addition of CORT (11βHSD1 oxidase substrate) into Leydig cells increases 17βHSD3 activity to generate testosterone and NADP+ which stimulates 11βHSD1 oxidation in a positive cycle (Figure 2)[63].
This coupling of reactions demonstrates the rapid effects of glucocortcoids (at physiologic concentrations) on the stimulation of testosterone production in Leydig cells. However, under the stress condition, 11βHSD1 is saturated and excessive CORT will cause genomic repression of testosterone production (a delayed glucocorticoid effect) or a rapid mechanism suppressing cAMP levels thus involving a rapid nongenomic repression of testosterone production.
Taken together, these studies summarized above support the hypothesis that there is rapid regulation of glucocorticoids on Leydig cell steroidogenesis. This rapid effect of glucocorticoids is mediated via 11βHSD1 enzyme and also possibly by plasma membrane receptors of glucocorticoids, as demonstrated by the BSA-CORT experiments.
Acknowledgements
The technical assistance of Ms. Chantal Manon Sottas is gratefully acknowledged. The review is written in memory of Dr. Matthew P. Hardy.
* Supported in part by NIH grant HD33000
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schmid W, Cole TJ, Blendy JA, Schutz G. Molecular genetic analysis of glucocorticoid signaling in development. J Steroid Biochem Mol Biol. 1995;53:33–35. doi: 10.1016/0960-0760(95)00038-2. [DOI] [PubMed] [Google Scholar]
- 2.Landers JP, Spelsberg TC. New concepts in steroid hormone action: transcription factors, proto-oncogenes, and the cascade model for steroid regulation of gene expression. Crit Rev.Eukaryot.Gene Expr. 1992;2:19–63. [PubMed] [Google Scholar]
- 3.Jonat C, Rahmsdorf HJ, Park KK, Cato ACB, Gebel S, Ponta H, Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990;62:11189–11204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- 4.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama. 1992;262:1244–1252. [PubMed] [Google Scholar]
- 5.Selye H. The general adaptation syndrome and the disease of adaptation. J.Clin.Endocrinol. 1946;6:117–230. doi: 10.1210/jcem-6-2-117. [DOI] [PubMed] [Google Scholar]
- 6.Baldwin DM. Effects of glucocorticoids on estrogen-dependent LH release in the ovariectomized rat and on gonadotropin secretion in the intact female rat. Endocrinology. 1979;105:120–178. doi: 10.1210/endo-105-1-120. [DOI] [PubMed] [Google Scholar]
- 7.Suter DE, Schwartz NB. Effects of glucocorticoids on secretion of luteinizing hormone and follicle-stimulating hormone by female rat pituitary cell in vitro. Endocrinology. 1985;117:849–854. doi: 10.1210/endo-117-3-849. [DOI] [PubMed] [Google Scholar]
- 8.Orr TE, Mann DR. Effects of restraint stress on plasma LH and testosterone concentrations, Leydig cell LH/hCG receptors, and in vitro testicular steroidogenesis in adult rats. Horm Behav. 1990;24:324–341. doi: 10.1016/0018-506x(90)90013-n. [DOI] [PubMed] [Google Scholar]
- 9.Orr TE, Mann DR. Role of glucocorticoid in the stress-induced suppression of testicular steroidogenesis in adult male rats. Horm Behav. 1992;26:350–363. doi: 10.1016/0018-506x(92)90005-g. [DOI] [PubMed] [Google Scholar]
- 10.Orr TE, Taylor MF, Bhattacharyya AK, Collins DC, Mann DR. Acute immobilization stress disrupts testicular steroidogenesis in adult male rats by inhibiting the activities of 17a-hydroxylase and 17,20-lyase without affecting the binding of LH/hCG receptors. J Androl. 1994;15:302–308. [PubMed] [Google Scholar]
- 11.Dong Q, Sottas CM, Ge R-S, Akingbemi BT, Hardy MP. Serum testosterone levels and puberty in rats treated with di(2-ethylhexyl)phthalate (DEHP). 26, 00. Journal of Andrology. 2005 [Google Scholar]
- 12.Fenster L, Katz DF, Wyrobek AJ, Pieper C, Rempel DM, Oman D, Swan SH. Effects of psychological stress on human semen quality. J.Androl. 1997;18:194–202. [PubMed] [Google Scholar]
- 13.Yazawa H, Sasagawa I, Ishigooka M, Nakada T. Effect of immobilization stress on testicular germ cell apoptosis in rats. Human Reproduction. 1999;14:1806–1810. doi: 10.1093/humrep/14.7.1806. [DOI] [PubMed] [Google Scholar]
- 14.Smals AG, Kloppenborg PW, Benraad TJ. Plasma testosterone profiles in Cushing's syndrome. J.Clin.Endocrinol.Metab. 1977;45:240–245. doi: 10.1210/jcem-45-2-240. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima A, KK, TU, YM, YH, Kurachi K, Aono T, Mizutani S, Matsumoto K. Effects of general anesthesia and severity of surgical stress on serum LH and testosterone in males. Acta Endocrinol. 1975;78:258–269. doi: 10.1530/acta.0.0780258. [DOI] [PubMed] [Google Scholar]
- 16.Hales DB, Payne AH. Glucocorticoid-mediated repression of P450scc mRNA and de novo synthesis in cultured Leydig cells. Endocrinology. 1989;124:2099–2104. doi: 10.1210/endo-124-5-2099. [DOI] [PubMed] [Google Scholar]
- 17.Monder C, Sakai RR, Miroff Y, Blanchard DC, Blanchard RJ. Reciprocal changes in plasma corticosterone and testosterone in stressed male rats maintained in a visible burrow system: evidence for a mediating role of testicular beta-hydroxysteroid dehydrogenase. Endocrinology. 1994;134:1193–1198. doi: 10.1210/endo.134.3.8119159. [DOI] [PubMed] [Google Scholar]
- 18.Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67:1750–1755. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- 19.Stalker A, Hermo L, Antakly T. Covalent affinity labeling, radioautography, and immunocytochemistry localize the glucocorticoid receptor in rat testicular Leydig cells. Am J Anat. 1989;186:369–377. doi: 10.1002/aja.1001860406. [DOI] [PubMed] [Google Scholar]
- 20.Schultz R, Isola J, Parvinen M, Honkaniemi J, Wikstrom AC, Gustafsson JA, Pelto-Huikko M. Localization of the glucocorticoid receptor in testis and accessory sexual organs of male rat. Mol Cell Endocrinol. 1993;95:115–120. doi: 10.1016/0303-7207(93)90036-j. [DOI] [PubMed] [Google Scholar]
- 21.Ge RS, Hardy DO, Catterall JF, Hardy MP. Developmental changes in glucocorticoid receptor and 11b-hydroxysteroid dehydrogenase oxidative and reductive activities in rat Leydig cells. Endocrinology. 1997;138:5089–5095. doi: 10.1210/endo.138.12.5614. [DOI] [PubMed] [Google Scholar]
- 22.Payne AH, Sha LL. Multiple mechanisms for regulation of 3b-hydroxysteroid dehydrogenase/D5----D4-isomerase, 17a-hydroxylase/C17-20 lyase cytochrome P450, and cholesterol side-chain cleavage cytochrome P450 messenger ribonucleic acid levels in primary cultures of mouse Leydig cells. Endocrinology. 1991;129:1429–1435. doi: 10.1210/endo-129-3-1429. [DOI] [PubMed] [Google Scholar]
- 23.Wang XJ, Walsh LP, Reinhart AJ, Stocco DM. The role of arachidonic acid in steroidogenesis and steroidogenic acute regulatory (StAR) gene and protein expression. J Biol Chem. 2000;275:20204–20209. doi: 10.1074/jbc.M003113200. [DOI] [PubMed] [Google Scholar]
- 24.Gao HB, Tong MH, Hu YQ, Guo QS, Ge R, Hardy MP. Glucocorticoid induces apoptosis in rat Leydig cells. Endocrinology. 2002;143:130–138. doi: 10.1210/endo.143.1.8604. [DOI] [PubMed] [Google Scholar]
- 25.Gao HB, Tong MH, Hu YQ, You HY, Guo QS, Ge RS, Hardy MP. Mechanisms of glucocorticoid-induced Leydig cell apoptosis. Molecular & Cellular Endocrinology. 2003;199:153–163. doi: 10.1016/s0303-7207(02)00290-3. [DOI] [PubMed] [Google Scholar]
- 26.Sutter-Dub MT. Rapid non-genomic and genomic responses to progestogens, estrogens, and glucocorticoids in the endocrine pancreatic B cell, the adipocyte and other cell types. Steroids. 2002;67:77–93. doi: 10.1016/s0039-128x(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 27.Wiper-Bergeron N, Wu D, Pope L, Schild-Poulter C, Hache RJ. Stimulation of preadipocyte differentiation by steroid through targeting of an HDAC1 complex. EMBO J. 2003;22:2135–2145. doi: 10.1093/emboj/cdg218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. Journal of Andrology. 2004;25:972–981. doi: 10.1002/j.1939-4640.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 29.Hyde GN, Seale AP, Grau EG, Borski RJ. Cortisol rapidly suppresses intracellular calcium and voltage-gated calcium channel activity in prolactin cells of the tilapia (Oreochromis mossambicus) Am J Physiol Endocrinol Metab. 2004;286:626–633. doi: 10.1152/ajpendo.00088.2003. [DOI] [PubMed] [Google Scholar]
- 30.Rose JD, Moore FL, Orchinik M. Rapid neurophysiological effects of corticosterone on medullary neurons: relationship to stress-induced suppression of courtship clasping in an amphibian. Neuroendocrinology. 1993;57:815–824. doi: 10.1159/000126440. [DOI] [PubMed] [Google Scholar]
- 31.Rose JD, Moore FL. Behavioral neuroendocrinology of vasotocin and vasopressin and the sensorimotor processing hypothesis. Front Neuroendocrinol. 2002;23:317–341. doi: 10.1016/s0091-3022(02)00004-3. [DOI] [PubMed] [Google Scholar]
- 32.Norfleet AM, Thomas ML, Gametchu B, Watson CS. Estrogen receptor-alpha detected on the plasma membrane of aldehyde-fixed GH3/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology. 1999;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- 33.Lakshmi V, Monder C. Purification and characterization of the corticosteroid 11b-dehydrogenase component of the rat liver 11b-hydroxysteroid dehydrogenase complex. Endocrinology. 1988;123:2390–2398. doi: 10.1210/endo-123-5-2390. [DOI] [PubMed] [Google Scholar]
- 34.Agarwal AK, Monder C, Eckstein B, White PC. Cloning and expression of rat cDNA encoding corticosteroid 11b-dehydrogenase. J.Biol.Chem. 1989;264:18939–18943. [PubMed] [Google Scholar]
- 35.Naray-Fejes-Toth A, Rusvai E, Denault DL, St.Germain DL, Fejes-Toth G. Expression and characterization of a new species of 11 beta-hydroxysteroid dehydrogenase in Xenopus oocytes. Am J Physiol. 1993;265:896–900. doi: 10.1152/ajprenal.1993.265.6.F896. [DOI] [PubMed] [Google Scholar]
- 36.Rusvai E, Naray-Fejes-Toth A. A new isoform of 11b-hydroxysteroid dehydrogenase in aldosterone target cells. J Biol Chem. 1993;268:10717–10720. [PubMed] [Google Scholar]
- 37.Agarwal AK, Mune T, Monder C, White PC. NAD(+)-dependent isoform of 11b-hydroxysteroid dehydrogenase. Cloning and characterization of cDNA from sheep kidney. J Biol Chem. 1994;269:25959–25962. [PubMed] [Google Scholar]
- 38.Albiston AL, Obeyesekere VR, Smith RE, Krozowski ZS. Cloning and tissue distribution of the human 11b-hydroxysteroid dehydrogenase type 2 enzyme. Mol.Cell.Endocrinol. 1994;105:R11–R17. doi: 10.1016/0303-7207(94)90176-7. [DOI] [PubMed] [Google Scholar]
- 39.Stewart PM, Krozowski ZS. 11b-Hydroxysteroid dehydrogenase. Vitamins and Hormones. 1999;57:249–324. [PubMed] [Google Scholar]
- 40.Baillie AH, Ferguson MM, Calman KC, Hart DM. Histochemical demonstration of 11b-hydroxysteroid dehydrogenase. J Endocrinol. 1965;33:119–125. doi: 10.1677/joe.0.0330119. [DOI] [PubMed] [Google Scholar]
- 41.Edwards CR, Stewart PM, Burt D, Brett L, McIntyre MA, Sutanto WS, deKloet ER, Monder C. Localisation of 11b-hydroxysteroid dehydrogenase--tissue specific protector of the mineralocorticoid receptor. Lancet. 1988;2:986–989. doi: 10.1016/s0140-6736(88)90742-8. [DOI] [PubMed] [Google Scholar]
- 42.Phillips DM, Lakshmi V, Monder C. Corticosteroid 11b-dehydrogenase in rat testis. Endocrinology. 1989;125:209–216. doi: 10.1210/endo-125-1-209. [DOI] [PubMed] [Google Scholar]
- 43.Haider SG, Passia D, Rommerts FF. Histochemical demonstration of 11b-hydroxysteroid dehydrogenase as a marker for Leydig cell maturation in rat. Acta Histochemica. 1990;38:203–207. [PubMed] [Google Scholar]
- 44.Monder C, Sakai RR, Blanchard RJ, Blanchard DC, Lakshmi V, Miroff Y, Phillips DM, Hardy M. The mediation of testicular function by 11b-hydroxysteroid dehydrogenase. In: Boublik JH, Funder JW, Sheppard KE, editors. Stress and Reproduction. New York: Raven Press; 1992. pp. 145–155. [Google Scholar]
- 45.Ge RS, Gao HB, Nacharaju VL, Gunsalus GL, Hardy MP. Identification of a kinetically distinct activity of 11b-hydroxysteroid dehydrogenase in rat Leydig cells. Endocrinology. 1997;138:2435–2442. doi: 10.1210/endo.138.6.5165. [DOI] [PubMed] [Google Scholar]
- 46.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20:117–134. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka J, Fujita H, Matsuda S, Toku K, Sakanaka M, Maeda N. Glucocorticoid- and mineralocorticoid receptors in microglial cells: The two receptors mediate differential effects of corticosteroids. Glia. 1997;20:23–37. [PubMed] [Google Scholar]
- 48.Bambino T, Hsueh A. Direct inhibitory effect of glucocorticoids upon testicular luteinizing hormone receptor and steroidogenesis in vivo and in vitro. Endocrinology. 1981;108:2142–2148. doi: 10.1210/endo-108-6-2142. [DOI] [PubMed] [Google Scholar]
- 49.Welsh TH, Bambino TH, Hsueh AJW. Mechanism of glucocorticoid-induced suppression of testicular androgen biosynthesis in vitro. Biol Reprod. 1982;27:1138–1146. doi: 10.1095/biolreprod27.5.1138. [DOI] [PubMed] [Google Scholar]
- 50.Ge R-S, Dong Q, Niu E, Sottas CM, Hardy DO, Catterall JF, Latif SA, Morris DJ, Hardy MP. 11b-Hydroxysteroid dehydrogenase 2 in rat Leydig cells: its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology. 2005;146:2657–2664. doi: 10.1210/en.2005-0046. [DOI] [PubMed] [Google Scholar]
- 51.Ge RS, Hardy MP. Initial predominance of the oxidative activity of type I 11b-hydroxysteroid dehydrogenase in primary rat Leydig cells and transfected cell lines. J Androl. 2000;21:303–310. [PubMed] [Google Scholar]
- 52.Ferguson SE, Pallikaros Z, Michael AE, Cooke BA. The effects of different culture media, glucose, pyridine nucleotides and adenosine on the activity of 11b-hydroxysteroid dehydrogenase in rat Leydig cells. Molecular & Cellular Endocrinology. 1999;158:37–44. doi: 10.1016/s0303-7207(99)00186-0. [DOI] [PubMed] [Google Scholar]
- 53.Leckie CM, Welberg LA, Seckl JR. 11b-hydroxysteroid dehydrogenase is a predominant reductase in intact rat Leydig cells. J Endocrinol. 1998;159:233–238. doi: 10.1677/joe.0.1590233. [DOI] [PubMed] [Google Scholar]
- 54.Ge RS, Hardy MP. Protein kinase C increases 11b-hydroxysteroid dehydrogenase oxidation and inhibits reduction in rat Leydig cells. Journal of Andrology. 2002;23:135–143. doi: 10.1002/j.1939-4640.2002.tb02606.x. [DOI] [PubMed] [Google Scholar]
- 55.Banhegyi G, Benedetti A, Fulceri R, Senesi S. Cooperativity between 11b-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase in the lumen of the endoplasmic reticulum. J Biol Chem. 2004;279:27017–27021. doi: 10.1074/jbc.M404159200. [DOI] [PubMed] [Google Scholar]
- 56.Atanasov AG, Nashev LG, Schweizer RA, Frick C, Odermatt A. Hexose-6-phosphate dehydrogenase determines the reaction direction of 11b-hydroxysteroid dehydrogenase type 1 as an oxoreductase. FEBS Lett. 2004;571:129–133. doi: 10.1016/j.febslet.2004.06.065. [DOI] [PubMed] [Google Scholar]
- 57.Bujalska IJ, Draper N, Michailidou Z, Tomlinson JW, White PC, Chapman KE, Walker EA, Stewart PM. Hexose-6-phosphate dehydrogenase confers oxo-reductase activity upon 11b-hydroxysteroid dehydrogenase type 1. J Mol Endocrinol. 2005;34:675–684. doi: 10.1677/jme.1.01718. [DOI] [PubMed] [Google Scholar]
- 58.Draper N, Walker EA, Bujalska IJ, Tomlinson JW, Chalder SM, Arlt W, Lavery GG, Bedendo O, Ray DW, Laing I, Malunowicz E, White PC, Hewison M, Mason PJ, Connell JM, Shackleton CH, Stewart PM. Mutations in the genes encoding 11b-hydroxysteroid dehydrogenase type 1 and hexose-6-phosphate dehydrogenase interact to cause cortisone reductase deficiency. Nat Genet. 2003;34:434–439. doi: 10.1038/ng1214. [DOI] [PubMed] [Google Scholar]
- 59.Hewitt KN, Walker EA, Stewart PM. Minireview: hexose-6-phosphate dehydrogenase and redox control of 11{beta}-hydroxysteroid dehydrogenase type 1 activity. Endocrinology. 2005;146:2539–2543. doi: 10.1210/en.2005-0117. [DOI] [PubMed] [Google Scholar]
- 60.Lavery GG, Walker EA, Draper N, Jeyasuria P, Marcos J, Shackleton CH, Parker KL, White PC, Stewart PM. Hexose-6-phosphate dehydrogenase knock-out mice lack 11 beta-hydroxysteroid dehydrogenase type 1-mediated glucocorticoid generation. J.Biol.Chem. 2006;281:6546–6551. doi: 10.1074/jbc.M512635200. [DOI] [PubMed] [Google Scholar]
- 61.Tanahashi K, Hori SH. Immunohistochemical localization of hexose 6-phosphate dehydrogenase in various organs of the rat. J.Histochem.Cytochem. 1980;28:1175–1182. doi: 10.1177/28.11.7430614. [DOI] [PubMed] [Google Scholar]
- 62.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Fonfara G, Krozowski Z, Gomez-Sanchez EP. Immunohistochemical distribution of hexose-6-phosphate dehydrogenase and 11beta-hydroxysteroid dehydrogenase; Annual meeting of the Endocrine Society; 2005. pp. P1–P335. [Google Scholar]
- 63.Ge R-S, Dong Q, Sottas CM, Morris DJ, Hardy MP. Coupling between Type 1 11beta-Hydroxysteroid Dehydrogenase and Enzymes of Testosterone Synthesis in Rat Leydig Cells. Annual meeting of the Endocrine Society; San Diego. 2005. pp. p2–p238. p2-238. [Google Scholar]